Translate this page into:
The mechanism of L1 cell adhesion molecule interacting with protein tyrosine kinase 2 to regulate the focal adhesion kinase–growth factor receptor-bound protein 2–son of sevenless–rat sarcoma pathway in the identification and treatment of type I high-risk endometrial cancer

*Corresponding author: Wenhua Tan, Department of Gynaecology and Obstetrics, The 2nd Affiliated Hospital of Harbin Medical University, Harbin, China. tanwenhua1962@126.com
-
Received: ,
Accepted: ,
How to cite this article: He W, Liu W, Liu X, Tan W. The mechanism of L1 cell adhesion molecule interacting with protein tyrosine kinase 2 to regulate the focal adhesion kinase–growth factor receptor-bound protein 2–son of sevenless–rat sarcoma pathway in the identification and treatment of type I high-risk endometrial cancer. CytoJournal. 2024; 21:34. doi: 10.25259/Cytojournal_50_2024
Abstract
Objective:
The objective of this study was to investigate how L1 cell adhesion molecule (L1CAM) interacting with protein tyrosine kinase 2 (PTK2) affects endometrial cancer (EC) progression and determine its association with the focal adhesion kinase (FAK)–growth factor receptor-bound protein 2 (GRB2)–son of sevenless (SOS)–rat sarcoma (RAS) pathway. EC is a female cancer of major concern in the world, and its incidence has increased rapidly in recent years. L1CAM is considered a reliable marker of poor prognosis in patients with EC.
Material and Methods:
A single-center and prospective study was conducted using data from the Cancer Genome Atlas and samples from normal and EC tissues to explore the differential expression of L1CAM. Additional experimental models included human immortalized endometrial epithelium cells (hEECs) and EC cell lines such as KLE, RL95-2, and Ishikawa. L1CAM expression was regulated using lentiviruses designed for either overexpression or interference, and PTK2/focal adhesion kinase (FAK) signaling was inhibited with PF431396. Transfected KLE cells were injected into mice, and tumor growth was monitored over 14 days. Cellular proliferation and survival were assessed using cell counting kit, colony formation, and terminal deoxynucleotidyl transferase-mediated 2’-deoxyuridine 5’-triphosphate (dUTP) nick-end labeling assays. Metastatic behavior was evaluated through Transwell assays for cell migration and invasion. The expression levels of matrix metallopeptidase (MMP) 2 and MMP9 were determined by Western blot. In addition, the activation of the FAK–GRB2–SOS–RAS pathway was examined by assessing the protein levels of FAK, GRB2, SOS, and RAS.
Results:
There was a significant difference in L1CAM expression between EC tumor tissues and normal tissues, and L1CAM messenger RNA (1.85-fold) and L1CAM protein (2.59-fold) were significantly more expressed in EC tissues (P < 0.01) than in normal tissues. The tumor growth of L1CAM overexpressing EC cells was faster than that of negative control EC cells (6.43 fold; P < 0.001). L1CAM promoted the expression of FAK (1.43-2.72-fold; P < 0.001); enhanced EC cell proliferation (P < 0.01), survival and motility (P < 0.001), migration (P < 0.001), and invasion (P < 0.001); and activated the FAK–GRB2–SOS–RAS pathway, all of which were reversed when FAK expression was not upregulated (P < 0.001).
Conclusion:
By upregulating PTK2 and its encoded protein FAK, L1CAM was found to promote tumor progression and increase the activation of the FAK–GRB2–SOS–RAS pathway. These findings establish L1CAM and PTK2 as reference genes for poor prognostic prediction in EC and as targets for EC therapy, providing a valuable basis for distinguishing between benign and malignant endometrial conditions and justifying the necessity of targeted therapeutic approaches.
Keywords
Endometrial cancer
L1 cell adhesion molecule
Protein tyrosine kinase 2
Focal adhesion kinase
Growth factor receptor-bound protein 2
INTRODUCTION
Endometrial cancer (EC) ranks as the sixth most common cancer among women worldwide and is the most prevalent gynecological malignancy in high-income countries.[1] Global reports in 2020 estimated 417,000 new cases and 97,000 deaths.[1] The incidence rate of EC is rapidly increasing; according to the International Agency for Research on Cancer, it is projected to rise by more than 50% worldwide by 2040.[2] Compared with low-income countries where the incidence rate of EC is around 3.3/100,000 females, high-income countries have a higher incidence rate of EC that is around 11.1/100,000 females.[3] The majority of EC is type I tumors, which account for 80% of all EC cases and are commonly presented as low-grade endometrioid histology.[4] Women with stage I EC mostly have favorable prognosis.[5] However, 15–20% of patients with EC, including patients in type I, are classified as high risk for the occurrence of distant metastasis- and disease-related death.[6] Therefore, an in-depth understanding of EC progression is needed, and novel therapeutic targets are required for developing promising adjuvant treatments to treat patients with EC, including patients in low stage but with high risk.
According to the previous studies, the L1 cell adhesion molecule (L1CAM), as a main unfavorable factor, is a reliable biomarker for predicting poor prognosis in patients with EC,[7-9] especially among patients with EC in Federation Internationale de Gynecologie et d’Obstetrique Stage I, type I.[10] However, the underlying mechanisms by which L1CAM confers the characteristic phenotype to cancer cells remain unclear.[11] Focal adhesion kinase (FAK), encoded by the protein tyrosine kinase 2 (PTK2) gene, plays key roles in cancers, driving invasion, migration, and poor prognosis.[12] In mouse models, FAK promotes the invasion of skin and breast cancer, as well as the metastasis from breast to lung.[13,14] In addition, the overexpression of FAK is associated with unfavorable prognostic outcomes and potentially induces resistance to treatments.[15,16] Moreover, studies have demonstrated that L1CAM mediates the activation of FAK through its interaction with PTK2, thereby promoting the motility of human glioma cells.[17-19] FAK signaling has been shown to promote the formation and progression of EC.[20-22] It has been reported that the increased phosphorylation of FAK leads to the activation of rat sarcoma (RAS) cascade through leading to the binding of growth factor receptor-bound protein 2 (GRB2)-son of sevenless (SOS) complex which is the key regulator of RAS pathway.[23]
Considering that metastasis is the predominant cause of mortality associated with EC,[22] we hypothesized that the facilitative role of L1CAM in the progression of type I EC is mediated through its interaction with PTK2, which subsequently triggers the FAK–GRB2–SOS–RAS signaling pathway. This investigation seeks to enhance our comprehension of L1CAM’s involvement in EC pathogenesis and to identify novel therapeutic targets, potentially leading to the development of innovative adjuvant treatments that may improve the prognosis of patients with EC.
MATERIAL AND METHODS
Ethical approval and consent to participate
This investigation was conducted in strict compliance with the principles outlined in the Declaration of Helsinki and received approval from the Ethics Committee of the 2nd Affiliated Hospital of Harbin Medical University (Approval No: YJSKY2023-316). Before their participation, all subjects were comprehensively briefed on the study parameters and required to provide written informed consent.
Cell culture
Human immortalized endometrial epithelium cells (hEECs) were obtained (MZ-3223, MingzhouBio, Ningbo, China), and human EC cell lines of KLE (iCell-h317), RL95-2 (iCell-h182), and Ishikawa (iCell-h113) were obtained (iCell Bioscience Inc., Shanghai, China). All cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; iCell-0005, iCell Bioscience Inc., Shanghai, China), which was supplemented with 1% penicillin, streptomycin (15070063, Thermo Fisher Scientific, Waltham, MA, USA), and 10% fetal bovine serum (FBS; iCell-0500), under the culture conditions of 37℃, 70–80% humidity, and 5% carbon dioxide (CO2). Cell lines have been authenticated by polymorphic short tandem repeat profiling. All cell lines were tested for mycoplasma infection on receipt using LookOut® Mycoplasma PCR Detection Kit (Sigma-Aldrich, Saint Louis, Missouri, USA).
Lentivirus cell transfection
The L1CAM overexpression lentivirus (OL-L1CAM) and corresponding negative control (OL-NC), L1CAM interference lentivirus (IL-L1CAM), and negative control lentivirus (IL-NC) were obtained from GenePharma Co., Ltd. (Shanghai, China). Cells were inoculated into a 24-well plate (1 × 105/well) at 37℃ and 5% CO2. The culture medium was removed when the number of cells reached about 2 × 105/well, and fresh medium of 0.5mL was added. In addition, polybrene (10μg/mL) and lentivirus suspension (1 × 108TU/mL, 10μL) were added into the fresh medium for transfection. After lentivirus transfection, the transfected cells were screened through puromycin selection. The cells were cultured in dulbecco’s modified eagle medium (DMEM) supplemented with 2 μg/mL puromycin (A1113802, Thermo Fisher Scientific, Waltham, MA, USA) for 5 days. Successfully transfected cells were identified by the expression of GFP, which was observed under a fluorescence microscope. The L1CAM isoform used in this study corresponded to the accession number NM_001278116.2. The sequences of the siRNAs used for L1CAM knockdown were as follows: siRNA1: 5’-GCUCAUCGAGGCUAUGUUA-3’, siRNA2: 5’-CGGAUAUCCUGCUAUAGGA-3’, and siRNA3:5’-GGCUAUGUCUGGCAUGUAA-3’.
Details of the patients’ baseline characteristics for the Cancer Genome Atlas (TCGA)
This study included a total of 305 patients with EC for The Cancer Genome Atlas (TCGA). The average age of these patients was 65.3 years (standard deviation [SD] = 8.6 years), the average weight was 63.1 kg (SD = 10.5 kg), and the average height was 157.5 cm (SD = 7.5 cm), with an average body mass index of 27.7 (SD = 7.8). Regarding menopausal status, 46 (15%) were premenopausal, 216 (71%) were postmenopausal, and 43 (14%) were perimenopausal.
Cell counting kit (CCK)-8 assay
In accordance with the manufacturer’s protocols, cells were initially seeded into 96-well plates at a density of 1 × 104 cells per well. Subsequently, 10 μL of CCK-8 solution (MA0218-5, Meilunbio, Dalian, China) was added to each well. Following incubation at 37°C for 24, 48, 72, and 96 h, absorbance at 450 nm was measured using a Cmax Plus spectrophotometer (Molecular Devices Corporation, Silicon Valley, USA).
Colony forming assay
Each well of 6-well plates was plated with 1 × 106 cells and allowed them to incubate until visible colonies formed, which typically took 21 days. Following a 15 min room temperature fixation with pure methanol, the cells were stained for 20 min with 0.1% crystal violet (C0121, Beyotime, Shanghai, China). Subsequently, colonies were examined and quantified using a CKX53 microscope (OLYMPUS, Tokyo, Japan).
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay
Following the TUNEL kit protocol (C1088, Beyotime, Shanghai, China), deparaffinized and rehydrated tissue sections were initially incubated with proteinase K (15 μg/mL) for 20 min at 37°C. Subsequent to rinsing the sections with phosphate buffer saline, they were stained with 2-(4-Amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; C1002, Beyotime, Shanghai, China). TUNEL-positive cells were then examined using a CKX53 microscope (OLYMPUS, Tokyo, Japan) and analyzed by Image J software (v1.8, National Institutes of Health, Bethesda, MD, USA).
Transwell assay
24-well Transwell plates with 8 μm pore inserts were used for the migration experiment. To conduct the invasion assay, 30 min were spent incubating 8 μm pore inserts that had been pre-coated with Matrigel (356234, BD Biosciences, Franklin Lakes, New Jersey, USA). The upper chambers were loaded with 2 × 104 cells in cell culture medium, while the lower chambers contained DMEM supplemented with 10% FBS. After 24 h of incubation, cells and Matrigel were removed using cotton-tipped swabs. Subsequently, filters were fixed with 4% formaldehyde (P0099, Beyotime, Shanghai, China) for 15 min at 25 °C and stained with 0.1% crystal violet solution (C0121, Beyotime, Shanghai, China) for 30 min at room temperature. The cells were then examined using a light contrast microscope (CKX53, OLYMPUS, Tokyo, Japan).
Western blot assay
Ice-cold radio immunoprecipitation assay buffer (P0013, Beyotime, Shanghai, China) was supplemented with 1× protease inhibitor (ST505, Beyotime, Shanghai, China) for cell lysate preparation. Cell lysates were centrifuged for 15 min at 4°C at 14,000 rpm. The supernatants containing total proteins were then collected and subjected to separation by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (P0012, Beyotime, Shanghai, China). Proteins were subsequently transferred onto polyvinylidene fluoride membranes (IPVH15150, Millipore Sigma, Billerica, MA, USA), which were initially blocked with 2% fat-free milk and subsequently incubated with primary antibodies specific for L1CAM (1:1000 dilution; ab270455, Abcam, Cambridge, Massachusetts, USA), FAK (1:1000 dilution; ab40794, Abcam, Cambridge, MassachusettsMA, USA), GRB2 (1:1000 dilution; ab32111, Abcam, Cambridge, Massachusetts, USA), SOS (1:1000 dilution; ab140621, Abcam, Cambridge, Massachusetts, USA), RAS (1:1000 dilution; ab108602, Abcam, Cambridge, Massachusetts, USA), MMP2 (1:1000 dilution; ab92536, Abcam, Cambridge, Massachusetts, USA), MMP9 (1:1000 dilution; ab76003, Abcam, Cambridge, Massachusetts, USA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:2000 dilution; ab8245, Abcam, Cambridge, Massachusetts, USA). Following primary antibody incubation, the membranes were washed 3 times with 0.1% washing buffer (ST673, Beyotime, Shanghai, China) and then incubated with horseradish peroxidase -conjugated secondary antibody (1:2000 dilution; ZB-2305, ZSGB-BIO, Beijing, China). Detection was performed using enhanced chemiluminescence reagents (1622301, Millipore, Billerica, Massachusetts, USA), and gray values were analyzed using ImageJ software (version 1.48, National Institutes of Health, Rockville, Maryland, USA).
Reverse transcription quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent (DP424, Tiangen, Beijing, China). Following the measurement of RNA concentration and purity, complementary DNA was synthesized using a kit (KR116, Tiangen, Beijing, China) at 65°C for 10 min. Quantitative PCR (qPCR) was conducted in accordance with the manufacturer’s instructions using SYBR Green (D7268S, Beyotime, Shanghai, China) on a LightCycler96 thermocycler (Roche, Shanghai, China). The amplification conditions were set as follows: Initial denaturation at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 s and annealing/extension at 60°C for 60 s. GAPDH was employed as the internal reference gene. The specific primer sequences are summarized in Table 1. Relative expression levels of target genes were quantified using the 2−ΔΔCt method.
| Gene | Forward | Reverse |
|---|---|---|
| L1CAM | 5'-CATACATGGCAGTTGAGGGC-3' | 5'-GGCAGAAATAGCGTCCAGTG-3' |
| PTK2 | 5'-TGCAGTCTTGGTCCTGCTAA-3' | 5'-GTGAGGATGGCTGAGGATTT-3' |
| GRB2 | 5'-AGGCGGGAAGGAATTCTTAC-3' | 5'-TCTGGTGACGGTAGGTTGAG-3' |
| SOS1 | 5'-CGAAGGAGACTCCGTGTGAA-3' | 5'-CCGTTGAGGGTGTTCTTGAT-3' |
| RAS | 5'-CGAGGGCAAGAGTGCACAT-3' | 5'-TGGCACTGTACTGGGAGGAG-3' |
| GAPDH | 5'-GAAGGTGAAGGTCGGAGTCA-3' | 5'-GAAGATGGTGATGGGATTTC-3' |
L1CAM: L1 cell adhesion molecule, PTK2: Protein tyrosine kinase 2, GGRBS2: Growth factor receptor-bound protein 2, RAS: Rat sarcoma, SOS 1: Son of sevenless 1, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, C: Cytosine, G: Guanine, T: Thymine, A: Adenosine.
In vivo experiments
A total of 12 female nude mice, aged 4–6 weeks and weighing 20–22 g, were acquired from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China, Certificate number: SCXK (Jing) 2016-0006). All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the 2nd Affiliated Hospital of Harbin Medical University (Approval No: YJSKY2023-316). Before experimentation, the mice were acclimatized for 1 week under free access to water and food, maintained at 22–25°C with 40–70% relative humidity, and subjected to a 12-h light/dark cycle. Subsequently, the 12 mice were randomly allocated into two groups, with 6 mice per group. Each mouse was subcutaneously injected into the right flank with 1 × 106 KLE cells that were transfected with either OL-NC or OL-L1CAM. Following a 14-day period of normal housing, the mice were euthanized through CO2 inhalation, and the tumors were harvested for analysis. The volume of each subcutaneous tumor was calculated using the following formula: tumor length × tumor width2/2.
Statistical analysis
Statistical analyses were performed using Prism 8.0.2 software (La Jolla, California, USA). Data obtained from repeated experiments were presented as mean ± SD. Comparisons between two groups were conducted using Student’s t-test. Multiple group comparisons were performed using analysis of variance followed by Bonferroni’s post hoc test. P < 0.05 was considered statistically significant.
RESULTS
L1CAM is overexpressed in EC
The messenger RNA (mRNA) expression profile, which included 24 normal healthy samples and 177 EC samples, was obtained from TCGA. A scatter plot illustrating L1CAM expression levels across all samples is presented in Figure 1a. This expression profile showed that EC tumors had much higher levels of L1CAM than did normal samples (0.78-fold; P < 0.01). Furthermore, L1CAM mRNA levels were markedly higher in EC tissues than in adjacent normal tissues [Figure 1b]; (1.85-fold; P < 0.01) and significantly elevated in human EC cells compared with hEECs (2.45-3.8 9-fold); [Figure 1c]; (P < 0.01). In addition, L1CAM protein expression was significantly higher in EC tissues than in peripheral normal tissue (2.59-fold); [Figure 1d and e]; (P < 0.01) and substantially increased in human EC cells than in normal endometrial epithelium cells (1.88–2.18-fold); [Figure 1f and g]; (P < 0.01)
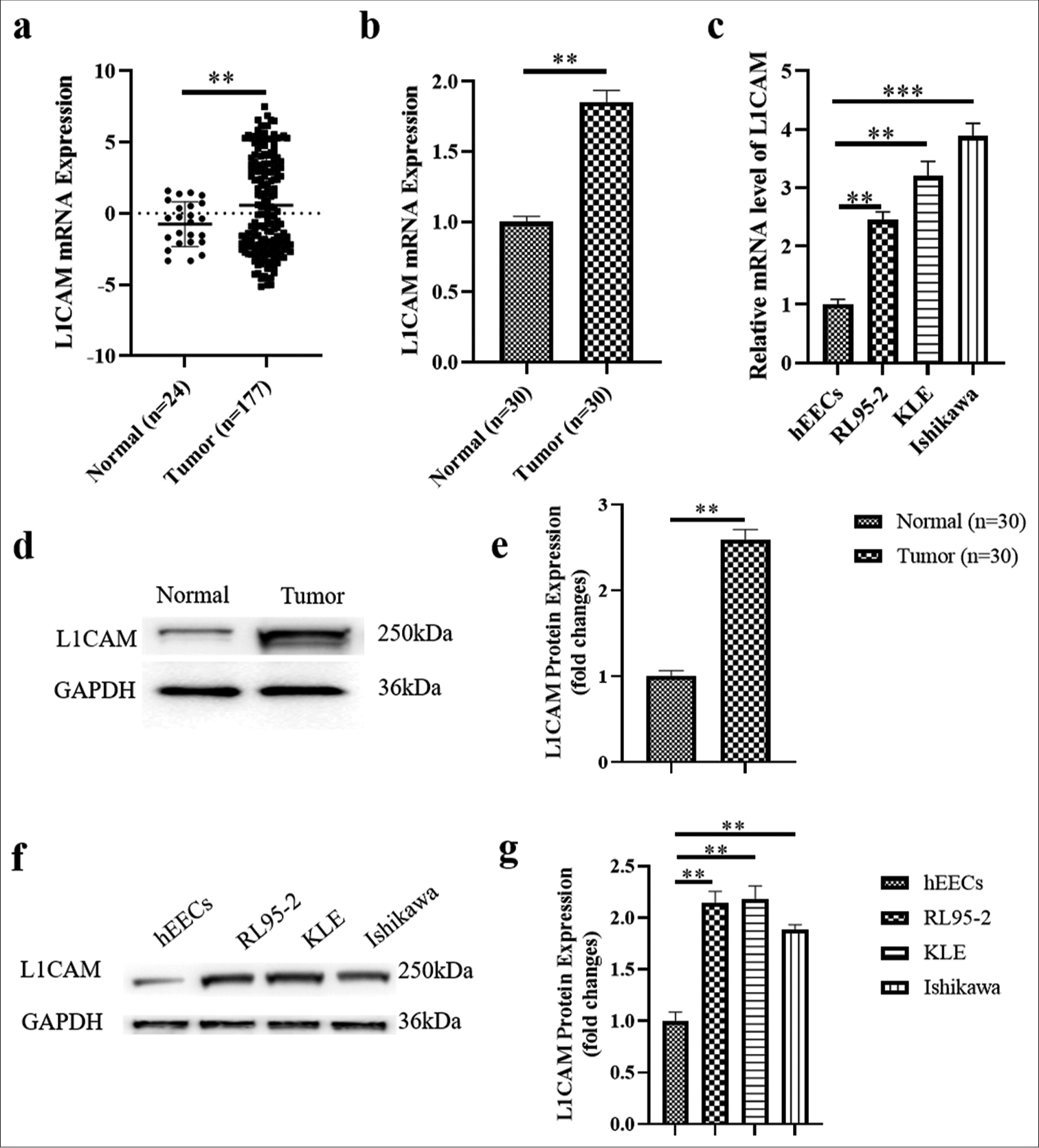
- L1CAM is overexpressed in EC. (a) Scatter diagram showing the L1CAM expression levels in normal samples and EC tumor samples based on the expression profile from TCGA. The results obtained by RT-qPCR showing L1CAM mRNA expression (b) in EC tumor tissue and peripheral normal tissues (n = 30), as well as (c) in hEECs and in human EC cell lines of RL95-2, KLE, and Ishikawa (n = 5). The results obtained by Western blot showed L1CAM protein expression (d and e) in EC tumor tissue and peripheral normal tissues (n = 30) and (f and g) in hEECs and in human EC cell lines of RL95-2, KLE, and Ishikawa (n = 5). **P < 0.01 indicated statistically highly significant differences. (***P < 0.001: Indicates very high statistical significance. L1CAM: L1 cell adhesion molecule, EC: Endometrial cancer, TCGA: The Cancer Genome Atlas, RT-qPCR: Reverse transcription quantitative real-time polymerase chain reaction, mRNA: Messenger RNA, hEECs: Human immortalized endometrial epithelium cells.)
L1CAM promotes the proliferation and survival of EC cells
In Figure 2a, the mRNA expression of L1CAM was successfully downregulated following transfection with IL-L1CAM (reduced to 0.20; P < 0.001). Conversely, mRNA levels of L1CAM were significantly upregulated after transfection with OL-L1CAM (2.17-fold; P < 0.001). The CCK-8 assay results in Figure 2b demonstrated that downregulation of L1CAM significantly reduced the proliferation of KLE EC cells at 48, 72(P < 0.05), and 96 h (P < 0.01). Conversely, upregulation of L1CAM significantly enhanced the proliferation of KLE EC cells at these time points (P < 0.05). Figure 2c and d indicated that colony formation was significantly reduced when L1CAM was downregulated (reduced to 0.3; P < 0.001), whereas colony formation significantly increased when L1CAM was upregulated (3.22-fold; P < 0.001). Figure 2e and f revealed a high number of apoptotic cells when L1CAM expression was reduced (P < 0.001), and we observed few apoptotic cells when L1CAM expression was increased (3.86-fold; P < 0.001). Collectively, these findings suggested that increased L1CAM expression is associated with enhanced proliferation and survival of EC cells
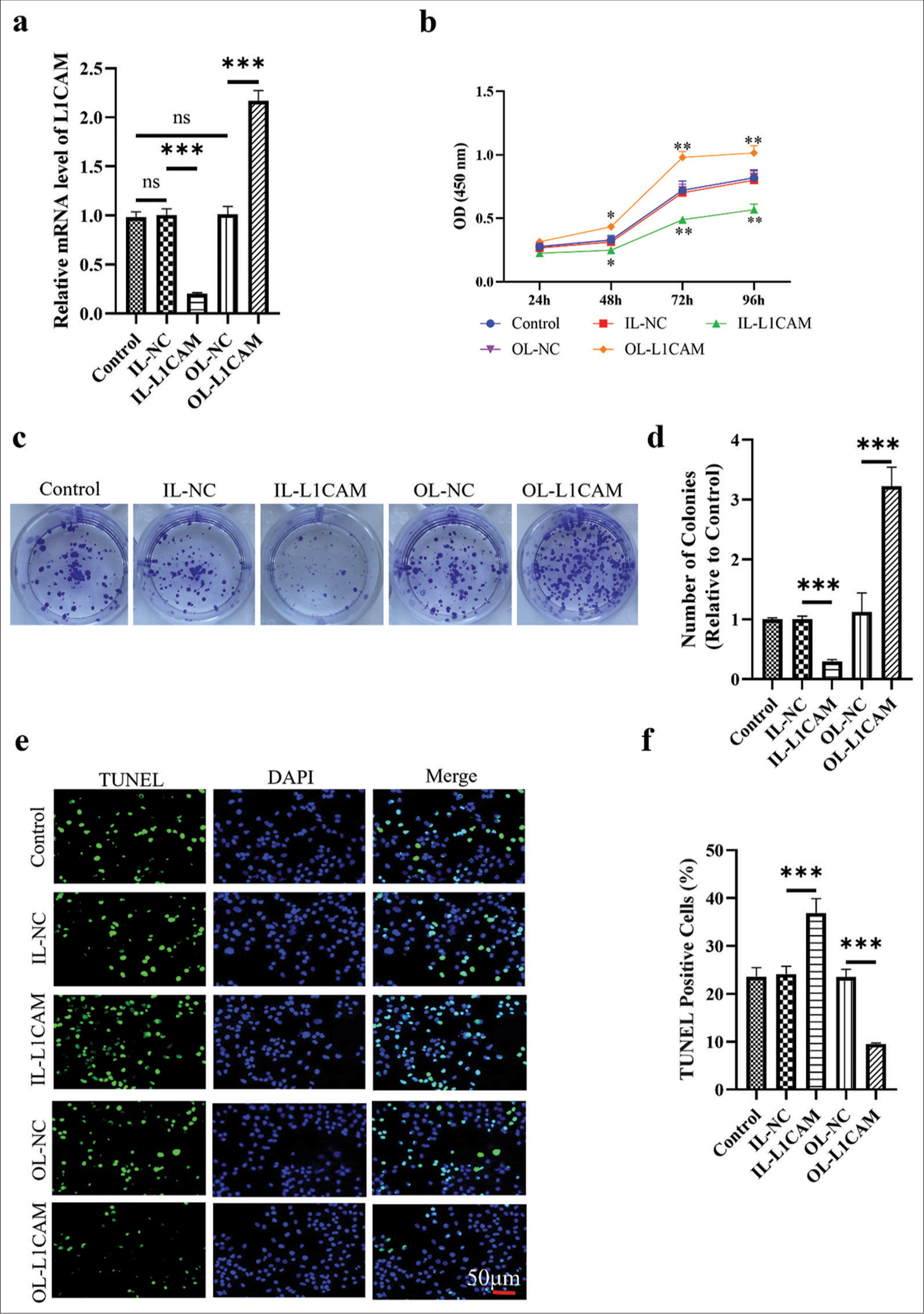
- L1CAM promotes the proliferation and survival of EC cells. (a) L1CAM mRNA expression levels in transfected KLE cells obtained by RT-qPCR (n = 5). Results of (b) CCK-8 assay (n = 5), (c and d) colony forming assay (n = 5), and (e and f) TUNEL assay (n = 5) for transfected KLE cells. *P < 0.05 indicates statistical significance. **P < 0.01 indicates high statistical significance. *** P < 0.001 indicates very high statistical significance. (ns stands for no significance, L1CAM: L1 cell adhesion molecule, EC: Endometrial cancer, RT-qPCR: Reverse transcription quantitative real-time polymerase chain reaction, mRNA: Messenger RNA, CCK: Cell counting kit, TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling, OD: Optical density, IL: Interference lentivirus, NC: Negative control, DAPI,4’,6-Diamidino-2’-phenylindole.)
L1CAM promotes the metastasis of EC cells
The results of Transwell assays [Figure 3a-d] demonstrated that the migration and invasion capabilities of KLE cells with downregulated L1CAM expression were significantly reduced (45–46%; P < 0.01), whereas these capabilities were significantly enhanced in KLE cells with upregulated L1CAM expression (1.51–2.55-fold; P < 0.001). Matrix metalloproteinases (MMPs) play crucial roles in the metastasis and invasion of various tumor cells.[24-26] In particular, MMP2 and MMP9 are most associated with tumor metastasis, migration, and invasion across various human cancers,[27-29] so further exploration was conducted on how L1CAM influences the metastasis of EC cells. Western blot analysis [Figure 3e and f] revealed that decreased L1CAM expression corresponded with reduced protein levels of MMP2 (P < 0.001) and MMP9 (P < 0.01) (reduced to 0.19 and 0.49), whereas increased L1CAM expression was associated with elevated levels of these proteins (1.55–2.07-fold; P < 0.001).
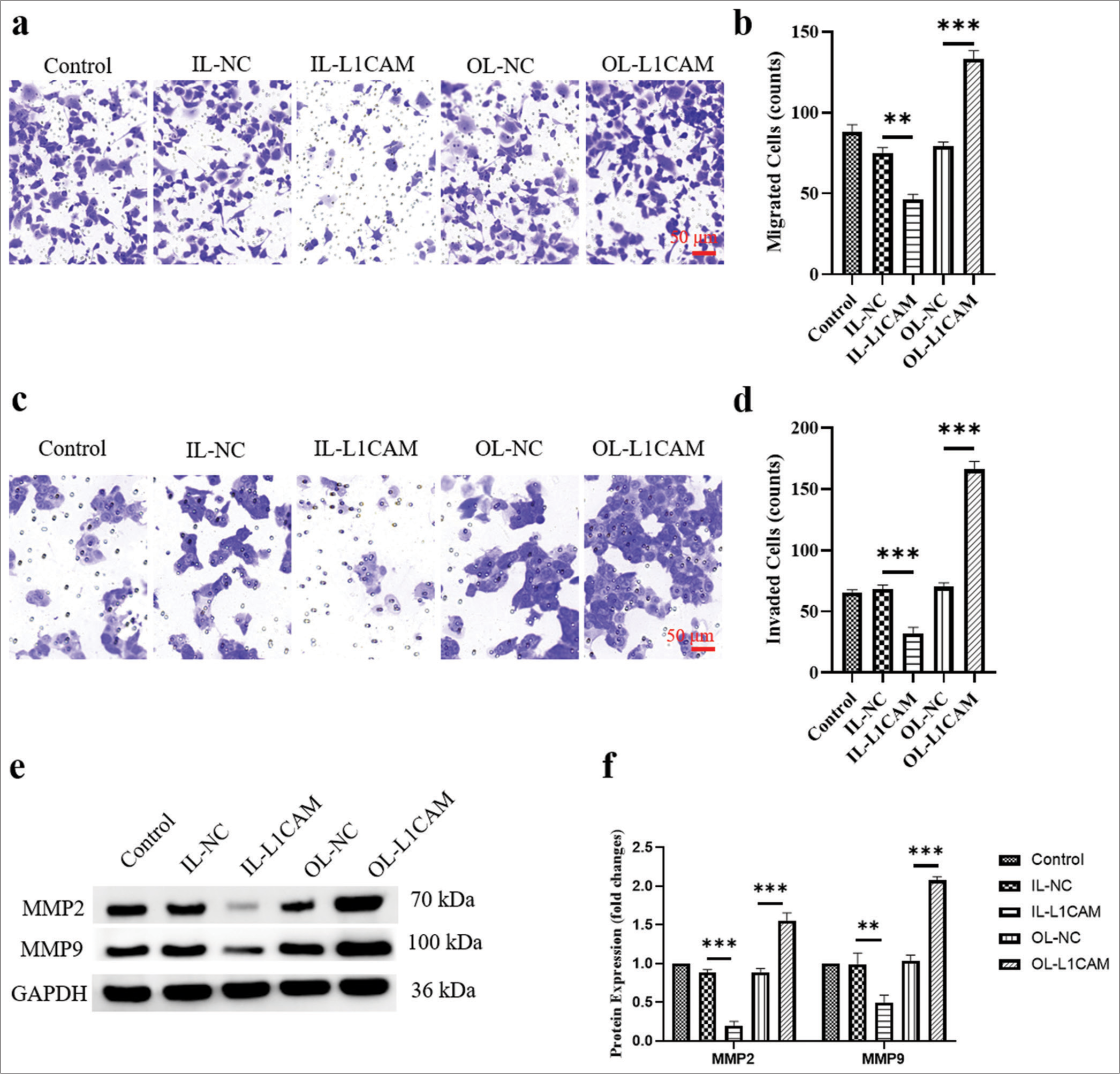
- L1CAM promotes the metastasis of EC cells. (a and b) Transwell assay for migration (n = 5) and (c and d) Transwell assay for invasion (n = 5) for transfected KLE cells. (e and f) Protein levels of MMP2 and MMP9 obtained by Western blot (n = 5). (**P < 0.01 indicates high statistical significance.***P < 0.001 indicates very high statistical significance. L1CAM: L1 cell adhesion molecule, EC: Endometrial cancer, MMP: Matrix metalloproteinase, OL: Overexpression lentivirus, NC: negative control, MMP: Matrix metalloproteinase, GADPH: Glyceraldehyde-3-phosphate dehydrogenase.)
L1CAM promotes the proliferation and survival of EC cells by promoting FAK
We used the FAK inhibitor PF431396 (S7644, Selleck Chemicals, Houston, TX, USA) at a concentration of 50 nM to examine the function of FAK in the control of EC progression by L1CAM.[19] Figure 4a-c illustrates that the levels of mRNA of PTK2 (reduced to 0.117) and its encoded FAK protein were significantly downregulated (reduced to 0.114; P < 0.001) in KLE cells transfected with IL-L1CAM, where L1CAM expression was reduced. By contrast, the levels of PTK2 mRNA and its encoded FAK protein were both significantly upregulated (1.65–2.19-fold; P < 0.001) in OL-L1CAM-transfected KLE cells in which L1CAM was upregulated. In OLL1CAM-transfected KLE cells, the administration of PF431396 successfully decreased the expression of PTK2 mRNA (reduced to 0.3302) and its encoded FAK protein (reduced to 0.5065) compared with control group. The expression levels of L1CAM mRNA and L1CAM protein are shown in Figure 4d-f, demonstrating that PF431396, which inhibited FAK expression, could not affect L1CAM expression. Accordingly, L1CAM could promote the expression of FAK, while FAK could not change the expression level of L1CAM. The CCK-8 assay results [Figure 4g] demonstrated that the inhibition of FAK reversed the promoted proliferation of OL-L1CAM-transfected KLE cells (0.73 fold P < 0.01). Colony formation tests showed the same trend [Figure 4h and i]; (P < 0.01). Furthermore, the TUNEL assay results [Figure 4j and k] showed that the inhibition of FAK reversed the decreased apoptosis of OL-L1CAMtransfected KLE cells (P < 0.001). Thus, the failed upregulation of FAK disrupted the L1CAM-induced promotion on EC cell proliferation and survival. In other words, L1CAM promoted EC cell proliferation and survival by promoting FAK expression.
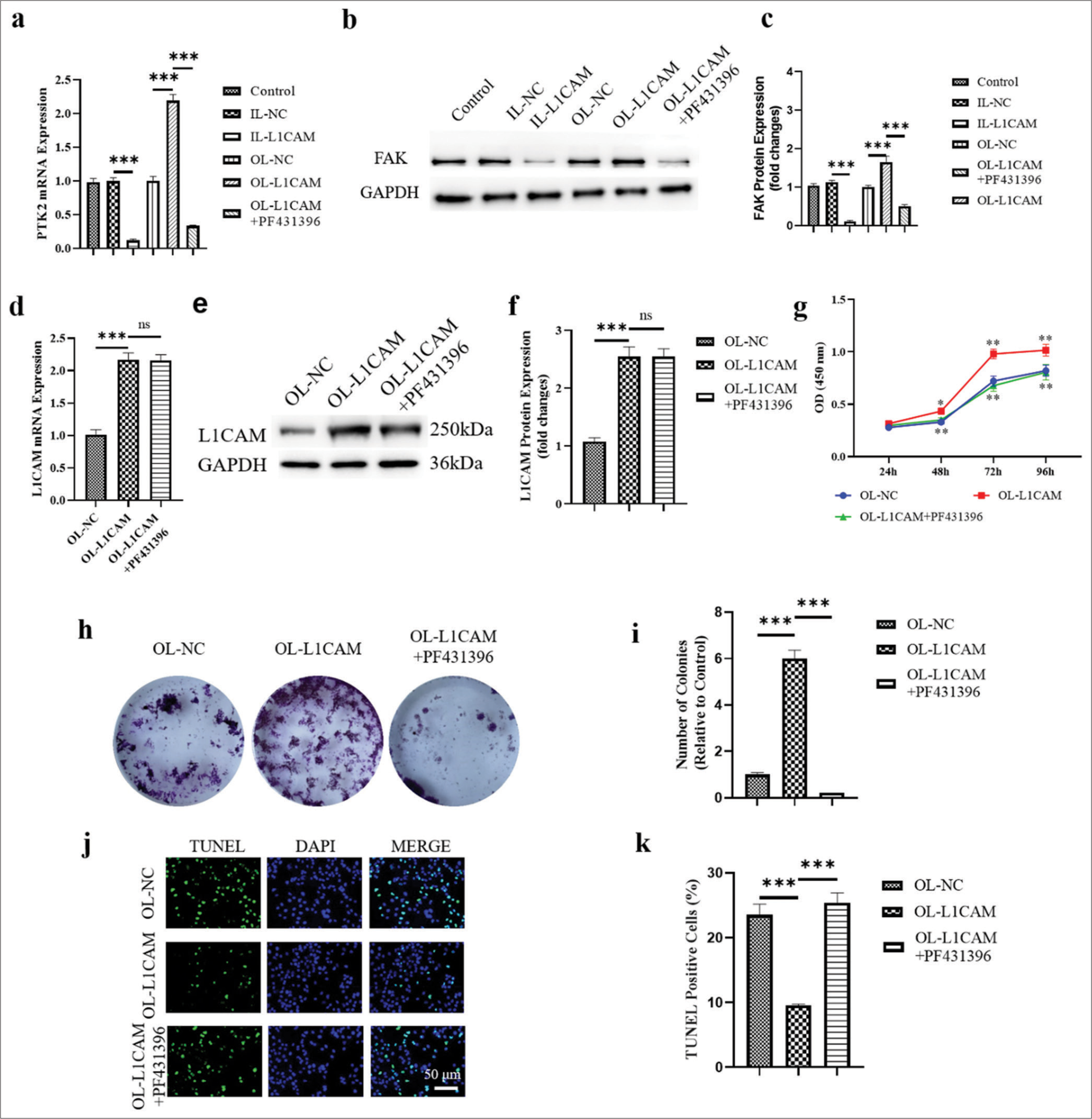
- L1CAM promotes the proliferation and survival of EC cells by upregulating FAK. (a) The PTK2 mRNA levels obtained by RT-qPCR (n = 5). (b and c) The levels of FAK protein encoded by PTK2 obtained by Western blot (n = 5). The expression levels of (d) L1CAM mRNA (n = 5) and (e and f ) L1CAM protein (n = 5). Results of (g) CCK-8 assay (n = 5), (h and i) colony forming assay (n = 5), and (j and k) TUNEL assay (n = 5) for KLE cells. (** P < 0.01 indicates high statistical significance. *** P < 0.001 indicates very high statistical significance. ns stands for no significance. L1CAM: L1 cell adhesion molecule, EC: Endometrial cancer, mRNA: Messenger RNA, RT-qPCR: Reverse transcription quantitative real-time polymerase chain reaction, CCK: Cell counting kit, TUNEL: Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling, FAK: Focal adhesion kinase, PKT2: Protein tyrosine kinase 2, NC: Negative control, OL: Overexpression lentivirus, L1CAM: L1 cell adhesion molecule, DAPI: 4’,6-Diamidino-2’-phenylindole.)
L1CAM promotes the metastasis of EC cells by upregulating FAK
The results of Transwell assay are shown in Figure 5a-d. The inhibition of PTK2/FAK reversed the increased migration, and the increased invasion of L1CAM upregulated KLE cells (reduced to 0.53- and 0.64-fold; P < 0.001). Moreover, the increased protein levels of MMP2 and MMP9 were decreased by the inhibition of FAK (reduced to 0.66- and 0.80-fold; [Figure 5e and f]; (P < 0.001). Thus, the failed upregulation of FAK disrupted the effect of L1CAM promoting metastasis of EC cells. In other words, L1CAM promoted EC cell metastasis by promoting FAK expression.
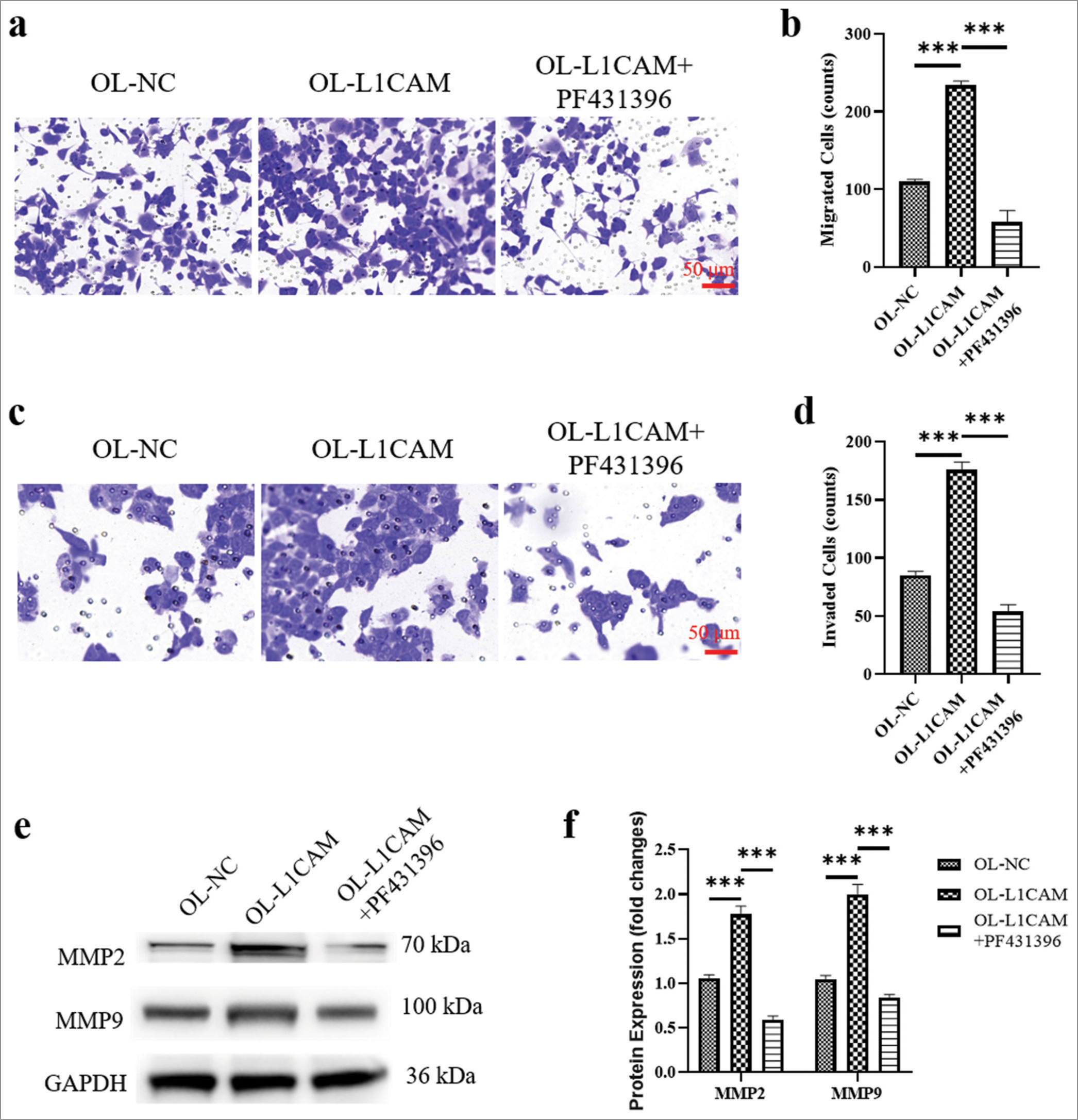
- L1CAM promotes the metastasis of EC cells by upregulating FAK. (a and b) Transwell assay for migration (n = 5) and (c and d) transwell assay for invasion (n = 5) for KLE cells. (e and f) Protein levels of MMP2 and MMP9 obtained by Western blot (n = 5). ***P < 0.001 indicates very high statistical significance. L1CAM: L1 cell adhesion molecule, EC: Endometrial cancer, FAK: Focal adhesion kinase, MMP: Matrix metalloproteinase, NC: Negative control, LICAM: L1 cell adhesion molecule, OL: Overexpression lentivirus, MMP: Matrix metalloproteinase, GADPH: lyceraldehyde-3-phosphate dehydrogenase.)
L1CAM functions by promoting the FAK–GRB2–SOS–RAS pathway
To further investigate how L1CAM promotes FAK and influences the downstream FAK–GRB2–SOS–RAS pathway, we analyzed the related pathway proteins in human EC cells from KLE, RL95-2, and Ishikawa lines. As depicted in Figure 6a and b, compared with IL-NC-transfected KLE cells, the proteins GRB2, SOS, and RAS were significantly downregulated in KLE cells transfected with IL-L1CAM (reduced to 0.46–0.49; P < 0.001). In contrast, OL-L1CAM-transfected KLE cells showed a considerable upregulation of these proteins when compared to OL-NC-transfected cells. (1.28-1.51-fold; P < 0.001). Compared with OL-L1CAM-transfected KLE cells, PF431396 administration decreased all protein levels of GRB2, SOS, and RAS (reduced by 0.69–0.81-fold; P < 0.001). We observed that L1CAM expression was downregulated in KLE cells transfected with IL-L1CAM and upregulated in those transfected with OL-L1CAM, regardless of PF431396 administration (P < 0.001). In addition, FAK expression was downregulated in KLE cells transfected with IL-L1CAM and upregulated in those transfected with OL-L1CAM (1.55 fold; P < 0.001). Notably, this upregulation was reversed in OL-L1CAM transfected KLE cells following the administration of PF431396 (decreased to 0.65 fold). Accordingly, L1CAM would promote the activation of the FAK–GRB2–SOS–RAS pathway by first promoting the expression of FAK. This result was further proven by the same phenomena presented in RL95-2 [Figure 6c and d] and Ishikawa [Figure 6e and f]. Compared with IL-NC-transfected cells, IL-L1CAM transfection decreased all protein levels of L1CAM, FAK, GRB2, SOS, and RAS (decreased by 0.22–0.46-fold; P < 0.01), whereas OL-L1CAM transfection increased all protein levels of L1CAM, FAK, GRB2, and SOS (P < 0.001). In addition, PF431396 administration had no effect on L1CAM protein level but decreased all protein levels of FAK, GRB2, SOS, and RAS (P < 0.001). We confirmed that L1CAM, when active, promoted the activation of the FAK–GRB2–SOS–RAS pathway primarily by enhancing the expression of FAK.
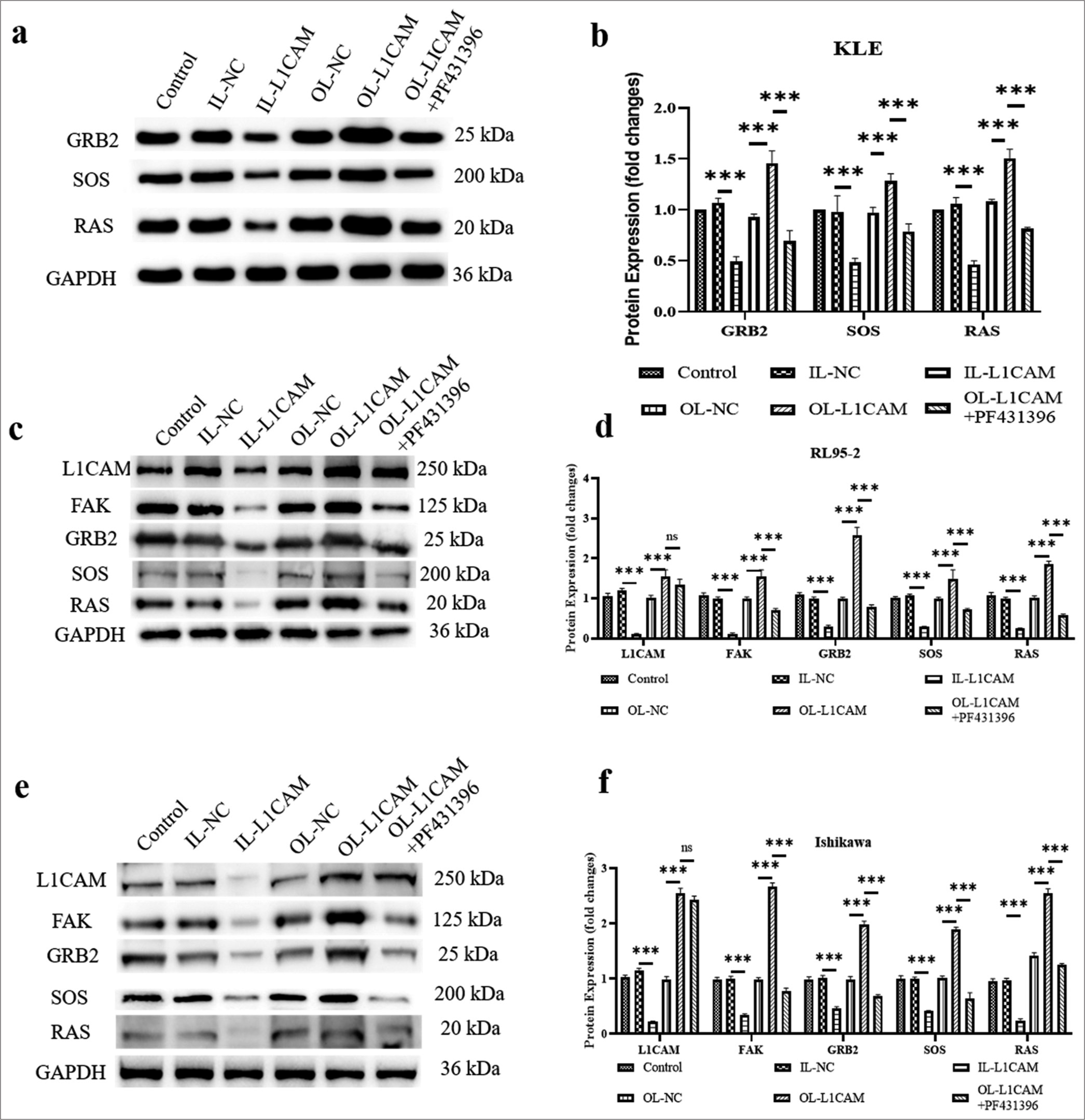
- L1CAM functions by promoting the FAK–GRB2–SOS–RAS pathway. (a and b) Protein levels of GRB2, SOS and RAS in KLE cells (n = 5). Protein levels of L1CAM, FAK, GRB2, SOS, and RAS in (c and d) RL95-2 cells (n = 5) and (e and f) Ishikawa cells (n = 5). (***P < 0.001 indicates very high statistical significance. ns stands for no significance. L1CAM: L1 cell adhesion molecule, FAK: Focal adhesion kinase, GRB2: Growth factor receptor-bound protein 2, SOS: Son of sevenless, RAS: Rat sarcoma, NC: Negative control, GADPH: lyceraldehyde-3-phosphate dehydrogenase.)
L1CAM promotes the progression of subcutaneous tumor
As shown in Figure 7a and b, the subcutaneous tumor of OE-L1CAM-transfected KLE cells was faster than that of OE-NC-transfected KLE cells (6.43-fold; P < 0.001), indicating that a high level of L1CAM was associated with the rapid progression of EC tumor. Besides, the mRNA expression levels of L1CAM, PTK2, GRB2, SOS, and RAS increased in the OE-L1CAM group compared with the OE-NC group [Figure 7c]; (P < 0.01).
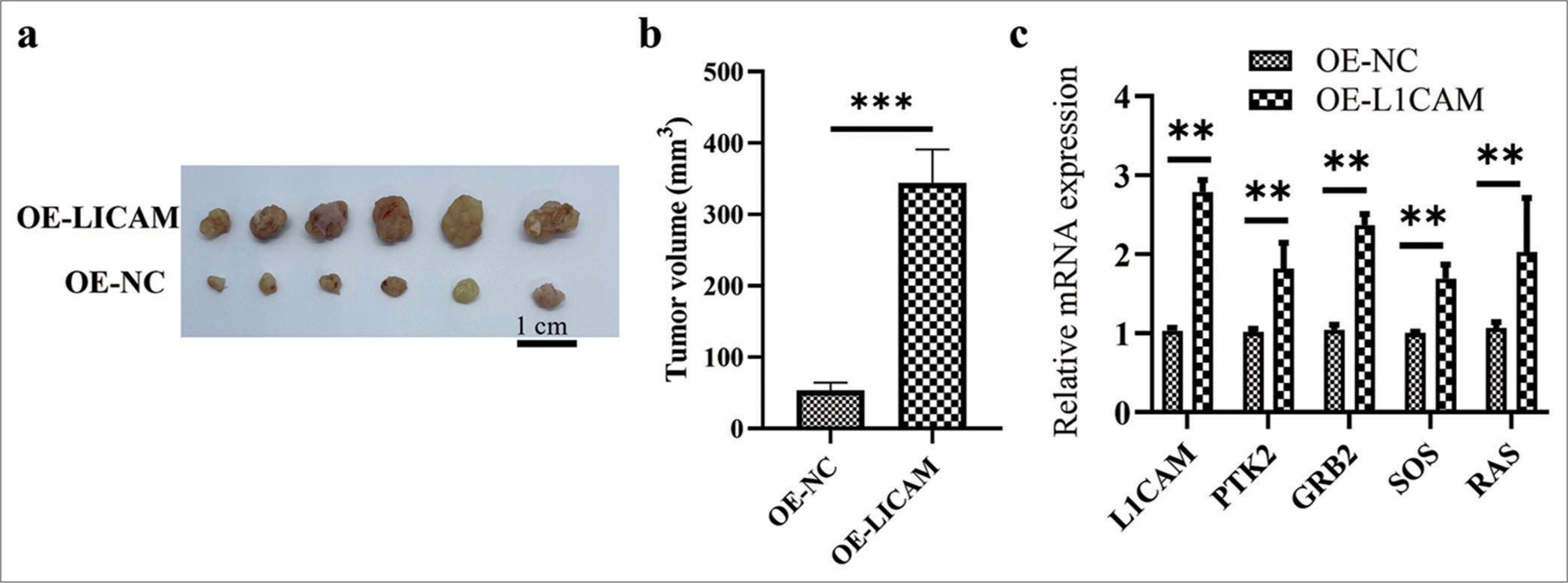
- L1CAM promotes the progression of subcutaneous EC tumor. (a) Images and (b) quantification of tumor growth after transfected KLE EC cells were subcutaneously injected into mice (n = 6). (c) The L1CAM, PTK2, GRB2, SOS, and RAS mRNA levels obtained by RT-qPCR. **P < 0.01 indicates high statistical significance. (***P < 0.001 indicates very high statistical significance. L1CAM: L1 cell adhesion molecule, GRB2: Growth factor receptor-bound protein 2, SOS: Son of sevenless, RAS: Rat sarcoma, EC: Endometrial cancer, mRNA: Messenger RNA, RT-qPCR: Reverse transcription quantitative real-time polymerase chain reaction, NC: Negative control, OE: Overexpression, PTK: Protein tyrosine kinase.)
DISCUSSION
EC, a malignancy arising from the inner epithelial lining of the uterus, has seen rising incidence rates and contributes significantly to cancer-related mortality worldwide.[30] Managing EC remains challenging,[31] and enhancing therapeutic precision in this complex malignancy necessitates a deep understanding of the genetic diversity that drives various pathological states. In this study, we explored the interaction between L1CAM and PTK2 in EC and examined how this interaction influences the progression of the disease.
L1CAM, a transmembrane glycoprotein of the immunoglobulin (Ig) superfamily, has a molecular weight ranging from 200 kDa to 220 kDa.[32] L1CAM functions through interactions with various binding partners in cis and trans configurations, and it is associated with actin, cytoskeletal proteins, and spectrin.[33-35] It can also bind to itself or to other integrins, neurocan, and cell adhesion molecules. According to earlier research, L1CAM expression is linked to a poor outcome in cases of ovarian cancer and EC.[36] In addition, these studies identified the presence of soluble L1CAM in the serum and ascites of patients, which is linked to the protein’s ability to be cleaved into a soluble form.[37,38] In particular, we investigated the group consisting of the most aggressive, high-grade cancers including all uterine carcinomas; in 25% of high-grade EC, L1CAM was found to be normally upregulated and related to poor outcomes.[39] Although L1CAM was shown to be a favorable predictive biomarker in children with neuroblastoma, the majority of studies also found that L1CAM is associated with poor prognosis, cancer progression, and metastasis and may be an independent prognostic factor[40] and in the mouse lymphoma model.[41] In this study, we confirmed that L1CAM was overexpressed in EC tumor tissues and cells, and it was associated with the progression of EC by promoting migration, invasion, growth, and motility.
The PTK2 gene encodes FAK, a cytoplasmic tyrosine kinase protein.[42] Previous research has demonstrated that overexpression of the PTK2 gene is common in metastatic cancers and is associated with progression, specific survival, pathological stage, and malignant clinical outcomes of cancer.[43,44] Furthermore, FAK has been shown to play roles in promoting the malignant proliferation, migration, and survival of various cancer cells in vitro.[45] Consequently, the FAK protein and the PTK2 gene are recognized as significant promoters of cancer progression.[46,47] In this study, we observed that the upregulation of L1CAM and its subsequent promotion of EC cell progression were coupled with an increase in FAK/PTK2 expression. Moreover, clinical trials have indicated that inhibiting FAK can suppress cancer cell growth.[48,49] Our experiments revealed that PF431396, a FAK inhibitor, effectively downregulated FAK protein expression and reversed the cancer-promoting effects induced by L1CAM. Interestingly, we discovered that the reversal of cancer cell progression by inhibiting FAK did not occur through the downregulation of L1CAM but rather through the diminished activation of the FAK–GRB2–SOS–RAS signaling pathway.
Accordingly, the findings in this study revealed that L1CAM could promote the progression of EC by increasing PTK2/ FAK expression and activating the FAK–GRB2–SOS–RAS pathway. FAK is a downstream component of L1CAM and cannot affect the expression of L1CAM. However, it can regulate EC tumor progression by affecting the downstream GRB2–SOS–RAS pathway. These findings also identified the genes of L1CAM and PTK2 as the targets for EC therapy because they act as the upstream switches of this chain for EC progression regulation. Especially, for L1CAM acting as a therapeutic target, apart from that L1CAM plays roles in promoting cell motility, invasion, metastatic formation, and tumor progression and is a factor of poor prognosis; another advantage of L1CAM being a therapeutic target is that it has a favorable tissue distribution in adult organisms.[50-52] Thus, the improvement of antibodies for L1CAM could be used for ameliorating not only EC but also other cancers.
SUMMARY
This study revealed that L1CAM promotes tumor progression in EC by upregulating PTK2 and its encoded protein of FAK, resulting in the increased activation of the FAK–GRB2– SOS–RAS pathway. These findings on how the interaction between L1CAM and PTK2 influences EC progression and its association with disease progression demonstrated that L1CAM and PTK2 can serve as biomarkers for poor prognosis prediction and as potential therapeutic targets in EC treatment.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author on reasonable request.
ABBREVIATIONS
L1CAM: L1 cell adhesion molecule
PTK2: Protein tyrosine kinase 2
EC: Endometrial cancer
FAK: Focal adhesion kinase
GRB2; Growth factor receptor-bound protein 2
SOS: Son of sevenless
RAS: Rat sarcoma
hEECs: Human immortalized endometrial epithelium cells
AUTHOR CONTRIBUTIONS
WH and WL: Interpreted the data and wrote the manuscript. WT and WL: Designed the study. WT and XL contributed to writing the manuscript. All authors read and approved the final version of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of The 2nd Affiliated Hospital of Harbin Medical University (Approval No: YJSKY2023-316).
Before patients enrolled in this study, they were well informed about this study and required to provide the written consents.
All animal procedures were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals of the 2nd Affiliated Hospital of Harbin Medical University (Approval No: YJSKY2023-316).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
Not applicable.
References
- Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209-49.
- [CrossRef] [Google Scholar]
- Cancer of the corpus uteri: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):45-60.
- [CrossRef] [Google Scholar]
- Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569-78.
- [CrossRef] [Google Scholar]
- Endometrial cancer state of the science meeting. Int J Gynecol Cancer. 2009;19:134-40.
- [CrossRef] [Google Scholar]
- ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int J Gynecol Cancer. 2016;26:2-30.
- [CrossRef] [Google Scholar]
- Adjuvant therapy for endometrial cancer in the era of molecular classification: Radiotherapy, chemoradiation and novel targets for therapy. Int J Gynecol Cancer. 2021;31:594-604.
- [CrossRef] [Google Scholar]
- Immunohistochemical biomarkers are prognostic relevant in addition to the ESMOESGO-ESTRO risk classification in endometrial cancer. Gynecol Oncol. 2021;161:787-94.
- [CrossRef] [Google Scholar]
- L1CAM in early-stage type I endometrial cancer: Results of a large multicenter evaluation. J Natl Cancer Inst. 2013;105:1142-50.
- [CrossRef] [Google Scholar]
- L1CAM expression in endometrial carcinomas: An ENITEC collaboration study. Br J Cancer. 2016;115:716-24.
- [CrossRef] [Google Scholar]
- The role of L1CAM as predictor of poor prognosis in stage I endometrial cancer: A systematic review and meta-analysis. Arch Gynecol Obstet. 2024;309:789-99.
- [CrossRef] [Google Scholar]
- Linking L1CAM-mediated signaling to NF-κB activation. Trends Mol Med. 2011;17:178-87.
- [CrossRef] [Google Scholar]
- FAK structure and regulation by membrane interactions and force in focal adhesions. Biomolecules. 2020;10:179.
- [CrossRef] [Google Scholar]
- Mammary epithelial-specific disruption of the focal adhesion kinase blocks mammary tumor progression. Proc Natl Acad Sci U S A. 2007;104:20302-7.
- [CrossRef] [Google Scholar]
- Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998-3003.
- [CrossRef] [Google Scholar]
- FAK activity sustains intrinsic and acquired ovarian cancer resistance to platinum chemotherapy. Elife. 2019;8:e47327.
- [CrossRef] [Google Scholar]
- Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell. 2015;27:574-88.
- [CrossRef] [Google Scholar]
- FAK-MAPK-dependent adhesion disassembly downstream of L1 contributes to semaphorin3A-induced collapse. EMBO J. 2008;27:1549-62.
- [CrossRef] [Google Scholar]
- The L1 cell adhesion molecule constrains dendritic spine density in pyramidal neurons of the mouse cerebral cortex. Front Neuroanat. 2023;17:1111525.
- [CrossRef] [Google Scholar]
- Small-molecule inhibitors of FGFR, integrins and FAK selectively decrease L1CAM-stimulated glioblastoma cell motility and proliferation. Cell Oncol (Dordr). 2016;39:229-42.
- [CrossRef] [Google Scholar]
- Retraction: Epithelial membrane protein-2 promotes endometrial tumor formation through activation of FAK and Src. PLoS One. 2022;17:e0276151.
- [CrossRef] [Google Scholar]
- FGA controls VEGFA secretion to promote angiogenesis by activating the VEGFR2-FAK signalling pathway. Front Endocrinol (Lausanne). 2022;13:791860.
- [CrossRef] [Google Scholar]
- Therapeutic exosomes loaded with SERPINA5 attenuated endometrial cancer cell migration via the integrin β1/FAK signaling pathway. Cell Oncol (Dordr). 2022;45:861-72.
- [CrossRef] [Google Scholar]
- Integrin-mediated activation of MAP kinase is independent of FAK: Evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385-95.
- [CrossRef] [Google Scholar]
- Matrix metalloproteinase inhibitor RO 28-2653 decreases liver metastasis by reduction of MMP-2 and MMP-9 concentration in BOP-induced ductal pancreatic cancer in Syrian Hamsters: Inhibition of matrix metalloproteinases in pancreatic cancer. Prostaglandins Leukot Essent Fatty Acids. 2006;75:429-34.
- [CrossRef] [Google Scholar]
- The significance of MMP-1 and MMP-2 in peritoneal disseminated metastasis of gastric cancer. Surg Today. 2000;30:614-21.
- [CrossRef] [Google Scholar]
- The relation of gelatinase (MMP-2 and-9) expression with distant site metastasis and tumour aggressiveness in colorectal cancer. Br J Cancer. 2000;82:249.
- [CrossRef] [Google Scholar]
- Extracellular matrix metalloproteinase inducer (EMMPRIN) and matrix metalloproteinases (MMPs) as regulators of tumor-host interaction in a spontaneous metastasis model in rats. Histochem Cell Biol. 2008;130:1155-64.
- [CrossRef] [Google Scholar]
- Src kinase regulates metalloproteinase-9 secretion induced by type IV collagen in MCF-7 human breast cancer cells. Matrix Biol. 2008;27:220-31.
- [CrossRef] [Google Scholar]
- Ets-1 is up-regulated together with its target gene products matrix metalloproteinase-2 and matrix metalloproteinase-9 in atypical and anaplastic meningiomas. Histopathology. 2006;48:836-45.
- [CrossRef] [Google Scholar]
- Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer. 2020;126:2225-49.
- [CrossRef] [Google Scholar]
- Neural adhesion molecule L1 as a member of the immunoglobulin superfamily with binding domains similar to fibronectin. Nature. 1988;334:701-3.
- [CrossRef] [Google Scholar]
- Neural recognition molecules and synaptic plasticity. Curr Opin Cell Biol. 1997;9:627-34.
- [CrossRef] [Google Scholar]
- Neural cell recognition molecule L1: From cell biology to human hereditary brain malformations. Curr Opin Neurobiol. 1998;8:87-97.
- [CrossRef] [Google Scholar]
- L1CAM malfunction in the nervous system and human carcinomas. Cell Mol Life Sci. 2010;67:2425-37.
- [CrossRef] [Google Scholar]
- L1 cell adhesion molecule is a strong predictor for distant recurrence and overall survival in early stage endometrial cancer: Pooled PORTEC trial results. Eur J Cancer. 2014;50:2602-10.
- [CrossRef] [Google Scholar]
- L1 cell adhesion molecule (L1CAM) as a pathogenetic factor in endometriosis. Hum Reprod. 2008;23:1053-62.
- [CrossRef] [Google Scholar]
- Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J Biol Chem. 2000;275:15490-7.
- [CrossRef] [Google Scholar]
- Prognostic significance of L1CAM expression and its association with mutant p53 expression in high-risk endometrial cancer. Mod Pathol. 2016;29:174-81.
- [CrossRef] [Google Scholar]
- THOC2 expression and its impact on 5-fluorouracil resistance in glioblastoma multiforme. Am J Cancer Res. 2023;13:2410-25.
- [Google Scholar]
- L1cam promotes tumor progression and metastasis and is an independent unfavorable prognostic factor in gastric cancer. J Hematol Oncol. 2013;6:43.
- [CrossRef] [Google Scholar]
- Focal adhesion kinase (FAK) gene amplification and its clinical implications in gastric cancer. Hum Pathol. 2010;41:1664-73.
- [CrossRef] [Google Scholar]
- Focal adhesion kinase overexpression and its impact on human osteosarcoma. Oncotarget. 2015;6:31085-103.
- [CrossRef] [Google Scholar]
- Rab11a is overexpressed in gastric cancer and regulates FAK/AKT signaling. J Oncol. 2020;2020:3494396.
- [CrossRef] [Google Scholar]
- Baicalein inhibits gastric cancer cell proliferation and migration through a FAK interaction via AKT/mTOR signaling. Am J Chin Med. 2021;49:525-41.
- [CrossRef] [Google Scholar]
- Expression of ASAP1 and FAK in gastric cancer and its clinicopathological significance. Oncol Lett. 2020;20:974-80.
- [CrossRef] [Google Scholar]
- Promotive effect of Talin-1 protein on gastric cancer progression through PTK2-PXN-VCL-E-cadherin-CAPN2-MAPK1 signaling axis. J Clin Lab Anal. 2020;34:e23555.
- [CrossRef] [Google Scholar]
- Protein tyrosine kinase 2: A novel therapeutic target to overcome acquired EGFR-TKI resistance in non-small cell lung cancer. Respir Res. 2019;20:270.
- [CrossRef] [Google Scholar]
- Role of focal adhesion kinase in small-cell lung cancer and its potential as a therapeutic target. Cancers (Basel). 2019;11:1683.
- [CrossRef] [Google Scholar]
- Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp Mol Med. 2020;52:877-86.
- [CrossRef] [Google Scholar]
- L1-CAM as a target for treatment of cancer with monoclonal antibodies. Anticancer Res. 2009;29:4919-31.
- [Google Scholar]
- Therapeutic antibodies to human L1CAM: Functional characterization and application in a mouse model for ovarian carcinoma. Cancer Res. 2010;70:2504-15.
- [CrossRef] [Google Scholar]








