Translate this page into:
Ubiquitin-specific peptidase 14 promotes neuron injury by stabilizing acyl-CoA synthetase long-chain family member 4 through deubiquitination

*Corresponding author: Ying Liu, Department of Anesthesia, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang, China. liuying_hbmu@163.com
-
Received: ,
Accepted: ,
How to cite this article: Hao X, Liu Y. Ubiquitin-specific peptidase 14 promotes neuron injury by stabilizing acyl-CoA synthetase long-chain family member 4 through deubiquitination. CytoJournal. 2025;22:11. doi: 10.25259/Cytojournal_52_2024
Abstract
Objective:
Ubiquitin-specific peptidase 14 (USP14) may be a target for stroke treatment. Our study aims to explore the molecular mechanism of USP14 in the stroke process.
Material and Methods:
A stroke cell model was constructed using oxygen–glucose deprivation/reoxygenation (OGD/R)-induced SK-N-SH cells, and cell growth was assessed using cell counting kit 8 assay, EdU assay, and flow cytometry. Proinflammatory cytokine levels were tested through an enzyme-linked immunosorbent assay. The levels of USP14 and acyl-CoA synthetase long-chain family member 4 (ACSL4) were determined through Western blot and quantitative real-time polymerase chain reaction, whereas the interaction of USP14 and ACS14 was evaluated by co-immunoprecipitation assay.
Results:
OGD/R-induced SK-N-SH cell injury by enhancing ferroptosis and the knockdown of USP14 inhibited OGD/R-induced cell inflammation, apoptosis, and ferroptosis. Moreover, USP14 enhanced ACSL4 protein expression through deubiquitination. ACSL4 silencing mitigated neuron injury, and ACSL4 upregulation abolished USP14 knockdown-mediated inhibition of neuron injury.
Conclusion:
USP14 can enhance neuron injury through stabilizing ACSL4 protein expression.
Keywords
Deubiquitination
Stroke
Ubiquitin-specific peptidase 14
INTRODUCTION
Stroke has emerged as a major disease that endangers the quality of life of middle-aged and older people in China and is the second leading cause of death.[1,2] The main type of stroke onset is ischemic stroke (IS), which is characterized by high morbidity, disability, mortality, and recurrence rates.[3,4] In recent years, the oxygen-glucose deprivation/reoxygenation (OGD/R) models of neurons have been used in simulating the clinical stroke process at the cellular level.[5] Therefore, searching for the underlying molecular mechanisms that affect OGD/R-induced neuron injury is of great significance for the development of novel treatments for stroke.
Ubiquitin-specific proteases (USPs) can recognize the ubiquitination signals of specific target proteins and deubiquitinate them.[6] Ubiquitin-specific peptidase 14 (USP14) dysregulation regulates cell inflammation, apoptosis, and ferroptosis in colon cancer and atherosclerosis-related diseases by influencing protein degradation.[7,8] The inhibition of USP14 protects neurons from ferritinophagy-mediated ferroptosis, thereby alleviating IS progression.[9] Moreover, treatment with USP14 inhibitor attenuates ischemia– reperfusion-induced neuronal injury.[10] However, USP14’s roles and mechanisms in IS need to be further investigated.
As a novel independent cell death pathway, ferroptosis mediates the development of IS.[11,12] Acyl-CoA synthetase long-chain family member 4 (ACSL4) causes ferroptosis and is considered a key gene in the pathogenesis of IS,[13,14] facilitating neuronal death in IS by augmenting lipid peroxidation.[15] Baicalein mitigates ischemia-reperfusion injury by suppressing ACSL4-mediated ferroptosis.[16] Moreover, RNF146 alleviates OGD/R-induced neuronal damage in IS by regulating ACSL4,[17] and E3 ligase MDM2 promotes ACSL4 degradation and thus mediates ischemic brain injury by promoting its ubiquitination.[18] These findings show that ACSL4 is associated with ubiquitin modification. We found that USP14 can affect ACSL4 protein expression, but whether USP14 regulates the stroke process by mediating ACSL4 expression has not been studied.
In this study, we explored the role and mechanism of USP14 in OGD/R-induced stroke cell injury models. We proposed and tested the hypothesis that USP14 promotes neuron injury by stabilizing ACSL4.
MATERIAL AND METHODS
Cell culture and treatment
SK-N-SH cells (catalog number: HTB-11; American Type Culture Collection (ATCC), Manassas, VA, USA) and PC12 cells (catalog number: CRL-1721; ATCC) were cultured in Dulbecco’s Modified Eagle’s Medium (SH30022.01B, HyClone, Logan, UT, USA) or Roswell Park Memorial Institute-1640 medium (SH30027.01, HyClone) containing 10% fetal bovine serum (SH30370.03, HyClone) and 1% penicillin/streptomycin (SV30010, HyClone). All cells were tested by STR and mycoplasma. For OGD/R treatment, the cells were grown in a glucose-free medium under the conditions of 95% N2 and 5% CO2 for 6 h and then reoxygenated in a standard medium under the conditions of 95% air and 5% CO2 for 24 h.
Cell transfection and treatment
Using Lipofectamine 3000 (L3000075, Invitrogen, Carlsbad, CA, USA), we transfected the cells with ACSL4/USP14 small interfering RNA (si-ACSL4: F 5'-UUUCUCUUAAGAAGAACACGA-3', R 5'-GUGUUCUUCUUAAGAGAAAAA-3'; si-USP14: F 5'-UCUGUAUUCAAUUCUACACCU-3', R 5'-GUGU AGAAUUGAAUACAGAUG-3'), polyclonal DNA ACSL4/USP14 overexpression vector, and negative controls (si-NC: 5'-GGAGUAGGGAGCAAACCUAUAGGAA-3' and 5'-UUCCUAUAGGUUUGCUCCCUACUCC-3' and pcDNA; GenePharma, Shanghai, China) before OGD/R induction. The transfected cells were then treated with cycloheximide (CHX, 50 μg/L; Selleck, Houston, TX, USA), and the half-life of ACSL4 was determined by measuring its protein expression at 0, 3, 6, 9, and 12 h or ubiquitinproteasome inhibitor MG132 level (5 μM; Beyotime, Shanghai, China) for 4 h.
Cell counting kit 8 (CCK8) assay
Cells were seeded into 96-well plates and treated with a CCK8 reagent (CK04-500T, Dojindo, Kumamoto, Japan). Cell viability was determined at 450 nm with a microplate reader (Epoch, BioTek, Winooski, Vermont, USA).
EdU assay
An EdU detection kit (C0078S, Beyotime) was used in staining cells according to the manufacturer’s instructions. The cells were spread in 24-well plates and induced with EdU, Apollo, and DAPI solution (C1005, Beyotime). Fluorescent images were then captured under a microscope (SMZ18; Nikon, Tokyo, Japan), and EdU-positive cell rate (%) was calculated using ImageJ software (version 1.8.0, NIH, Bethesda, MD, USA).
Flow cytometry
The cells were transferred to a tube, suspended with binding buffer, and stained with annexin V-FITC and PI (C1062S, Beyotime) for 15 min. Cell apoptosis rate was then determined through flow cytometry (LSRFortessa TM X-20; BD Biosciences, San Diego, CA, USA) with CellQuest Pro software (BD Biosciences).
Enzyme-linked immunosorbent assay (ELISA)
Inflammatory factor levels were detected using corresponding ELISA kits (SEKH-0013, SEKH-0002, SEKH-0047, Solarbio, Beijing, China) according to manufacturers’ instructions. The cell culture medium was centrifuged, and the supernatant was added to the culture plate and incubated with the corresponding antibody and enzyme conjugate. After incubation with the chromodeveloping substrate 3,3,5’,5’-Tetramethyl benzidine, a termination solution was added to the culture plate. Absorbance at 450 and 630 nm was analyzed with a microplate reader (Epoch, BioTek), and inflammatory factor levels were calculated according to the standard curve.
Detection of ferroptosis
The levels of Fe2+, malondialdehyde (MDA), and reactive oxygen species (ROS) in cells were detected using assay kits (ab83366, ab11897, and ab113851, respectively); Abcam, Cambridge, MA, USA). For Fe2+ level detection, cells were collected, added to wells, and incubated with assay buffer and iron probe. Then, the absorbance at 593 nm was analyzed using the microplate reader. For MDA level detection, a cell culture extract was incubated with TBA solution, and the absorbance was analyzed with the microplate reader at 532 nm. For ROS level detection, cells were stained with 2’,7’-dichlorodihydrofluorescein diacetate and washed with buffer, and absorbance at 485 or 535 nm was analyzed with the microplate reader.
Western blot (WB)
Cells were lysed with radio-immunoprecipitation assay lysis buffer (P0013B, Beyotime) and the proteins were extracted after quantified by bicinchoninic acid protein assay kit (P0010S, Beyotime). The proteins were then separated with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel and transferred to PVDF membranes (FFP20, Beyotime). The membrane was closed and incubated with anti-USP14 (ab192618, 1:2000, Abcam), anti-ACSL4 (ab155282, 1:10000, Abcam), anti-glyceraldehyde-3-phosphate dehydrogenase (ab9485, 1:2500, Abcam), and secondary antibody (ab205718, 1:50000, Abcam). Protein signals were exposed using an enhanced chemiluminescence reagent (P0018S, Beyotime) and Doc XR Imaging System (Bio-Rad, Hercules, CA, USA). The gray value was analyzed with ImageJ software (version 1.8.0, NIH).
Quantitative real-time polymerase chain reaction (PCR)
Total RNA was isolated using TRIzol reagent (15596026CN, Invitrogen) and used for synthesizing complementary DNA (cDNA) with cDNA synthesis kit (6210A, Takara, Tokyo, Japan). SYBR Green (RR820A, Takara) was mixed with the cDNA and specific primers [Table 1] for PCR amplification with a PCR instrument (LightCycler480; Roche, Basel, Switzerland).
| Name | Primers for PCR (5’-3’) |
|---|---|
| USP14 | |
| Forward | TCCAGAAGAACCCTCAGCCA |
| Reverse | TGAAGCTCTCAAGGCACCTG |
| ACSL4 | |
| Forward | AAGAGGACATTTAAGAAAAACGCTA |
| Reverse | GTCCCAAGGCTGTCCTTCTT |
| GAPDH | |
| Forward | AAGGCTGTGGGCAAGGTCATC |
| Reverse | GCGTCAAAGGTGGAGGAGTGG |
USP14: Ubiquitin-specific peptidase 14, ACSL4: Acyl-CoA synthetase long-chain family member 4, GAPDH: Glyceraldehyde-3-phosphate dehydrogenase, A: Adenine, C: Cytosine, G: Guanine, T: Thymine
Co-immunoprecipitation analyses
SK-N-SH cells were collected and lysed with lysis buffer. Then, the cell lysates were incubated with anti-immunoglobulin G (ab172730, Abcam), anti-USP14 (ab71165, Abcam), or anti-ACSL4 (ab155282, Abcam) in TBS buffer (28358, Thermo Scientific, Waltham, MA, USA). Then, the cell lysates were incubated with protein A agarose (20334, Thermo Scientific) with rotation. The immunoprecipitates were eluted with an SDS-PAGE buffer and then used for WB analysis.
Detection of ubiquitination
SK-N-SH cells were transfected with Flag-ACSL4, si-NC, or si-USP14 for 24 h. Then, the cell lysates were incubated with anti-USP14, HA-tag labeled ubiquitin protein, and protein A/G agarose beads (20422; Thermo Scientific) overnight. The precipitated proteins were collected for WB and detection of ACSL4 ubiquitination level.
Statistical analysis
Data were expressed as mean ± SD with GraphPad Prism (version 7.0, La Jolla, CA, USA). Differences were compared using Student’s t-test or analysis of variance. P < 0.05 was considered statistically significant.
RESULTS
OGD/R can induce neuronal injury
OGD/R treatment suppressed SK-N-SH cell proliferation [Figure 1a and b] while increasing cell apoptosis rate and interleukin 6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor-alpha (TNF-α) levels [Figure 1c-f]. Further analysis showed that ferroptosis inhibitor-1 reversed OGD/R-mediated inhibition of cell viability [Figure 1g], indicating that OGD/R treatment mediates cell injury by promoting ferroptosis. Therefore, we examined ferroptosis-related indicators and found that OGD/R treatment elevated Fe2+, ROS, and MDA levels [Figure 1h-j].
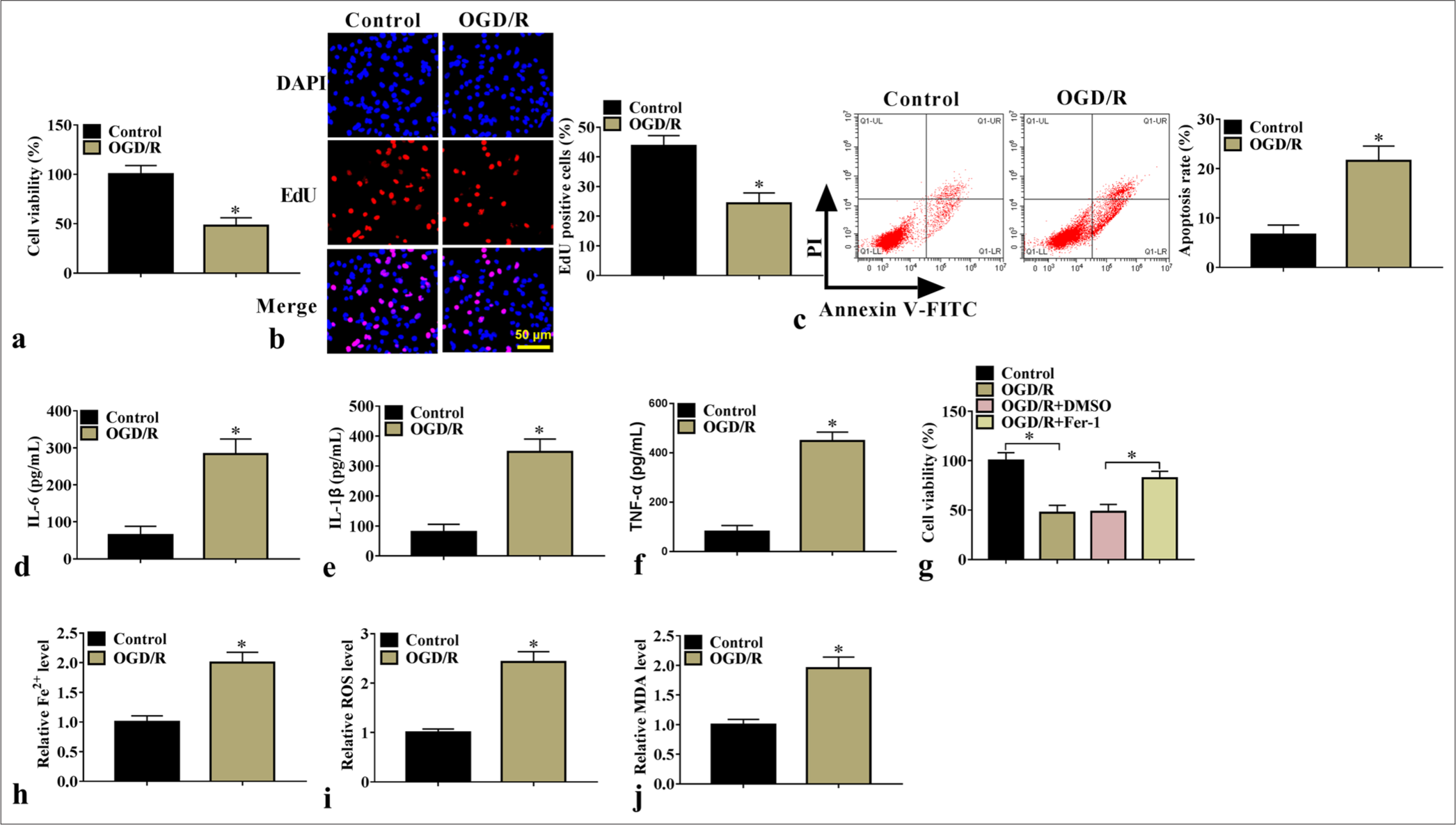
- OGD/R-induced cell injury. Cell proliferation and apoptosis (n=3) were examined using (a) CCK8 and (b) EdU assays and (c) flow cytometry. (d-f) IL-6, IL-1β, and TNF-α levels (n=3) were detected through ELISA. (g) Cells were induced with OGD/R and then treated with ferroptosis inhibitor, and cell viability was detected with CCK8 (n=3). (h-j) Fe2+, ROS, and MDA levels were detected (n=3). ✶P<0.05. OGD/R: Oxygen–glucose deprivation/reoxygenation, CCK8: Cell counting kit 8, IL-6: Interleukin 6, IL-1β: Interleukin-1 beta, TNF-α: Tumor necrosis factor-alpha, ELISA: Enzyme-linked immunosorbent assay, ROS: Reactive oxygen species, MDA: Malondialdehyde.
USP14 knockdown inhibited neuronal injury
We performed rescue experiments to confirm the effect of USP14 inhibition on neuronal damage. By detecting USP14 protein expression, we found that OGD/R increased USP14 protein expression in the SK-N-SH cells, but si-USP14 offset this effect [Figure 2a]. Functional experiments showed that si-USP14 offset OGD/R treatment-induced suppression of cell proliferation and enhancement of apoptosis rate [Figure 2b-d]. Furthermore, the OGD/R treatment-induced enhancement of IL-6, IL-1β, TNF-α, Fe2+, ROS, and MDA levels were reversed by si-USP14 [Figure 2e-j]. To further confirm our results, we performed the same experiments on another cell line (PC12) and siRNA (si-USP14#1). After silencing USP14 expression with si-USP14, we found that cell viability was enhanced, whereas TNF-α and Fe2+ levels were reduced in OGD/R-induced PC12 cells [Supplementary Figure 1a-d]. Furthermore, we confirmed that si-USP14#1 was able to decrease USP14 expression, and further analysis indicated that si-USP14#1 increased cell viability, inhibited apoptosis rate, and reduced TNF-α and Fe2+ levels in OGD/R-induced SK-N-SH cells [Supplementary Figure 2a-f]. The USP14-specific inhibitor 1-[1-(4-fluorophenyl)-2,5-dimethylpyrrol-3-yl]-2-pyrrolidin-1-ylethanone (IU1) was used for further analysis, and the results confirmed that IU1 treatment markedly decreased USP14 expression [Supplementary Figure 3a], promoted cell viability, and repressed TNF-α and Fe2+ levels [Supplementary Figure 3b-d].
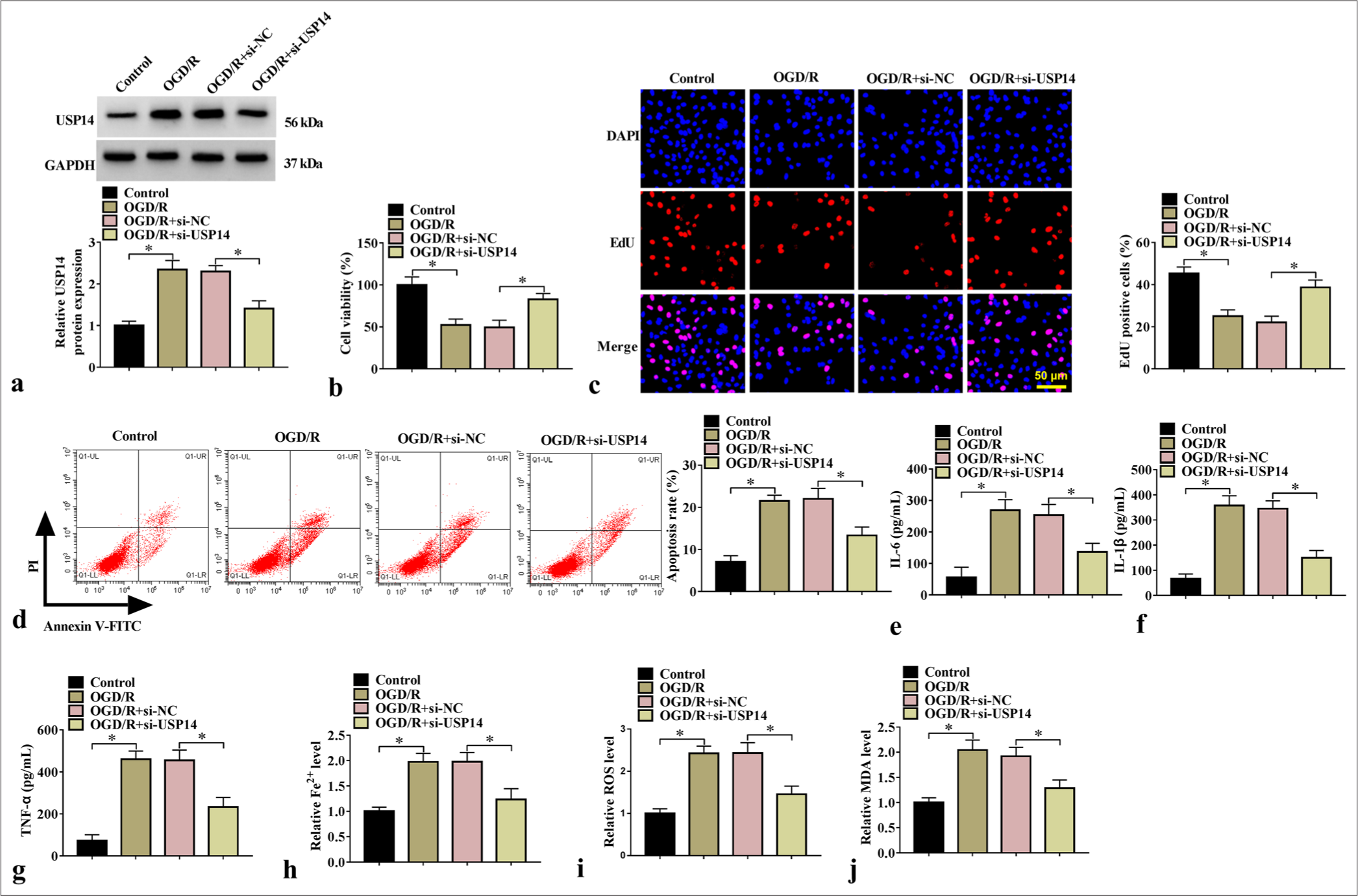
- Effects of si-USP14 on cell injury. (a) WB analysis for USP14 protein expression (n=3). Cell proliferation and apoptosis were examined by (b) CCK8, (c) EdU assay, and (d) flow cytometry (n=3). (e-g) ELISA for testing IL-6, IL-1β, and TNF-α levels (n=3). (h-j) Fe2+, ROS, and MDA levels were examined (n=3). USP14, ubiquitin-specific peptidase 14. ✶P<0.05. USP14: Ubiquitin-specific peptidase 14, CCK8: Cell counting kit 8, ELISA: Enzyme-linked immunosorbent assay, IL-6: Interleukin 6, IL-1β: Interleukin-1 beta, TNF-α: Tumor necrosis factor-alpha, ROS: Reactive oxygen species, MDA: Malondialdehyde.
Deubiquitinating enzyme USP14 could stabilize ACSL4 expression
To investigate the effect of USP14 on ACSL4, we constructed si-USP14 and USP14 vectors to inhibit and promote USP14 protein expression, respectively [Figure 3a]. Further analysis showed that si-USP14 or USP14 vectors reduced or promoted ACSL4 protein expression, respectively, without affecting its messenger RNA level [Figure 3b and c], indicating that USP14 affects ACSL4 protein level through deubiquitination. In addition, the co-immunoprecipitation analysis revealed that ACSL4 level was enriched in anti-USP14, and USP14 level was enriched in anti-ACSL4 [Figure 3d], suggesting that USP14 bound to the ACSL4 protein. USP14 overexpression prolonged ACSL4 half-life and stabilized ACSL4 protein synthesis [Figure 3e and f], and si-USP14 decreased ACSL4 expression. Meanwhile, the ubiquitin-proteasome inhibitor MG132 increased ACSL4 expression [Figure 3g]. USP14 knockdown reduced ACSL4 expression by increasing the ubiquitination level of ACSL4 [Figure 3h]. These results indicated that USP14 can enhance ACSL4 protein expression through deubiquitination.
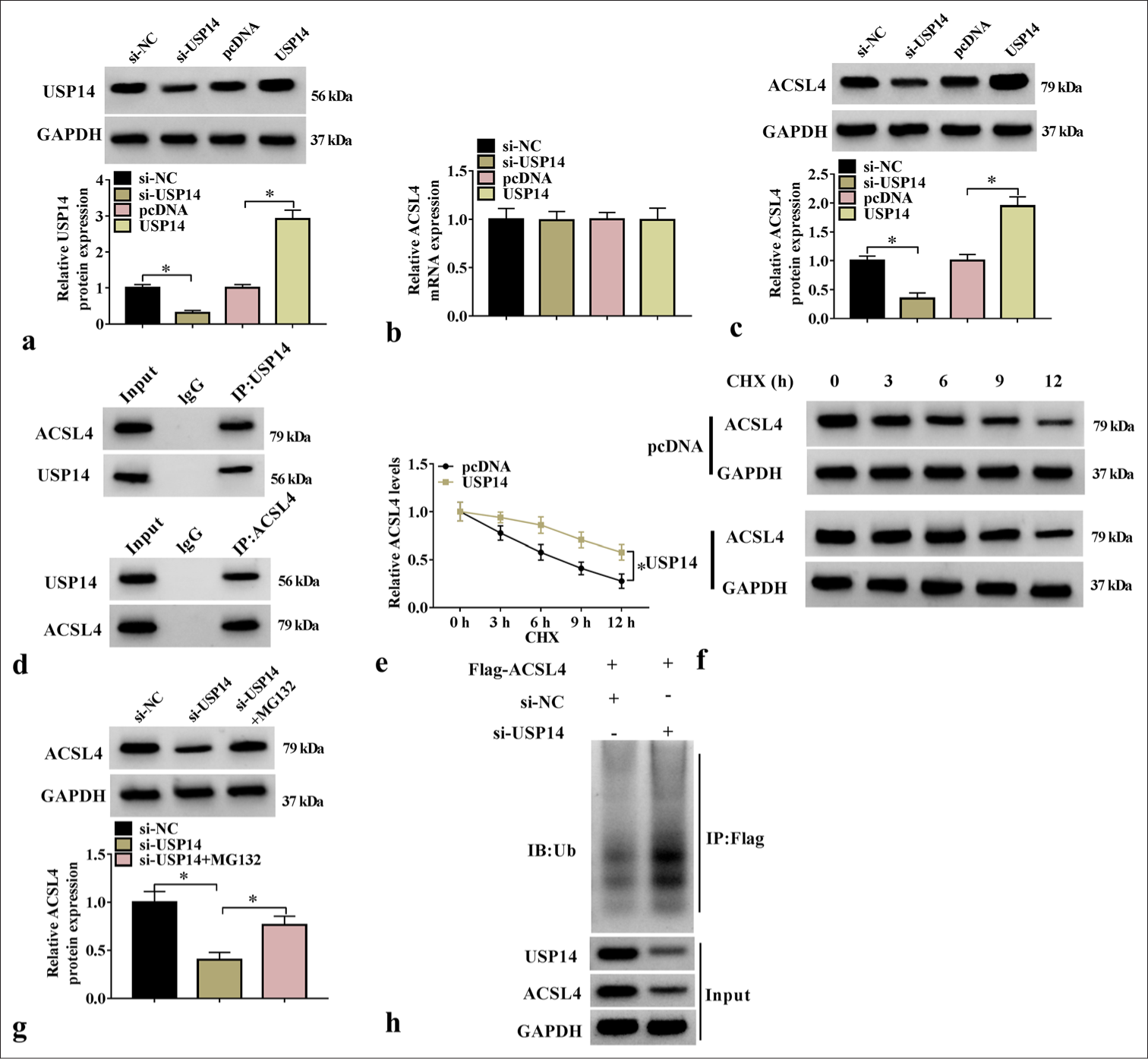
- USP14-enhanced ACSL4 expression through deubiquitination. (a) The transfection efficiency of si-USP14 and USP14 overexpression vectors were assessed with WB assay (n=3). (b and c) ACSL4 Messenger RNA and protein expression levels were tested through qRT-PCR and WB (n=3). (d) The interaction between ACSL4 and USP14 was detected through Co-IP (n=3). (e and f) ACSL4 half-life and protein levels were detected through WB analysis (n=3). (g) ACSL4 protein expression was tested through WB analysis (n=3). (h) The detection of the ubiquitination level of ACSL4 after transfection withsi-NC/si-USP14 (n=3). USP14, ubiquitin-specific peptidase 14; CHX, cycloheximide. ✶P<0.05. USP14: Ubiquitin-specific peptidase 14, ACSL4: Acyl-CoA synthetase long-chain family member 4, WB: Western blot, qRT-PCR: Quantitative real-time polymerase chain reaction.
Silencing of ACSL4 mitigated OGD/R-induced neuronal injury
We investigated the effect of ACS14 silencing on the OGD/R-treated SK-N-SH cells. The transfection of si-ACSL4 markedly reduced ACSL4 protein level [Figure 4a], and si-ACSL4 abolished OGD/R treatment-induced inhibition of cell proliferation and enhancement of apoptosis rate [Figure 4b-d]. In addition, ACSL4 silencing repressed IL-6, IL-1β, TNF-α, Fe2+, ROS, and MDA levels [Figure 4e-j]. All these results suggested that ACSL4 enhances OGD/R-induced neuronal injury.
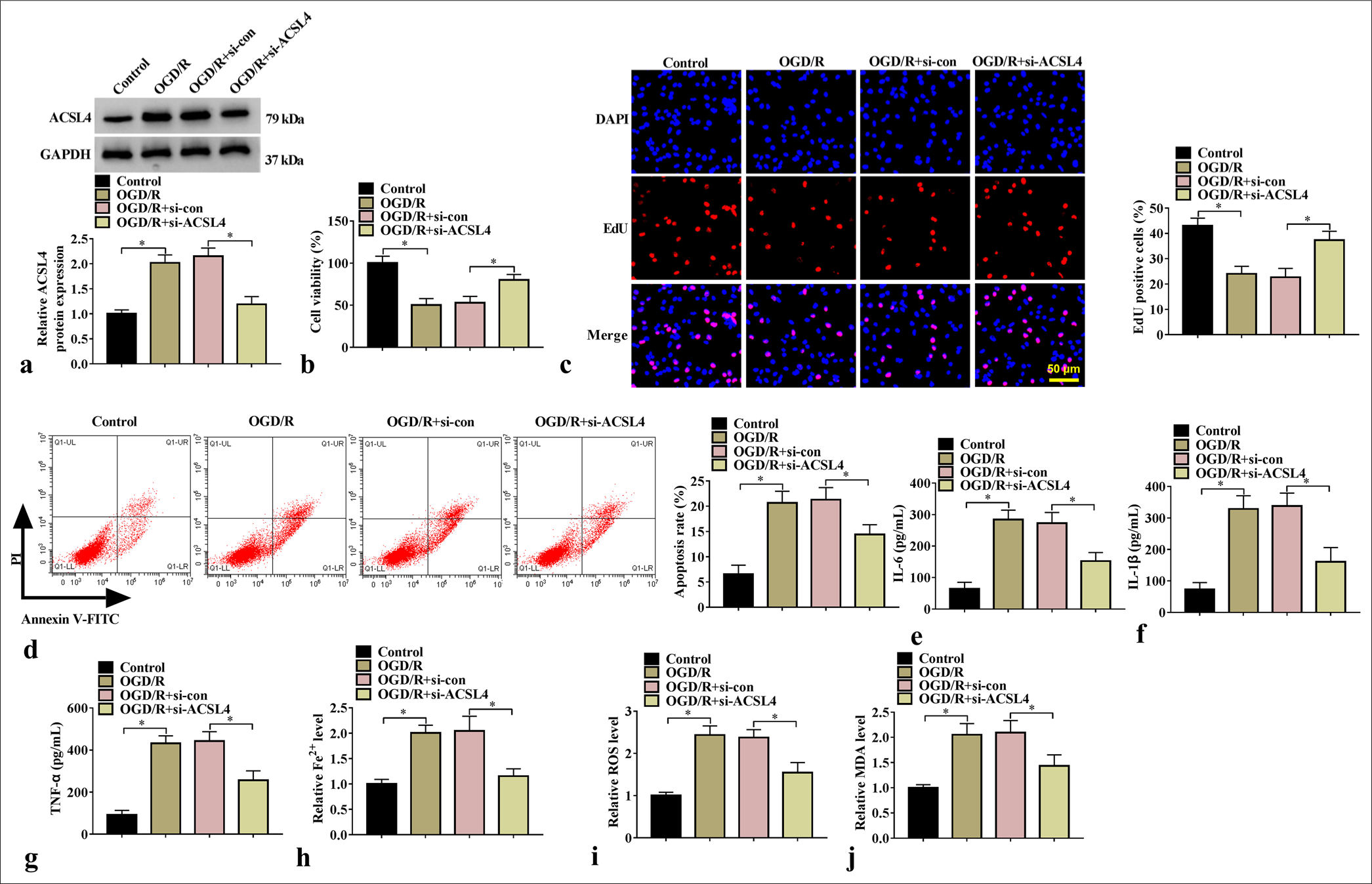
- Effect of si-ACSL4 on SK-N-SH cell injury. (a) ACSL4 protein expression was tested through WB analysis (n=3). Cell proliferation and apoptosis were determined through (b) CCK8, (c) EdU assay, and (d) flow cytometry (n=3). (e-g) IL-6, IL-1β, and TNF-α levels were assessed through ELISA (n=3). (h-j) Fe2+, ROS, and MDA levels were tested (n = 3). ✶P<0.05. ACSL4: Acyl-CoA synthetase long-chain family member 4, CCK8: Cell counting kit 8, WB: Western blot, IL-6: Interleukin 6, IL-1β: Interleukin-1 beta, TNF-α: Tumor necrosis factor-alpha, ROS: Reactive oxygen species, MDA: Malondialdehyde.
ACSL4 overexpression restored the effects of si-USP14 on neuronal injury
We further studied whether USP14 affects OGD/R-induced neuronal injury by regulating ACS14. We found that the ACSL4 overexpression vector offset the si-USP14-induced reduction in ACSL4 protein level [Figure 5a] and enhancement in proliferation [Figure 5b and c]. Meanwhile, ACSL4 overexpression reversed si-USP14-mediated decreases in apoptosis rate, proinflammatory cytokine levels, and ferroptosis-associated marker content [Figure 5d-j]. Overall, USP14 promoted neuronal injury by stabilizing ACSL4.
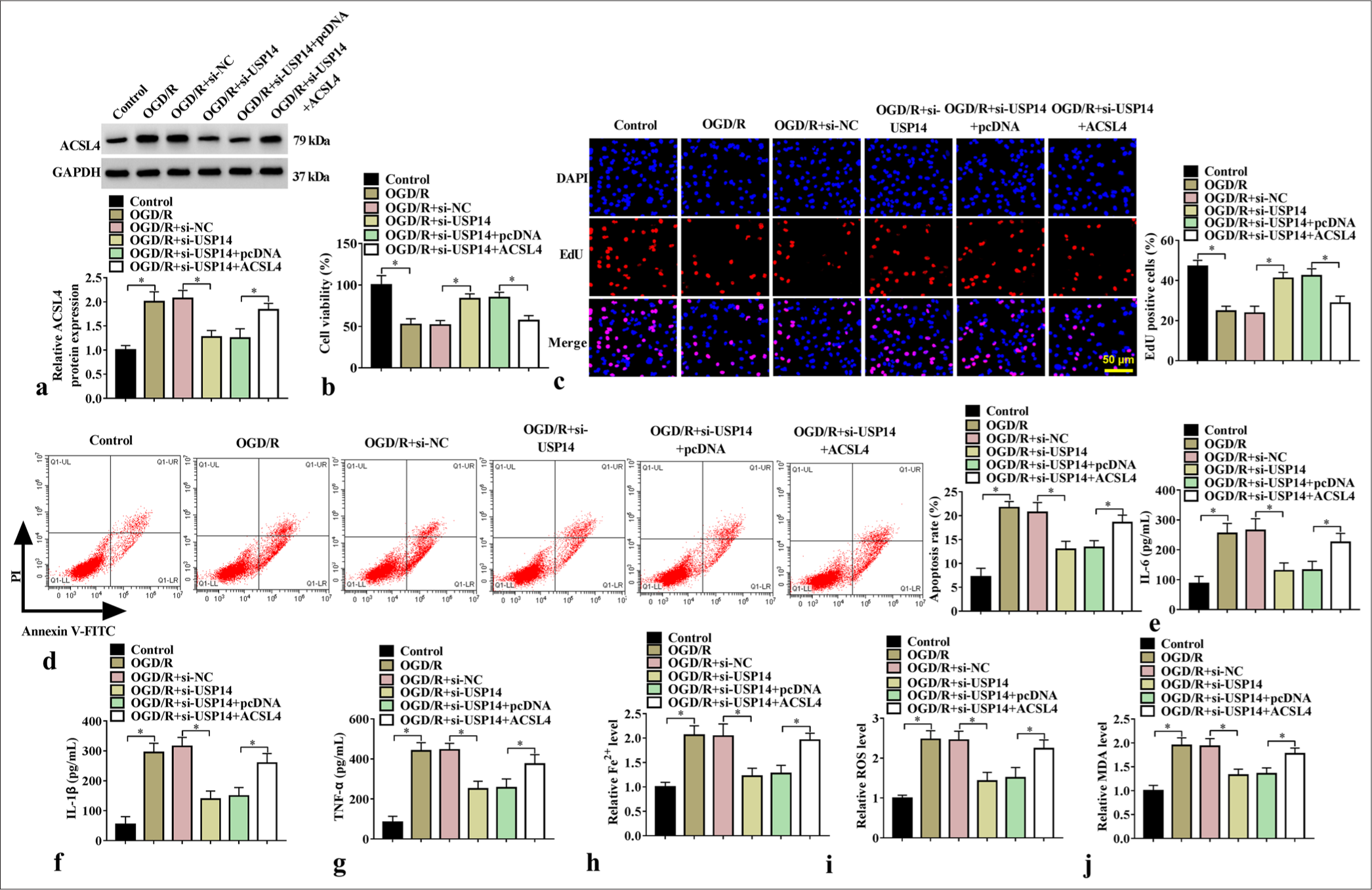
- Effects of si-USP14 and ACSL4 on cell injury. (a) WB analysis for ACSL4 protein expression (n=3). (b) CCK8, (c) EdU assay, and (d) flow cytometry were performed for cell proliferation and apoptosis tests (n=3). (e-g) ELISA for detecting IL-6, IL-1β, and TNF-α levels (n=3). (h-j) Fe2+, ROS, and MDA levels were detected (n=3). USP14, ubiquitin-specific peptidase 14. ✶P<0.05. USP14: Ubiquitin-specific peptidase 14, ACSL4: Acyl-CoA synthetase long-chain family member 4, CCK8: Cell counting kit 8, WB: Western blot, IL-6: Interleukin 6, IL-1β: Interleukin-1 beta, TNF-α: Tumor necrosis factor-alpha, ROS: Reactive oxygen species, MDA: Malondialdehyde.
DISCUSSION
The aging society and rapid urbanization have led to a considerable increase in stroke cases, and the number of younger individuals experiencing stroke is increasing.[19] Human SK-N-SH is a neuroblastoma cell line considered a core cell model for assessing neurons in vitro.[20] To date, OGD/R-treated SK-N-SH cells are considered classical in vitro cellular models of stroke.[21,22] We found that silencing USP14 alleviated OGD/R-induced neuron injury through ACSL4 deubiquitination. This data provides evidence of USP14 as a therapeutic target for stroke.
Owing to its important role in stabilizing disease-causing proteins, the deubiquitinating enzyme USP14 has received considerable interest. The effect of USP14 on cellular ferroptosis has been explored. CSE attenuates ferroptosis by downregulating USP14,[23] and the USP14 inhibitor IU1 alleviates H/R-induced cell death and ROS-dependent ferroptosis in HK-2 cells.[24] In addition, USP14 plays a positive role in IS-induced neuronal injury.[10,25] Consistent with previous study, our study demonstrated that USP14 knockdown attenuated OGD/R treatment mediated the enhancement effect on SK-N-SH cell inflammation, apoptosis, and ferroptosis, indicating that USP14 accelerates the progression of stroke.
Ferroptosis is a mechanism of cell death after ischemia in many organs, such as the kidney and heart.[26,27] ACSL4 has been implicated as a promoter of ferroptosis. For example, the attenuation of the PKCβII–ACSL4 axis can block ferroptosis in vitro,[28] and ACSL4 knockdown suppresses lipid peroxidation and ferroptosis after reperfusion.[29] Our study showed that ACSL4 knockdown suppressed apoptosis, inflammation, and ferroptosis in OGD/R-induced SK-N-SH cells. USP14 mediates human disease progression by deubiquitinating a specific gene,[30,31] and CHX inhibits protein synthesis. Moreover, our results showed that USP14 increased the half-life and protein level of ACSL4 after CHX treatment [Figure 3e and f], indicating that USP14 improved the protein stability of ACSL4. MG132 is a protease inhibitor that prevents protease activity during degradation. As shown in Figure 3g, ACSL4 protein levels were reduced by si-USP14, whereas ACSL4 protein levels increased after MG132 treatment and decreased ubiquitination, indicating that si-USP14 reduced ACSL4 levels by promoting ubiquitination. Therefore, USP14 can stabilize ACSL4 protein through deubiquitination. Further investigation revealed that USP14 promoted OGD/R-induced neuron injury by stabilizing ACSL4.
Although the role of USP14 in stroke has been studied, its role in neuron injury models and the underlying molecular mechanisms still need to be further explored. Our study revealed that USP14 enhances OGD/R-induced neuron injury by stabilizing ACSL4 protein expression, providing novel insights into the regulation of USP14 during stroke progression. In addition, our study showed the USP14/ACSL4 molecular axis for the first time, providing evidence of USP14 as a potential molecular target for stroke treatment. However, despite studying this axis at the cellular level, we did not perform in vivo experiments. A mouse MCAO model is needed to explore the effect of the USP14/ACSL4 axis on stroke progression.
SUMMARY
Our study showed that USP14 facilitates OGD/R-induced neuronal injury by deubiquitinating ACSL4. The discovery of these targets, which affect the stroke process, has profound clinical implications for stroke treatment.
AVAILABILITY OF DATA AND MATERIALS
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
ABBREVIATIONS
ACSL4: Acyl-CoA synthetase long-chain family member 4
CCK8: Cell counting kit 8
Co-IP: Co-immunoprecipitation
MDA: Malondialdehyde
OGD/R: Oxygen–glucose deprivation/reoxygenation
qRT-PCR: Quantitative real-timePCR
ROS: Reactive oxygen species
USP14: Ubiquitin-specific peptidase 14
USPs: Ubiquitin-specific proteases
WB: Western blot
ACKNOWLEDGMENT
Not applicable.
AUTHOR CONTRIBUTIONS
XTH: Experiments work, clinical research, data analysis, and manuscript writing. YL: Research design, literature research, and manuscript review. All authors read and approved the final manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethical approval and consent to participate is not required as this study does not involve animal or human experiments.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
This work was funded by The Scientific and Technological Project of Xiangyang City of Hubei Province (NO. 2022YL25B).
References
- Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023;6:e231455.
- [CrossRef] [PubMed] [Google Scholar]
- Management of acute ischemic stroke. Crit Care Med. 2020;48:1654-63.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and management of acute ischemic stroke: Speed is critical. CMAJ. 2015;187:887-93.
- [CrossRef] [PubMed] [Google Scholar]
- Ubiquitin-specific peptidases: Players in bone metabolism. Cell Prolif. 2023;56:e13444.
- [CrossRef] [PubMed] [Google Scholar]
- USP14 promotes tryptophan metabolism and immune suppression by stabilizing IDO1 in colorectal cancer. Nat Commun. 2022;13:5644.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of USP14 suppresses the formation of foam cell by promoting CD36 degradation. J Cell Mol Med. 2020;24:3292-302.
- [CrossRef] [PubMed] [Google Scholar]
- Nuclear receptor coactivator 4-mediated ferritinophagy contributes to cerebral ischemia-induced ferroptosis in ischemic stroke. Pharmacol Res. 2021;174:105933.
- [CrossRef] [PubMed] [Google Scholar]
- USP14 inhibitor attenuates cerebral ischemia/reperfusion-induced neuronal injury in mice. J Neurochem. 2017;140:826-33.
- [CrossRef] [PubMed] [Google Scholar]
- Enriched environment attenuates ferroptosis after cerebral ischemia/reperfusion injury via the HIF-1α-ACSL4 pathway. Oxid Med Cell Longev. 2023;2023:5157417.
- [CrossRef] [PubMed] [Google Scholar]
- Snap25 attenuates neuronal injury via reducing ferroptosis in acute ischemic stroke. Exp Neurol. 2023;367:114476.
- [CrossRef] [PubMed] [Google Scholar]
- Cottonseed oil alleviates ischemic stroke injury by inhibiting ferroptosis. Brain Behav. 2023;13:e3179.
- [CrossRef] [PubMed] [Google Scholar]
- Astragaloside IV alleviates neuronal ferroptosis in ischemic stroke by regulating fat mass and obesity-associated-N6-methyladenosineacyl-CoA synthetase long-chain family member 4 axis. J Neurochem. 2023;166:328-45.
- [CrossRef] [PubMed] [Google Scholar]
- ACSL4 exacerbates ischemic stroke by promoting ferroptosis-induced brain injury and neuroinflammation. Brain Behav Immun. 2021;93:312-21.
- [CrossRef] [PubMed] [Google Scholar]
- Baicalein ameliorates cerebral ischemia-reperfusion injury by inhibiting ferroptosis via regulating GPX4/ACSL4/ACSL3 axis. Chem Biol Interact. 2022;366:110137.
- [CrossRef] [PubMed] [Google Scholar]
- Ring finger protein 146-mediated long-chain fatty-acid-coenzyme a ligase 4 ubiquitination regulates ferroptosis-induced neuronal damage in ischemic stroke. Neuroscience. 2023;529:148-61.
- [CrossRef] [PubMed] [Google Scholar]
- Melatonin improves stroke through MDM2-mediated ubiquitination of ACSL4. Aging (Albany NY). 2024;16:1925-37.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemic stroke in young adults. Continuum (Minneap Minn). 2020;26:386-414.
- [CrossRef] [PubMed] [Google Scholar]
- Circ_0101874 overexpression strengthens PDE4D expression by targeting miR-335-5p to promote neuronal injury in ischemic stroke. J Stroke Cerebrovasc Dis. 2022;31:106817.
- [CrossRef] [PubMed] [Google Scholar]
- Circ_0001360 absence alleviates oxygen-glucose deprivation/reoxygenation-induced SK-N-SH cell injury via controlling the miR-671-5p/BMF pathway. Int J Neurosci. 2024;134:492-502.
- [CrossRef] [PubMed] [Google Scholar]
- LncRNA nuclear-enriched abundant transcript 1 aggravates cerebral ischemia/reperfusion injury through activating early growth response-1/RNA binding motif protein 25 axis. J Neurochem. 2022;163:500-16.
- [CrossRef] [PubMed] [Google Scholar]
- MFG-E8 stabilized by deubiquitinase USP14 suppresses cigarette smoke-induced ferroptosis in bronchial epithelial cells. Cell Death Dis. 2023;14:2.
- [CrossRef] [PubMed] [Google Scholar]
- Inhibition of USP14 suppresses ROS-dependent ferroptosis and alleviates renal ischemia/reperfusion injury. Cell Biochem Biophys. 2023;81:87-96.
- [CrossRef] [PubMed] [Google Scholar]
- USP14 inhibition promotes recovery by protecting BBB integrity and attenuating neuroinflammation in MCAO mice. CNS Neurosci Ther. 2023;29:3612-23.
- [CrossRef] [PubMed] [Google Scholar]
- Thrombin induces ACSL4-dependent ferroptosis during cerebral ischemia/reperfusion. Signal Transduct Target Ther. 2022;7:59.
- [CrossRef] [PubMed] [Google Scholar]
- ACSL4 deficiency confers protection against ferroptosis-mediated acute kidney injury. Redox Biol. 2022;51:102262.
- [CrossRef] [PubMed] [Google Scholar]
- PKCβII phosphorylates ACSL4 to amplify lipid peroxidation to induce ferroptosis. Nat Cell Biol. 2022;24:88-98.
- [CrossRef] [PubMed] [Google Scholar]
- Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 2019;26:2284-99.
- [CrossRef] [PubMed] [Google Scholar]
- USP14 regulates heme metabolism and ovarian cancer invasion through BACH1 deubiquitination and stabilization. Biochem Biophys Res Commun. 2023;667:186-93.
- [CrossRef] [PubMed] [Google Scholar]
- USP14 promotes the malignant progression and ibrutinib resistance of mantle cell lymphoma by stabilizing XPO1. Int J Med Sci. 2023;20:616-26.
- [CrossRef] [PubMed] [Google Scholar]








