Translate this page into:
The value and practical utility of intraoperative touch imprint cytology of sentinel lymph node(s) in patients with breast cancer: A retrospective cytology-histology correlation study

*Corresponding author: Hidehiro Takei, MD, PhD Department of Diagnostic Pathology, Asahikawa Medical University Hospital, Asahikawa, Hokkaido, Japan. htakei@asahikawa-med.ac.jp
-
Received: ,
Accepted: ,
How to cite this article: Uno Y, Akiyama N, Yuzawa S, Kitada M, Takei H. The value and practical utility of intraoperative touch imprint cytology of sentinel lymph node(s) in patients with breast cancer: A retrospective cytology-histology correlation study. CytoJournal 2020;17:11.
Abstract
Objective:
Intraoperative evaluation of sentinel lymph nodes (SLNs) for patients with breast cancer is widely performed with frozen section (FS), cytology, or a combination of both. Touch imprint cytology (TIC) reportedly has an equivalent sensitivity to FS. We studied its diagnostic utility to detect SLN metastases.
Materials and Methods:
Cases of 367 patients with breast cancer who underwent intraoperative valuation of SLNs (507 LNs) were evaluated. All FS and corresponding TIC slides of SLNs of each case were reviewed microscopically for the presence of metastases of any size. If present, the metastatic focus was measured on the FS.
Results:
Of these 507 SLNs, 82 LNs (16.2%) from 69 women were found to have metastases in the FS and consisted of 5 LNs of isolated tumor cells, 15 of micrometastasis, and 62 of macrometastasis. TIC identified metastases in 69 of these 82 SLNs (sensitivity: 84.1%, specificity: 100%, and accuracy: 97.4%). All macrometastases could be detected by TIC, whereas TIC identified approximately 50% of micrometastases and none of isolated tumor cells. The size detection limit of metastatic foci, defined as the smallest dimension of metastasis detected without false negatives, was 2 mm. The smallest metastatic focus identified was 0.8 mm.
Conclusions:
TIC of SLNs is of great use given its negative predictive value of 100% for identification of macrometastasis in our study. For intraoperative evaluation of SLNs, based on our data, a practical two-step approach is proposed: SLN evaluation should be initially performed by TIC and then proceed to FS histological analysis only when cytologically positive to determine the size of metastatic focus.
Keywords
Breast cancer
Frozen section
Sentinel lymph node
Tough imprint cytology
INTRODUCTION
The prognosis of breast cancer is directly correlated with a quantitative nodal tumor burden, which is a continuous variable and is far more important than the simple presence or absence of nodal disease. More specifically, the number of metastatic foci, size of metastasis, and number of involved lymph nodes all contribute unfavorably to the patient’s outcome. The stratification of nodal tumor burden is codified in the current AJCC/UICC N-staging system.[1]
Examination of the sentinel lymph nodes (SLNs) is known to accurately predict the status of the entire axillary basin and is currently the standard of care for staging in breast cancer patients with clinically negative axilla (cN0). Intraoperative determination of the SLN status provides the surgeon with crucial information on whether immediate axillary lymph node dissection (ALND) should be completed, avoiding second anesthesia. It is reported that women with microscopic metastatic SLNs (pN0 [i+] and pN1 mi or metastatic foci ≤2 mm) do not benefit from completion ALND in overall and disease-free survival and axillary recurrence rate.[2,3] Thus, in the intraoperative SLN examination, it is of importance to determine the size of metastatic focus, more specifically, whether the metastasis is macrometastatic (>2 mm) or not.
Intraoperative SLN evaluation is widely performed in a daily oncosurgical practice, and its methodology includes cytology (scrape, smear, or touch), histologic frozen section (FS), rapid immunohistochemistry, and rapid molecular biology (nucleic acid such as CK-19 messenger RNA amplification by reverse transcription–polymerase chain reaction). Of these, the most important and widely utilized methods are FS, touch imprint cytology (TIC), and a combination of both.
TIC is less expensive, quicker, and easier to perform without losing valuable tissues compared to FS and reportedly has an equivalent sensitivity to FS tissue diagnosis for the intraoperative SLN evaluation in breast cancer.[4-10] In this study, we investigated the diagnostic sensitivity and specificity of TIC and its utility to detect SLN metastases in patients with early breast cancer, primarily clinically significant ones. The size detection limit of metastatic foci was also assessed. Based on our data, we proposed a practical and efficient intraoperative approach to SLNs, best-utilizing TIC.
MATERIALS AND METHODS
A retrospective search for the pathology database files at Asahikawa Medical University Hospital, Asahikawa, Japan, revealed a total of 367 patients with breast cancer (cN0) who underwent intraoperative SLN examinations (507 lymph nodes), assessed by both FS and TIC, in a period between June 2015 and June 2018. Both FS and TIC slides of the SLNs from all these cases were retrieved and microscopically reviewed independently by two observers (YU and HT). All FS slides were examined for the presence or absence of metastasis of any size, and if present, the largest focus was measured and its histologic type was recorded (If metastatic deposits were multiple, the largest contiguous tumor deposit was measured). Then, all TIC slides were also examined for the presence or absence of metastatic cancer cells. The discrepancy between the two observers was resolved by joint review. Based on these data, the diagnostic sensitivity, specificity, and accuracy of TIC to detect SLN metastases of any size as well as clinically significant ones were calculated, and identifiable minimal size of metastatic focus was also assessed. The size detection limit of metastatic foci, defined as the smallest dimension of metastasis detected by TIC without false negatives, was determined as well. In addition, the patient demographics and breast tumor characteristics of each case were recorded.
At the time of intraoperative consultation, each SLN received was serially cross-sectioned in its entirety perpendicularly to its long axis with intervals of 2 mm. Touch imprint was taken from each cut surface and stained with rapid Papanicolaou. Then, all of the sectioned tissue pieces were entirely processed for FS using Cryofilm® transfer method (Leica Biosystems).[11] In this peculiar FS method, a 5-μm-thick FS was prepared with a special attached adhesive film, together stained with H and E, transferred together to a glass slide, and mounted under a coverslip. This method allows for better sectioning and evaluation of fatty lymph nodes, especially their periphery, compared to the conventional FS method, leading to a possible increase in sensitivity to detect small metastasis.
This study has been approved by the Institutional Review Board at Asahikawa Medical University (#18269).
RESULTS
A total of 507 SLNs from 367 patients (all women; average age: 59.6 years, range: 28–89 years) were examined intraoperatively during this 37-month study period. No male cases were present. The SLNs measured 5–28 mm in the greatest dimension. The number of SLNs examined per case ranged from 1 to 4 (mostly one). Of these 507 SLNs, 82 lymph nodes (16.2%) from 69 women were histologically found to have breast cancer metastases of any size in the FS examined and consisted of 5 nodes of isolated tumor cells (ITCs: metastatic focus ≤0.2 mm or <200 cells), 15 nodes of micrometastasis (0.2< ≤2 mm), and 62 nodes of macrometastasis (>2 mm) [Table 1]. In terms of histological types, 70 cases were invasive carcinoma of no special type (IC-NST), 9 invasive lobular carcinoma (ILC), and 3 other types of carcinoma (e.g., invasive micropapillary carcinoma). All of the micrometastasis and ITC cases were IC-NST, except for one case of micrometastasis, which was ILC.
| SLNs with metastasis (patients) | SLNs without metastasis (patients) | ||
|---|---|---|---|
| Histology | Detected by TIC (%) | ||
| Macrometastasis (>2.0 mm) | 62 (49) | 62 (100) | |
| Micrometastasis (0.2 < ≤2.0 mm) | 15 (15) | 7 (46.7) | |
| Isolated tumor cells (≤0.2 mm or <200 cells) | 5 (5) | 0 | |
| Subtotal | 82 (69) | 69 (84.1) | |
| - | 425 (298) | ||
| Total | 507 (367) | ||
SLNs: Sentinel lymph nodes, TIC: Touch imprint cytology
As shown in Table 1 and Figure 1, TIC could detect metastasis in 69 of these 82 SLNs (84.1%) with histologically identifiable metastases, whereas no false-positive cases (i.e., positive cytology but negative histology) were present (sensitivity: 84.1%, specificity: 100%, and accuracy: 97.4%). All of the macrometastases could be successfully detected by TIC (sensitivity and specificity: 100%). On the other hand, TIC could identify approximately 50% of micrometastases, which included one case of ILC micrometastasis (1 mm focus). None of ITCs were detected by TIC. The smallest size of metastatic focus detected by TIC was 0.8 mm (one case). The size detection limit of metastatic foci was determined to be 2 mm.
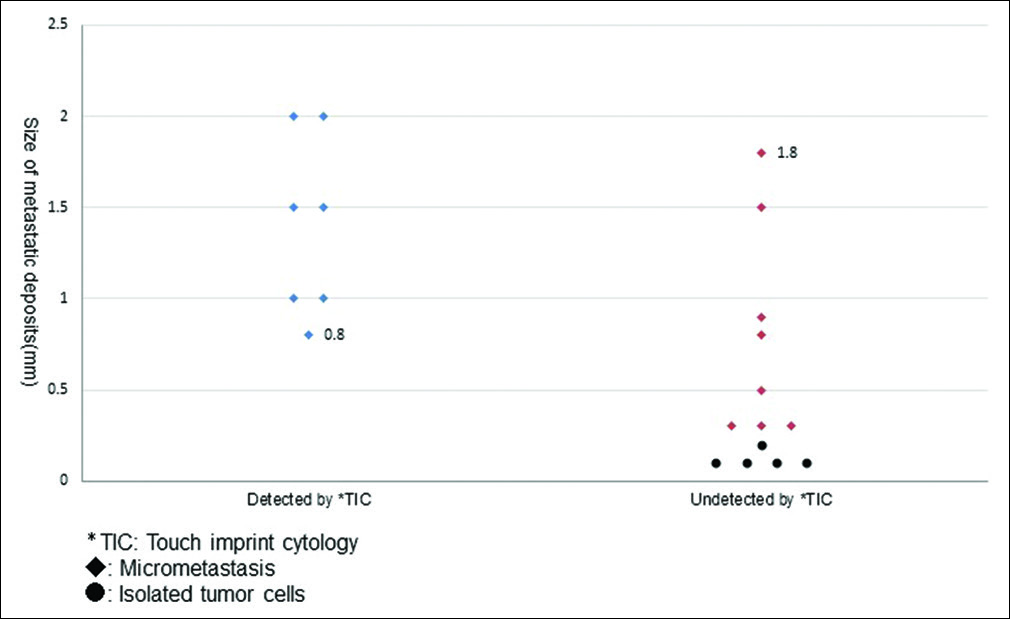
- Size distribution of metastatic deposits (≦2 mm), stratified by detected vs. undetected by touch imprint cytology.
DISCUSSION
TIC is rapid, simple, inexpensive, and does not require sophisticated instruments or loose tissues. In surgical pathology practice, it is routinely used for intraoperative consultation as an adjunct to histopathology (FS). For an intraoperative SLN examination of breast cancer, TIC reportedly showed high concordance with FS in diagnosis.[4-10] In our investigation, we could successfully detect all of macrometastatic foci by TIC (i.e., negative predictive value [NPV] of 100% for cytological identification of macrometastasis). The lower size limit of detection, the smallest metastatic dimension that could be identified by TIC without false negatives (i.e., positive histology but negative cytology), was 2 mm. Moreover, TIC was sensitive enough to identify about half of micrometastatic foci, mostly of ≥1 mm, with the minimal size of detection of 0.8 mm [one case only, as shown in Figure 2].

- (a) The smallest metastatic focus (0.8 mm) detected by touch imprint cytology (Frozen section using Cryofilm® transfer method; hematoxylin and eosin stain). (b) Corresponding touch imprint cytology showing a cluster of cancer cells (rapid Papanicolaou stain).
Recently, Bruzzone et al. conducted a similar study using intraoperative scrape cytology (ISC) of bisected SLNs and reported its sensitivity of 100% for macrometastasis, which is in keeping with our imprint-based results.[12] On the other hand, their reported sensitivity for micrometastasis was much higher than ours (84% vs. 46.7%). For detection of macrometastasis in SLNs, there is probably no methodological difference between TIC and ISC, whereas for detection of micrometastasis, touch imprint might not be able to transfer fewer cancer cells to the slides as effectively as scraping even if multiple serially sectioned surfaces are touch imprinted.
Cytological identification of metastatic LC in SLN is known to be challenging given its low-grade cytomorphology with some resemblance to surrounding lymphocytes and its tendency to lose cellular cohesiveness. In our study, nine cases of ILC (eight macrometastases and one micrometastasis) were found in 82 metastatic SLNs, and our TIC examination, with enough knowledge of the above-mentioned cytological characteristics, could successfully detect all of these LC cases. This is in keeping with the earlier study by Creager et al.,[13] in which there were no statistically significant differences in sensitivity, specificity, or accuracy for the intraoperative detection of LC versus ductal carcinoma in SLNs using TIC.
For breast cancer patients with microscopic metastatic spread (i.e., pN0 [i+] and pN1 mi) in SLN(s), completion ALND is usually not performed. The presence of macrometastasis, even one focus, in the SLN(s) traditionally mandated to complete ALND. This strategy is still followed as the standard of management in some countries such as Japan. However, novel study results of the multicenter American College of Surgeons Oncology Group Z0011 trial for patients with early-stage invasive breast cancer (cT1-T2 cN0), having one or two SLNs with macrometastasis, revealed noninferior rates of overall, disease-free, and locoregional recurrence-free survivals between ALND group and SLN biopsy alone group as long as they were treated with breast-conserving surgery followed by tangential adjuvant radiation.[14] These novel findings were adopted by the current NCCN guidelines.[15] This novel study caused a drastic paradigm shift, and ALND is no longer necessary or needed for the majority of early breast cancer cases. Likewise, it is reported that cases of intraoperative SLN examination markedly declined after the Z0011 criteria were applied as a standard in axillary management.[16,17] The intraoperative SLN examination most likely tends to be limited to cases that do not meet the Z0011 criteria.
Regardless of the number of SLNs examined intraoperatively, accurate identification of macrometastasis, which is “clinically significant,” is important. Moreover, it would be far more important in countries where the traditional axillary management strategy is still applied (such as Japan). In this setting, TIC is of great use given the NPV of 100% for cytological identification of macrometastasis in our study. Based on our results, a practical and efficient intraoperative two-step approach to the SLN(s) is proposed as follows: TIC is initially performed on each serially sectioned piece of LN(s) and is examined cytologically, and when it is negative, an intraoperative diagnosis of “no macrometastasis identified” can be rendered. When it is positive, FS should be proceeded, using only cytologically positive tissue piece(s), and the metastatic focus should be histologically measured since TIC is sensitive enough to detect about half of micrometastases. In this approach, TIC might be more effective than ISC since the former can detect fewer cases of micrometastasis (i.e., lower false positivity) albeit 100% sensitivity for macrometastasis in both methodologies. Given a low rate of SLN macrometastasis in early breast cancers, this strategy is easier to perform, quicker, and more cost-effective than the conventional FS examination.
CONCLUSIONS
We demonstrated a 100% sensitivity for macrometastasis in intraoperative SLN cytological examination with TIC, and utilizing this cytological method, proposed a practically useful intraoperative two-step strategy to examine the SLNs in breast cancer patients.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
YU designed the study and wrote the initial draft of the manuscript. HT contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. All other authors have contributed to data collection and interpretation and critically reviewed the manuscript. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
ETHICS STATEMENT BY ALL AUTHORS
This study has been approved by the Institutional Review Board at Asahikawa Medical University (#18269).
LIST OF ABBREVIATIONS (In alphabetic order)
ALND – Axillary lymph node dissection
FS – Frozen section
IC-NST – Invasive carcinoma of no special type
ILC – Invasive lobular carcinoma
ISC – Intraoperative scrape cytology
ITCs – Isolated tumor cells
NPV – Negative predictive value
SLNs – Sentinel lymph nodes
TIC – Touch imprint cytology.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
References
- Breast In: Amin MB, ed. AJCC Cancer Staging Manual (8th Edition). Switzerland: Springer International Publishing AG; 2017. p. :589-628. Edited by
- [CrossRef] [Google Scholar]
- Do all patients with sentinel node metastasis from breast carcinoma need complete axillary node dissection? Ann Surg. 1999;229:536-41.
- [CrossRef] [PubMed] [Google Scholar]
- Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: Overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927-33.
- [CrossRef] [Google Scholar]
- Sentinel lymph nodes for breast carcinoma: An update on current practice. Histopathology. 2016;68:152-67.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative touch imprint cytology for the diagnosis of sentinel lymph node metastases in breast cancer. Br J Surg. 2006;93:572-6.
- [CrossRef] [PubMed] [Google Scholar]
- Sentinel lymph node biopsy assessment using intraoperative imprint cytology in breast cancer patients: Results of a validation study. Asian J Surg. 2004;27:294-8.
- [CrossRef] [Google Scholar]
- The evaluation and optimization of intraoperative touch imprint cytology for sentinel lymph nodes in early-stage breast cancer in China. World J Surg. 2010;34:2325-32.
- [CrossRef] [PubMed] [Google Scholar]
- The value of touch imprint cytology and frozen section for intra-operative evaluation of axillary sentinel lymph nodes. Pol J Pathol. 2010;61:161-5.
- [Google Scholar]
- The use of touch preparation for the evaluation of sentinel lymph nodes in breast cancer. Am J Surg. 2010;199:792-6.
- [CrossRef] [PubMed] [Google Scholar]
- Testing the feasibility of intra-operative sentinel lymph node touch imprint cytology. Ann R Coll Surg Engl. 2009;91:336-9.
- [CrossRef] [PubMed] [Google Scholar]
- Use of a new adhesive film for the preparation of multi-purpose fresh-frozen sections from hard tissues, whole-animals, insects and plants. Arch Histol Cytol. 2003;66:123-43.
- [CrossRef] [PubMed] [Google Scholar]
- Synergy of cytological methods in the pathological staging of breast cancer: Axillary fine-needle aspiration and intraoperative scrape cytology of the sentinel lymph node. Diagn Cytopathol. 2018;46:919-26.
- [CrossRef] [PubMed] [Google Scholar]
- Intraoperative imprint cytologic evaluation of sentinel lymph nodes for lobular carcinoma of the breast. Ann Surg. 2004;239:61-6.
- [CrossRef] [PubMed] [Google Scholar]
- Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: A randomized clinical trial. JAMA. 2011;305:569-75.
- [CrossRef] [PubMed] [Google Scholar]
- NCCN Guidelines Insights: Breast Cancer, Version 3. 2018. J Natl Compr Canc Netw. 2019;17:118-26.
- [CrossRef] [PubMed] [Google Scholar]
- American College of Surgeons Oncology Group (ACOSOG) Z0011: Impact on surgeon practice patterns. Ann Surg Oncol. 2012;19:3144-51.
- [CrossRef] [PubMed] [Google Scholar]
- Measuring the impact of the American College of Surgeons Oncology Group Z0011 trial on breast cancer surgery in a community health system. Am J Surg. 2015;209:240-5.
- [CrossRef] [PubMed] [Google Scholar]








