Translate this page into:
Unsatisfactory rate in liquid-based cervical samples as compared to conventional smears: A study from tertiary care hospital
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Developed countries adopted liquid-based cytology (LBC) cervical cytology, partly because of its lower proportions of unsatisfactory (U/S)/inadequate samples. This study was carried out to evaluate effect on the rate of U/S samples after introduction of LBC in our laboratory.
Materials and Methods:
An audit of U/S cervical samples was performed, which included split samples (n = 1000), only conventional Pap smear (CPS) smears (n = 1000), and only LBC samples (n = 1000). The smears were reviewed by two observers independently, and adequacy for the samples was assessed as per The Bethesda System 2001. The reasons for U/S rate in split samples were categorized into various cytologic and/or technical reasons.
Results:
U/S rate was far less in only LBC samples (1.2%) as compared to only CPS (10.5%) cases. Cases in the satisfactory but limited category were also less in only LBC (0.4%) as compared to only CPS (3.2%) samples. The main reasons for U/S smears in split samples were low cell count (37.2% in CPS; 58.8% in LBC). The second main reason was low cellularity with excess blood and only excess blood in CPS samples.
Conclusion:
There was a significant reduction of U/S rate in LBC samples as compared to CPS samples, and the difference was statistically significant. The main cause of U/S samples in LBC was low cellularity indicating a technical fault in sample collection. The main cause of U/S rate in CPS was low cellularity followed by low cellularity with excess blood. Adequate training of sample takers and cytologists for the precise cell count to determine adequacy in smears can be of great help in reducing U/S rate.
Keywords
Cervical Pap smear
inadequate rate
liquid-based smears
SurePath™
unsatisfactory smear
INTRODUCTION
Unsatisfactory (U/S)/inadequate samples are those samples where a meaningful result cannot be issued. In such situations, women are invited back for the second test. The National Institute for Clinical Excellence (NICE) in the UK recommended the adoption of liquid-based cytology (LBC) in part because it was associated with significantly lower proportions of U/S smears from 9% in conventional Pap smear (CPS) cytology to 1.6% in LBC.[1]
The reduction in U/S rates from using LBC is of considerable benefit to women in terms of reducing anxiety, uncertainty, and the need for repeat tests. The reasons for U/S samples can be cytologic, technical, or both and therefore, it is of importance to recognize the cause of U/S samples to further reduce their number. High numbers of U/S samples may be due to problems with techniques used by the sample takers or in the laboratory, the screening staff incorrectly reporting inadequate samples, i.e., samples being reported as inadequate should have been reported as negative and, in either case, retraining of the staff may be needed. This study was undertaken to evaluate the effect on U/S rate in cervical samples after the introduction of LBC and identify the reasons for U/S samples in CPS and LBC cases.
MATERIALS AND METHODS
This was a prospective study involving 1000 “split samples,” 1000 only (CPS) samples, and 1000 LBC samples from patients coming to the Department of Obstetrics and Gynecology. The samples taken were part of routine hospital-based screening of patients for cervical epithelial abnormalities. The samples were taken with cytobrush and divided into two parts (split-sample technique). First, a conventional smear was prepared and was immediately alcohol-fixed. After that, the same brush head was detached and suspended in LBC vial containing preservative fluid, which was transferred to the cytopathology laboratory where further processing took place as per the protocol of the company providing the LBC equipment.
In addition to 1000 split samples, 1000 only CPS smears, and 1000 only LBC samples were taken for comparison, which was obtained during the study period. Cervical samples were compared for U/S rates. The protocol for assessing cellularity of CPS as well as LBC samples was as per The Bethesda System (TBS) 2001, i.e., the sample should contain a minimum of 8000 and 5000 squamous cells, respectively. The cell count for LBC samples was performed as per Table 1.

All the U/S cases in “split samples” were reviewed to evaluate the cause of inadequacy. Coding used to categorize U/S samples is as following:
Cytomorphology-related reasons
C1: Obscured by polymorphs
C2: Obscured by blood
C3: Obscured by blood and polymorphs
C4: Obscured by mucus
C5: Marked cytolysis
C6: Cells clumped and obscured by polymorphs
C7: Obscured by bacteria
C8: Marked clumping of cells
C9: Obscuration by polymorphs and bacteria
C10: Smear on both sides.
Technical reasons
T1: Poor preservation
T2: Insufficient cells.
The reasons for U/S samples were recorded according to codes for each case by two observers. The results of initial and reviewed cytology were analyzed to identify factors associated with U/S samples in both CPS and SurePath™ (SP) cervical cytology in “split samples.”
RESULTS
U/S rate was compared between split samples (n = 1000), only CPS (n = 1000), and only LBC (n = 1000) samples collected during the study period [Table 2]. In “split samples,” there were 43/1000 (4.3%) U/S cases in CPS and 17 (1.7%) cases in the LBC samples with 53.5% reduction in U/S smears. These split samples were routinely reported simultaneously. U/S rate in only CPS cases was 10.5% and satisfactory but limited (S/L) was 3.2%. In only LBC samples, U/S rate was 1.2% with S/L rate being 0.4%. U/S rate in only CPS (10.5%) was statistically significant (P < 0.0001) as compared to U/S rate in split samples (4.3%). The difference of U/S and S/L rates in only CPS and only LBC cases was highly statistically significant.

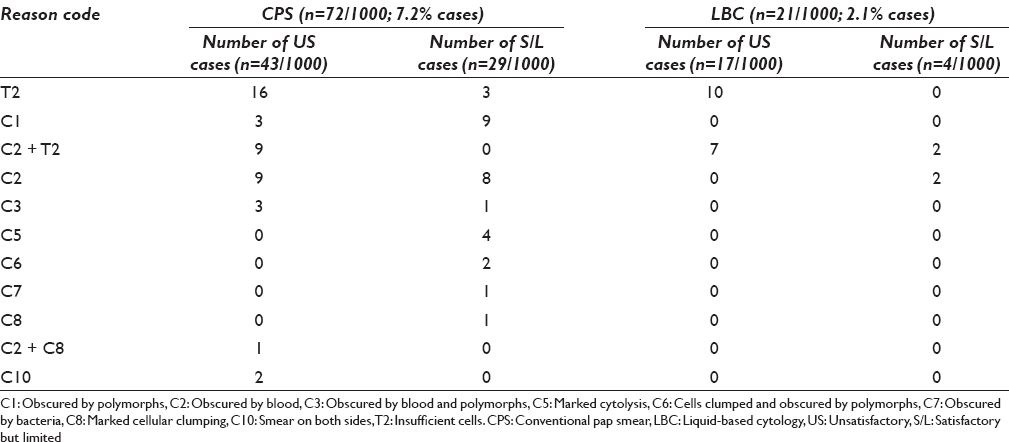
The reasons for U/S smears in split samples were coded as mentioned in the materials and methods. In split samples, both in CPS and LBC smears, the main cause of U/S smears was insufficient cells (code T2) - 16/43 (37.2%) in CPS and 10/17 (58.8%) in LBC samples [Table 3]. The second major cause was low cellularity with excess blood (C2+T2) - 9/43 cases in CPS and only excess blood (code C2) - 9/43 in CPS. 7/17 U/S cases in LBC were due to low cellularity and excess blood (C2+T2). 29 (2.9%) cases in CPS and 4 (0.4%) cases in LBC samples were reported as S/L due to excess blood/obscuration by polymorphs or excessive cytolysis, etc., and this difference was found to be significant with P < 0.001.
DISCUSSION
The term “U/S” was initially proposed in 1988 at the National Cancer Institute Workshop in Bethesda for reporting cervical cytological diagnoses.[2] The rate of U/S/inadequate samples was more in the conventional (CPS) cervical cytology because of obscuring of cells in thick, poorly fixed smears, or obscuring by blood/mucus/pus or because of inadequate sampling of the transformation zone. The British Society for Clinical Cytology (BSCC) and British Society for Colposcopy and Cervical Pathology published a statement in 1990 on “the Cell Content of Cervical Smears,”[3] which was followed by another document from the BSCC, Guidelines for judging the adequacy of a cervical smear for standardisation of definition of adequacy.[4] The 1988 Bethesda System included specimen adequacy component for cervical cytology, replaced by the term “S/L by…” in 1991, but the same was in turn deleted in 2001 edition as it leads to unnecessary repeat samples.[56] Despite the elimination of the term “S/L by,” it was recommended that the cytology report should mention the sample adequacy and describe the presence or absence of the endocervical/transformation zone component and any other quality indicators.[7] Although, it is the responsibility of the sample-taker to ensure that the entire transformation zone has been sampled.[8]
The number of inadequate rates was reported to be lower after the introduction of LBC, and there have been many studies in the literature comparing LBC with CPS cervical cytology.[91011121314151617] The UK NICE reported that in a pilot study in England of 1,78,000 slides, percentages of U/S slides decreased from 9·1% to an average of 1·6% after conventional cytology was replaced with LBC.[1] Greater reproducibility and the capacity for human papillomavirus DNA testing on the same sample have made LBC more desirable than conventional cytology in screening programs. Davey et al., 2006 reviewed the published work to assess the performance of LBC relative to conventional cytology and found that there was no meaningful difference in the percentages of U/S slides between LBC and CPS cytology.[18] However; this report was followed by comments from different authors negating their findings.[1920] TBS recommended 5000 as minimum cellularity based on the studies that showed that abnormal cells were less likely to be detected below that level mainly using SP technique rather than ThinPrep™[212223] One study quoted in TBS guidelines showed that abnormal cells were less likely to be detected in LBC preparations with <20,000 total cells.[24] Therefore, TBS allowed a “quality indicator comment” for preparations with cell counts between five and 20,000 cells so that clinicians can decide whether a repeat is indicated. McQueen and Duval raised questions for defining adequacy of LBC preparations for cervical screening.[25] The protocol for assessing adequacy used in our laboratory was according to TBS 2001; however, S/L category was retained in cervical cytology reporting. The sample takers also should be trained to identify and sample the transformation zone and provide the relevant clinical information along with the adequate cervical sample.
CONCLUSION
The results in the present study have highlighted that the most common reason for sample taker inadequates is insufficient cells present, and this is due to sample taking technique, followed by cytologic factors that are out of the sample takers control. Cytologists screening cytology slides should precisely undertake cell counts to determine adequacy, especially in samples containing an excess of polymorphs, blood, and bacteria. If sufficient numbers of squamous cells can be visualized; these smears should not be reported as U/S. U/S report in such situations leads to unnecessary anxiety for women, dissatisfaction for sample takers and additional cost. The inclusion of refresher training on sample adequacy during update courses for cytologists will be beneficial. Cellular clumping can be due to the hormonal changes, but this may be handled at technical level for cervical samples to improvise on the results of the cervical cytology.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that we have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
To give appropriate credit to each author of paper, the individual contributions of all authors to the manuscript have been specified.
According to International Committee of Medical Journal Editors (ICMJE http://www.icmje.org), “author” is generally considered to be someone who has made substantive intellectual contributions to a published study.
Authorship credit is based on (1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; (2) drafting the article or revising it critically for important intellectual content, and (3) final approval of the version to be published. Authors meet conditions 1, 2, and 3.
We declare that each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content.
All authors of this article declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
NG and VB were instrumental in collection and interpretation of data. We reviewed all smears and interpreted them with counting of cells to assess adequacy. Final interpretation was done by NG and AR. Initial acquisition of data, PubMed search, and modification in the manuscript was done by VB and NG. Clinical information was contributed by VS.
All authors read and approved the final manuscript.
Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board of the institution associated with this study as applicable. This study was a retrospective study not directly involving the patients. Authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
BSCC - British Society for Clinical Cytology
CPS - Conventional Pap Smear
LBC - Liquid-Based Cytology
NICE - National Institute for Clinical Excellence
SP - SurePath™
TBS - The Bethesda System
U/S - Unsatisfactory.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- National Institute for Clinical Excellence: NHS Technology Appraisal Guidance 69. Guidance on the Use of Liquid-Based Cytology for Cervical Screening. London: National Institute for Clinical Excellence; 2003.
- [Google Scholar]
- The 1988 Bethesda system for reporting cervical/vaginal cytological diagnoses. National cancer institute workshop. JAMA. 1989;262:931-4.
- [Google Scholar]
- British Society of Clinical Cytology (BSCC) Editorial. Cell content of cervical smears. Cytopathology. 1990;1:129-30.
- [Google Scholar]
- Should the laboratory assess the sampling adequacy of cervical smears? Cytopathology. 1997;8:409-16.
- [Google Scholar]
- The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9.
- [Google Scholar]
- Influence of specimen adequacy on the diagnosis of ASCUS. Diagn Cytopathol. 2004;31:155-8.
- [Google Scholar]
- National Health Service Cervical Screening Programme (NHSCSP) Publication No 1. Achievable Standards, Benchmarks for Reporting and Criteria for Evaluating Cervical Cytopathology. Sheffield: NHSCSP Publications; 1995. p. :1-44.
- [Google Scholar]
- Evaluation of the ThinPrep Pap test as an adjunct to the conventional Pap smear. Med J Aust. 1997;167:466-9.
- [Google Scholar]
- ThinPrep Pap test: Performance and biopsy follow-up in a university hospital. Cancer. 1999;87:105-12.
- [Google Scholar]
- Liquid-based cervical cytologic smear study and conventional Papanicolaou smears: A metaanalysis of prospective studies comparing cytologic diagnosis and sample adequacy. Am J Obstet Gynecol. 2001;185:308-17.
- [Google Scholar]
- Liquid-based cytology: Is this the way forward for cervical screening? Cytopathology. 2002;13:71-82.
- [Google Scholar]
- Are fluid-based cytologies superior to the conventional Papanicolaou test?. A systematic review. J Fam Pract. 2001;50:1040-6.
- [Google Scholar]
- Accuracy of the Papanicolaou test in screening for and follow-up of cervical cytologic abnormalities: A systematic review. Ann Intern Med. 2000;132:810-9.
- [Google Scholar]
- Performance of ThinPrep liquid-based cervical cytology in comparison with conventionally prepared Papanicolaou smears: A quantitative survey. Gynecol Oncol. 2003;90:137-44.
- [Google Scholar]
- Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systematic review. Lancet. 2006;367:122-32.
- [Google Scholar]
- How should adequacy of liquid-based cytology be defined? Cytopathology. 2006;17:165-7.
- [Google Scholar]
- The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria and Explanatory Notes. (2nd ed). New York: Springer-Verlag; 2004.
- [Google Scholar]
- Cellular constitution of Autocyte PREP® cervico-vaginal samples with biopsy-confirmed HSIL. Acta Cytol. 2000;44:505. Abstract
- [Google Scholar]
- Effect of cellularity on the sensitivity of detecting squamous lesions in liquid-based cervical cytology. Acta Cytol. 2003;47:605-10.
- [Google Scholar]
- Using a quality control approach to define an ‘adequately cellular’ liquid-based cervical cytology specimen. Cytopathology. 2006;17:168-74.
- [Google Scholar]








