Translate this page into:
Utility of immunocytochemistry in diagnosing abdominopelvic washings from patients undergoing radical surgery for endometrial cancer

*Corresponding author: Fang Yang, Department of Anorectal Surgery in Traditional Chinese Medicine, The First Affiliated Hospital of Hengyang Medical School, Hengyang, China. yangfangsina@163.com
-
Received: ,
Accepted: ,
How to cite this article: Lan Z, Zhang J, Yang F, Ma X, He R. Utility of immunocytochemistry in diagnosing abdominopelvic washings from patients undergoing radical surgery for endometrial cancer. CytoJournal. 2024;21:20. doi: 10.25259/Cytojournal_10_2024
Abstract
Objective:
This study aimed to explore the efficacy of immunocytochemistry in diagnosing abdominopelvic washings (APWs) and evaluate the superiority of cytology combined with immunocytochemistry over cytology alone.
Material and Methods:
Data on APW cytology and available cell blocks from patients who underwent radical surgery for endometrial cancer between January 2021 and December 2022 were reviewed. Cytology was re-evaluated according to a five-tier system. Immunocytochemistry analysis for targets such as Sry box transcription factor 1(SOX17), Paired box gene 2 (Pax-2) protein, Phosphatase and tensin (PTEN), and β-catenin was performed on each case with non-negative cytology. Mismatch repair (MMR) protein and P53 immunocytochemistry analyses were performed using cell blocks from cases with abnormal MMR or P53 expression in their primary lesion. The accuracies of cytology combined with immunocytochemistry and cytology alone were calculated.
Results:
Overall, 126 patients were included in this study, 18 of whom demonstrated non-negative cytology of APW. Cell blocks were successfully prepared for 16 cases. SOX17 positivity was observed in 16 cases, including 1 of serous carcinoma, 1 of clear cell carcinoma, and 14 of endometrioid carcinoma (EC). Loss of Pax-2 and PTEN expression was observed in the APWs of the 14 patients with EC. MMR deficiency was noted in two patients with EC, and P53 mutation was noted in another two patients with EC. Compared with 10 metastatic carcinomas (10/18, 55.56%) diagnosed by cytology alone, 15 malignant APWs (15/18, 83.33%) were confirmed through combination cytology and immunocytochemistry. APWs were more likely to be observed in cases with more than half myometrial invasion than those with no or less than half myometrial invasion (P = 0.0067). The probability of malignant APW occurrence was slightly elevated in cases of EC exhibiting microcystic, elongated, and fragmented(MELF) infiltrative growth (P = 0.039).
Conclusion:
SOX17 is a useful Müllerian marker for distinguishing endometrial epithelium in APW. Loss of Pax-2 and PTEN expression offers evidence of metastatic endometrial carcinoma. Furthermore, positive APWs retained molecular features similar to primary lesions. The use of multiple immunocytochemical markers can effectively enhance the diagnostic efficiency of APWs.
Keywords
Endometrial cancer
Abdominopelvic washings
Cytology
Immunocytochemistry
INTRODUCTION
In the context of the International Federation of Gynecology and Obstetrics (FIGO) 1988 staging for endometrial cancer, cytology of abdominopelvic washings (APWs) was initially used as part of the staging process to assess whether cancer cells had spread beyond the uterus into the peritoneal cavity.[1] According to the FIGO 1988, cases with positive cytology were staged as IIIA.[2] However, some subsequent studies showed no survival difference between stage I and stage IIIA with only positive cytology.[3] The 2009 staging system stated that APW cytology still needs to be reported separately but is not associated with the staging.[2,4] Nevertheless, some studies have suggested a different perspective, that is, positive cytology serves as an independent prognostic factor.[5,6] Positive peritoneal cytology has been associated with decreased overall survival of women with stage I/II endometrial cancer.[7] Evidently, the two contrasting viewpoints demand more evidence for substantiation. Nonetheless, for subspecialists in cytopathology, ensuring precise cytological diagnoses is crucial to furnishing accurate analytical material.
APW cytology interpretation primarily relied on the cytologist’s expertise. However, accurate diagnosis was often hindered by challenges such as differentiating mesothelial cell hyperplasia, collagen balls, endometriosis, and endosalpingiosis.[8] Moreover, the presence of mesothelial cells in APW hinders immunocytochemistry analysis using cell blocks. Mesothelial cells express various cytokeratins,[9] some of which are also expressed in cancer cells. Mesothelial cell markers, including calretinin, CK5/6, podoplanin, HBME-1, and WT-1, typically do not show positivity in all mesothelial cells. In other words, some mesothelial cells negative for these markers may cause confusion.
Although paired-box gene 8 (Pax-8) is positive in female Müllerian epithelial cells and is routinely employed as an adjunctive tool in distinguishing endometrial origin,[10] it is also expressed in some proliferative mesothelial cells.
Recently, SOX17 has been identified as a highly sensitive and specific marker for endometrial carcinomas.[11] A previous study also demonstrated the effectiveness of SOX17 immunohistochemistry in accurately distinguishing endometrial cancers and metastatic carcinomas from nongynecologic sources in cytological materials, such as pleural or peritoneal effusion and fine-needle aspiration samples.[12] Notably, mesothelial cells showed a negative stain for SOX17.
An immunohistochemical three-marker panel comprising Pax2, PTEN, and β-catenin was also confirmed as a useful diagnostic adjunct in identifying endometrioid pre-cancer and endometrial carcinomas from the benign endometrial epithelium.[13] In addition, according to the molecular classification proposed by the Cancer Genome Atlas, endometrial carcinoma has been classified into POLE mutation, mismatch repair deficient, p53 abnormal, and no specific molecular profile subgroups.[14] P53, MLH1, MSH2, MSH6, and PMS2 immunohistochemistry can be used as surrogates for initial classification.[15] These markers may be used in identifying the metastasis in the APW.
Therefore, the aims of our study were as follows: (1) validate the utility of immunocytochemistry using cell blocks of APW; (2) explore the consistency surrogate marker expression between primary lesions and positive APWs; and (3) determine the superiority of the combination of cytology and immunocytochemistry over cytology alone.
MATERIAL AND METHODS
Patients
Between January 2021 and December 2022, data on patients with endometrial carcinoma who underwent radical hysterectomy and APW examination at the First Affiliated Hospital, Hengyang Medical School, University of South China, were retrospectively analyzed. The study cohort included 126 cases. Patient information, tumor histological type, FIGO grade, depth of myometrial invasion, pelvic lymph node metastasis status, and other parameters were collected.
Specimen processing of the original material and cytology evaluation
During the period, APWs from the 126 cases were collected following radical hysterectomy and sent to the pathology department. Using the Sedimentation Cell Prep Plus Liquid-Based Cytology (LBC) Processor under the liquid-based preparation system (LBP-2601, Guangzhou Anbiping Medical Company Technology Co., Ltd.), cells were automatically sedimented onto a glass slide and subsequently stained using the Papanicolaou method.[16]
The original APW cytology slides were reviewed by two experienced cytologists blinded to each other. The cases were diagnosed according to a five-tier system: Descriptive/unsatisfactory; negative, no evidence of malignancy (Negative); atypical/inconclusive (A/I); suspicious for malignancy (Suspicious); and diagnostic of malignancy (DOM).[17] Interpretations were accepted when two cytopathologists yielded the same diagnoses. However, when disparities arose in interpretations, diagnoses were reached through a discussion between the two cytopathologists and another senior cytopathologist. Cases diagnosed as A/I, Suspicious, or DOM were defined as having non-negative cytology.
The remaining material in these cases was prepared for cell blocks following the manufacturer’s instructions (Cell Block Preparation Kit, Quanbo Medical Company Technology Co., Ltd, Changsha, Hunan, China). For quality control, cell blocks were also prepared for five cases with negative cytology. All cell blocks were embedded in paraffin, sliced, and stained with hematoxylin and eosin (H&E).
Immunocytochemistry
Immunocytochemistry was performed on each cell block containing suspicious cells. The following were the antibodies: SOX17 (catalog number: ab226862, clone ERP20684, Abcam,Waltham, MA, USA); MC (catalog number: MAB0130, clone HBME-1, Maixin, Fuzhou, Fujian, China); Pax-2 (catalog number: RMA-0816, clone EP235, Maixin); PTEN (catalog number: RMA-1074, clone 138G6, Maixin); and β-catenin (catalog number: MAB-0754, clone MX043, Maxin). Regarding the primary lesion with Mismatch repair (MMR)-deficient immunohistochemistry or abnormal P53 expression, the MLH1 (catalog number: MAB-0838, clone MX063, Maixin), PMS2 (catalog number: RMA-1051, clone MXR019, Maixin), MSH2 (catalog number: MAB-0836, clone MX061, Maixin,), MSH6 (catalog number: MAB-0831, clone MX056, Maixin), or P53 (catalog number: MAB-0674, clone DO7, Maixin) immunocytochemical stain was performed in the cell blocks. Besides, SOX17 should be diluted at a ratio of 1:1000, whereas other ready-to-use antibodies can be directly applied in immunocytochemistry.
The following were the steps of the procedure.
Paraffin-embedded cell blocks were sliced into 3-mm sections.
Deparaffinization and rehydration were performed.
Antigen retrieval was performed by ethylene diamine tetraacetic acid at 100°C under normal pressure for 20 min.
Endogenous peroxidase was removed by H2O2 for 10 min.
The first primary antibody was applied, and the slides were incubated overnight at 4°C.
A reaction amplification agent was added for 15 min.
Sections were incubated with a secondary antibody (raised against the species of the first primary antibody) for 15 min.
Slides were incubated with diaminobenzidine for 5 min at room temperature.
Washed in running domestic water supply for 10 min.
Hematoxylin counterstain was performed.
Washed in anhydrous alcohol and subsequently in xylene.
The slides were briefly drained and mounted in neutral gum.
Nuclear expression of SOX17, MMR protein, and P53 was considered positive. Immunoreactivity scores for SOX17 expression were calculated by multiplying the number representing the percentage of immunoreactive tumor cells (0, 0%; 1, <10%; 2, 10–49%; and 3, 50–100%) by the number representing the intensity (0, negative; 1, weak; 2, moderate; and 3, high).[12] Scores of 3–9 were considered positive, scores of 2 were classified as uncertain, and scores of 0–1 were categorized as negative. Pax-2 negativity was based on the absence of nuclear expression in tumor cells, whereas the absence of cytoplasmic expression showed that tumor cells were negative for PTEN. Aberrant β-catenin expression was defined as at least one nuclear positivity that could be observed in the cluster of atypical cells. Regarding MMR protein, >10% of the tumor cells showing moderate or strong positivity were considered to have intact functionality of the protein. Otherwise, the expression was diagnosed as MMR deficiency. The staining patterns of P53 were assigned for each tumor according to the following four categories: (1) wild type (admixture of positive and negative cells), (2) diffuse nuclear overexpression (diffuse strong nuclear staining in more than 80% of the tumor cells), (3) complete absence of nuclear staining, and (4) predominant cytoplasmic staining.
Pathology review
After completing cytomorphological categorization, the two cytologists reviewed both cytology slides and relevant cell block examinations (H&E-stained slides and immunocytochemistry). Similar to the diagnostic approach in cytology alone, the combination diagnoses were evaluated using the five-tier system and determined based on the agreement between the observers.
Statistical analysis
The diagnostic efficacy of cytology alone and cytology combined examination of cell blocks was calculated. The discordance between the cytology alone and the combined method was analyzed. Descriptive statistics were used to summarize clinicopathological information. The Fisher’s exact test and χ2 (Chi-square) test were employed to examine the correlation between the APW status and the final clinicopathological features of patients. Data were analyzed using Excel (Version 2020, Microsoft Corporation, Redmond, WA, USA) and R software (Version 4.1.2, R Foundation, Vienna, Austria), P < 0.05 was considered statistically significant data.
RESULTS
Patient demographics and pathological parameters of primary lesions
A total of 126 patients aged between 35 and 73 years (average of 55.29 years) underwent radical surgery for endometrial cancer during the study period. There were seven cases of serous carcinomas, one clear cell carcinoma, five mixed carcinomas, and three carcinosarcomas, and the remaining 110 were endometrioid adenocarcinomas [Table 1]. Of the 110 cases, 17 (15.45%) showed MMR deficiency and 15 demonstrated P53 mutation in immunohistochemistry.
| Characteristics | Total |
|---|---|
| Age (years) | |
| Mean (SD) | 55.2 (7.57) |
| Histopathology | |
| Endometrioid | 110 |
| Serous | 7 |
| Clear cell | 1 |
| Mixed | 5 |
| Carcinosarcoma | 3 |
| Molecular classification of endometrioid carcinoma | |
| MMR deficient | 17 |
| P53 mutation | 15 |
| POLE mutation and no specific molecular profile | 78 |
SD: Standard deviation, MMR: Mismatch repair, POLE: Polymerase epsilon
Cytology and immunocytochemistry
None of the cases were categorized into the descriptive/unsatisfactory group. Four, four, and ten cases were confirmed as A/I, Suspicious, and DOM, respectively [Figure 1a and b]. The other 108 cases showed negative results. Besides 1 metastatic serous carcinoma and 1 metastatic clear cell carcinoma, respectively, found in their APWs with definite cytomorphology, the remaining 16 endometrioid cancers demonstrated nonnegative cytology results [Table 2]. Out of the 16 cases, one fell into A/I, whereas other cases diagnosed as suspicious could not undergo cell block preparation due to rare residual material.
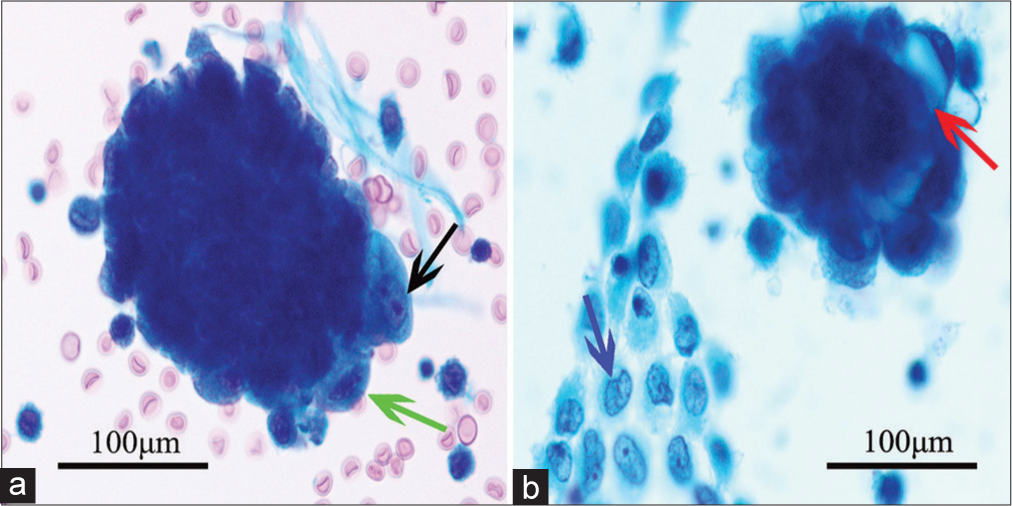
- Representative cytomorphological features of endometrioid cancer in abdominopelvic washing. Papanicolaou stain, ×400. (a) Tumor cells with prominent nucleoli (black arrow) and coarse chromatin (green arrow) and (b) tumor cells with “bubble” cytoplasm arranged in three-dimensional appearance (red arrow) and sheets of mesothelial cells with slightly irregular nuclear membrane (blue arrow).
| Cases | Age | FIGO grade | Depth of myometrial invasion | Lymph nodes metastasis | MELF invasion pattern | Cervical involvement | Cytology |
|---|---|---|---|---|---|---|---|
| 1 | 57 | 2 | >0.5 | Negative | None | Negative | A/I |
| 2 | 39 | 2 | <0.5 | Negative | None | Negative | A/I |
| 3 | 57 | 1 | >0.5 | Positive | MELF | Positive | DOM |
| 4 | 55 | 3 | >0.5 | Negative | None | Negative | Suspicious |
| 5 | 56 | 1 | <0.5 | Negative | None | Negative | Suspicious |
| 6 | 57 | 3 | <0.5 | Negative | None | Negative | A/I |
| 7 | 62 | 1 | >0.5 | Negative | None | Negative | A/I |
| 8 | 57 | 1 | >0.5 | Negative | MELF | Negative | DOM |
| 9 | 55 | 1 | <0.5 | Negative | None | Negative | DOM |
| 10 | 58 | 1 | >0.5 | Negative | None | Negative | Suspicious |
| 11 | 73 | 3 | >0.5 | Positive | MELF | Negative | DOM |
| 12 | 40 | 1 | <0.5 | Negative | None | Negative | Suspicious |
| 13 | 51 | 2 | >0.5 | Negative | None | Negative | DOM |
| 14 | 51 | 2 | <0.5 | Negative | None | Negative | DOM |
| 15 | 50 | 2 | <0.5 | Negative | None | Negative | DOM |
| 16 | 53 | 2 | <0.5 | Negative | None | Negative | DOM |
EC: Endometrioid carcinoma, FIGO: International federation of gynecology and obstetrics, A/I: Atypical/inconclusive, DOM: Diagnostic of malignancy, MELF: Microcystic, elongated, and fragmented
Sixteen cases with cell blocks underwent H&E staining [Figure 2a] and immunocytochemistry. Among them, the metastatic cells showed MC negativity [Figure 2b] and moderate-to-strong SOX17 positivity [Figure 2c]. Thirteen positive APWs from patients with endometrioid cancers demonstrated negativity to Pax-2 [Figure 2d] and PTEN. No aberrant nuclear expression of β-catenin was found in the study. One case with suspicious cytology had not been confirmed by immunocytochemistry because no suspicious cluster was found in the cell block. P53 overexpression was found in two positive APW cases [Figure 3a], and MMR deficiency was observed in another two positive APW cases [Figure 3b]. The negative group showed no aberrant expression of SOX17, Pax-2, PTEN, or β-catenin.
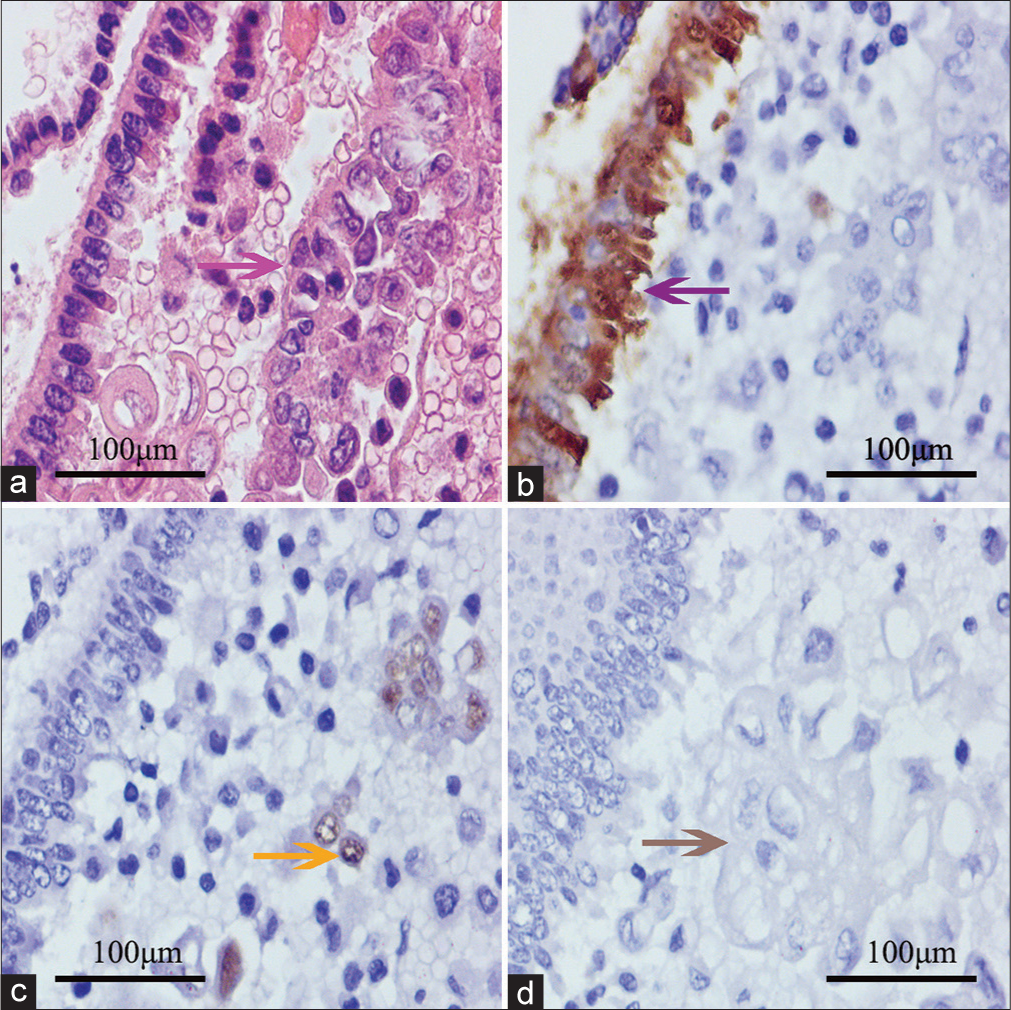
- Routine stain and immunocytochemistry of positive abdominopelvic washing. (a) Mesothelial cells in chain pattern (left) and endometrioid cancer cells (right, pink arrow); (b ) Hector Battifora Mesothelial-1 (HBME-1) demonstrating positive to mesothelial cells (purple arrow) and negative to endometrioid cancer cells (right); (c) SOX17 positive endometrioid cancer cells (yellow arrow); and (d) tumor cells negative to paired-box gene-2 (gray arrow). The images were collected from the same block of a patient.
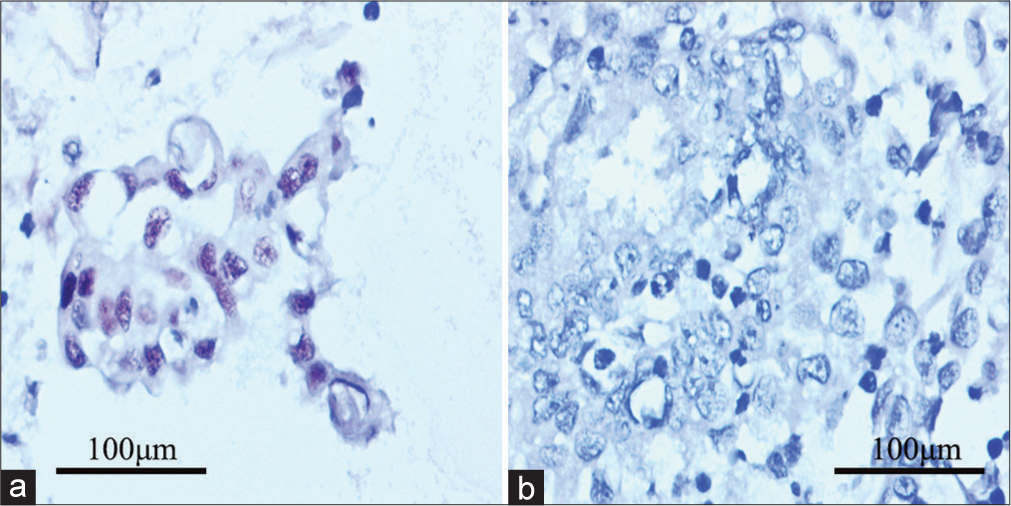
- P53 and mismatch repair immunocytochemistry for the positive abdominopelvic washing from cases with relevant molecular classification. (a) Tumor Protein p53 (P53) overexpression presenting mutational status; and (b) MutS Homolog 6 (MSH6) negativity in accordance with primary lesion.
Correlation between pathological parameters of primary lesions and positive APWs
The FIGO stage, lymph node metastasis, and MELF invasive pattern were not statistically associated with the presence of positive APWs [Table 3]. However, the depth of invasion exceeding half the thickness of the muscle layer was more likely to result in positive APWs (P = 0.0067). Cases of endometrioid carcinoma (EC) exhibiting MELF infiltrative growth showed a slightly elevated probability of malignant APW occurrence (P = 0.039).
| Characteristics | APW | Total | P | |
|---|---|---|---|---|
| Negative | A/I, suspicious, and DOM | |||
| FIGO stage | ||||
| 1 | 44 | 7 | 51 | 0.70 |
| 2 | 37 | 6 | 45 | |
| 3 | 11 | 3 | 14 | |
| Depth of myometrial invasion | ||||
| >0.5 | 16 | 8 | 24 | 0.0067 |
| <0.5 | 78 | 8 | 86 | |
| Lymph nodes metastasis | ||||
| Positive | 6 | 2 | 8 | 0.32 |
| Negative | 88 | 14 | 102 | |
| Cervical involvement | ||||
| Positive | 7 | 1 | 1.00 | |
| Negative | 87 | 15 | ||
| Growth pattern | ||||
| MELF | 3 | 3 | 0.039 | |
| Non-MELF | 91 | 13 | ||
APW: Abdominopelvic washing, FIGO: International federation of gynecology and obstetrics, A/I: Atypical/inconclusive, DOM: Diagnostic of malignancy, MELF: Microcystic, elongated, and fragmented
DISCUSSION
Endometrial cancer is a common gynecological cancer. In 2019, China recorded the second-highest number of incident cases and deaths related to endometrial cancer, with figures reaching 66,744 and 12,222 individuals, respectively.[18] Regarding cytologists, the correct interpretation of APW cytology is helpful for further investigations on the extrauterine spread; however, the correlation between the biological behavior of tumors and positive APW cytology has been controversial.
A previous study reported that the overall cellularity and number of atypical cells could be used to distinguish DOM category from other groups.[17] Admittedly, the cluster in APW cytology demonstrating overlapping cells with prominent nucleoli, irregular nuclear membranes, or vacuolated cytoplasm indicates potential malignant APW. The low cellularity of atypical cells, unlike other effusion specimens, posed a challenge in identifying APW. In the present study, the main reason that the cases were initially categorized into the A/I or suspicious group was the limited quantity of neoplastic cell aggregates. Therefore, the APW cytomorphology in these cases suggests the necessity of confirmation by other detection methods.
Regarding serous, clear cell, undifferentiated, and high-grade EC, the tumor cells could be easily identified in the positive APW cytology due to the significant cellular dysplasia. However, cytology from APW in patients with low-risk endometrium cancer presents difficulties.[19]
The mesothelial cells are the most prominent component of APW and the most common diagnostic pitfall of cytology. Due to the force of the flushing solution acting on the mesothelium, the mesothelial cells in the APW demonstrate some large and flat sheet patterns, which are different from those in the spontaneous effusions.[19,20] The cells in the sheet pattern show a mosaic-like pattern and benign nuclear features.[21] Occasionally, the presence of reactive mesothelial cells arranged in small clusters with slightly larger nuclear or/and cytoplasmic vacuoles can raise suspicions regarding their origin.[21] In general, the tumor cells manifesting a three-dimensional spatial structure demonstrate an irregular nuclear membrane and a “bubbly” cytoplasm. Therefore, these clusters in cytology can be easily found in a ×10 objective field of view. However, sometimes, the subtle morphological features of overlapped endometrial-like cells within the cluster are not easily discerned. Notably, the morphology of APW in cytology differs to some extent from that in cell block. Pseudo-glandular or sheet-patterned mesothelial cells and patched or glandular endometrial cells in H&E-stained slices of cell block exhibit a two-dimensional appearance. Cellular morphological features of tumor cells, such as increased nucleocytoplasmic, vacuolated nuclear chromatin, and an irregular nuclear membrane, can be discerned in a high-power objective field without the interference of stocked cells, which can be commonly observed in cytology.
Although morphology offers diagnostic clues for distinguishing endometrial cells from benign mesothelial cells, the confirmation of immunocytochemistry is necessary. In fact, the real endometrial cells in some cases may be quite scarce. In our study, only one cluster of endometrial cells was found in cytology and cell block, respectively. Pax-8 has been used as a useful Müllerian marker in diagnosis; however, it also shows expression in the kidney and thyroid. In effusion or APW specimens, the expression can be observed in some mesothelial cells. In the past, the identification of Pax-8-positive cells within cell blocks necessitated a morphological evaluation to distinguish whether they were mesothelial cells. However, SOX17 is not expressed in mesothelial cells; therefore, this issue does not exist.
Therefore, the highly sensitive and specific immunochemical reaction of SOX17 to endometrial cells in APWs contributes to unraveling the morphological simulation between the two and is also the first step in successfully identifying endometrial cancer cells.
Other confounding factors in APW include the presence of endometriosis and endosalpingiosis, which consist of normal Müllerian epithelium. This epithelium also exhibits a strong expression of SOX17. The coexistence of endometriosis and endometrial cancer has been reported in several studies, although at a very low proportion.[21,22] Although the occurrence of Müllerian cells in APW is scarce, the presence of these components may be suspicious. Glandular cells with scant cytoplasm and cytologically bland nuclei with finely granular chromatin and without prominent nucleoli from endometriosis commonly show one or several three-dimensional clusters.[23] Sometimes, endometrial stromal cells and hemosiderin-laden macrophages can be observed in cytology, thereby providing morphological evidence. The “bubble” cytoplasm and underdetermined chromatin can also be occasionally observed in endometriosis. Endometrial stromal cells, similar to lymphocytes and hemosiderin-laden macrophages, may result from other conditions with bleeding in the peritoneal cavity.[23] Regarding endosalpingiosis, the smooth ciliated border of the fallopian tube epithelium is a useful morphological feature; however, it is similar to the microvilli on the surface of mesothelial cells.[8,24]
At least one of the three markers (Pax-2, PTEN, and β-catenin) showed aberrant expression in 92.8% of endometrial atypical hyperplasia/endometrioid intraepithelial neoplasia.[13] Loss of Pax-2 and PTEN expression has been found in 77% and 68% of endometrial carcinoma, respectively.[25] Although the inactivation of Pax-2 and PTEN occurs in scattered glands of normal endometrial tissues, the coexistence of loss of expression in the same glands is rare. On the contrary, the phenomenon of the same tumor glands being negative for the two proteins is common. In our study, the metastatic carcinomas in the 12 cases of positive APW also lost the expression of the two proteins. However, they all demonstrated normal membrane expression of β-catenin, although aberrant nuclear expression was found in about 40% of endometrium carcinomas.[26] Regarding endosalpingiosis, the fallopian tube epithelium also retains the normal expression of Pax-2, PTEN, and β-catenin.
In routine procedures, the immunohistochemistry of MMR protein aligns perfectly with molecular detection of microsatellite instability status. Although the sensitivity of p53 immunohistochemistry in detecting TP53 mutation is not 100%, abnormal overexpression clearly indicates molecular abberations.[27] In our study, the positive APWs also retained the MMR deficiency or P53 mutation, similar to primary lesions.
To the best of our knowledge, the application of the abovementioned immunocytochemistry markers has not been reported in the English literature. Although no benign Müllerian components have been confirmed in the cell block, the abnormal immunocytochemistry offered sufficient evidence for the diagnosis of positive APWs.
Positive APWs are more likely to occur in cases with poor prognostic factors, including deep myometrial invasion, nodal metastasis, high tumor grade, and non-endometrioid histology.[28] Our study also demonstrated that the depth of myometrial invasion was positively correlated with positive APWs, although other factors were not statistically linked to metastasis in APWs. In addition, the MELF growth pattern may be a risk factor for positive APW based on statistical analysis of a small number of cases. Despite conflicting findings in studies examining the correlation between APW and the prognosis of endometrial cancer, a positive APW emerges as a prognostic indicator linked to short survival in non-endometrioid endometrial cancer.[29,30]
The debate regarding how malignant cells of uterus-confined endometrial malignancies reach the peritoneal cavity is ongoing.[18] Tumor heterogeneity had been observed in our study. For example, no mucinous component was detected in two cases of malignant APWs whose primary lesions had been defined as mucinous differentiation of conventional (Müllerian) type (data not shown). However, the immunocytochemical profile between primary lesions and APWs demonstrated high consistency. To explore the mechanism of positive APWs, further studies should focus on the relationship between the four molecular types and positive APWs.
Our study was mainly limited by the small sample size. The low percentage of positive APWs affected the statistical analysis. Alternative antibodies, including MLH1 and ARID1A, could not be applied to enhance accuracy. Furthermore, only cases with an initial diagnosis of negative APWs were prepared for cell blocks and categorized into the negative control group. The potential occurrence of positive carcinoma clusters noted in cell blocks but not in cytology had been ignored. Moreover, the two cases without confirmation from cell blocks due to insufficient residual materials may be linked to the inaccuracy. In addition, the detection of POLE ultramutation was not performed due to a lack of detection equipment. Therefore, we could not further explore the differences in positive APW occurrence among the four subtypes and the potential molecular mechanisms of APWs. Last but not least, the follow-up interval was insufficient for evaluating the link between positive APWs and prognosis parameters.
SUMMARY
Our study characterized the utilization of immunocytochemistry in diagnosing metastatic endometrial carcinoma within APW. As a Müllerian tumor marker with high sensitivity and specificity, SOX17 was utilized in the study for identifying the endometrial cell and other benign Müllerian cells and excluding the confusion of mesothelial cells. The panel of markers, including Pax-2, PTEN, and β-catenin, has been used for differentiating the malignant and benign cell clusters in the APWs of patients with EC. Regarding MMR and P53 immunocytochemistry, they provided further confirmation by their molecular accordance with primary lesions. Immunocytochemistry can promote the diagnostic efficacy of APWs.
AVAILABILITY OF DATA AND MATERIALS
The data analyzed in this study is available from the corresponding author upon reasonable request.
ABBREVIATIONS
A/I - Atypical/inconclusive
APW - Abdominopelvic washing
CB - Cell block
DOM - Diagnostic of malignancy
H&E - Hematoxylin and eosin
HBME-1 - Hector Battifora Mesothelial-1
LBC - Liquid-based cytology
MLH1 - MutL Homolog 1
MSH2 - MutS Homolog 2
MELF - Microcystic, elongated, and fragmented
MMR - mismatch repair protein
MSH6 - MutS Homolog 6
Negative - Negative, no evidence of malignancy
PMS2 - Postmeiotic Segregation Increased 2
Pax-2 - Paired box gene 2
Pax-8 - Paired box gene 8
POLE - Polymerase epsilon
PTEN - Phosphatase and tensin
P53 - Tumor Protein p53
SOX17 - Sry box transcription factor 17
Suspicious - Suspicious for malignancy
AUTHOR CONTRIBUTIONS
ZL: Study design, data collection and analysis, and manuscript drafting; JZ: Data collection, cytology review and CB evaluation; FY: Immunocytochemistry, statistical analysis, and manuscript review; XM: Cytology review and CB evaluation; RH: CB evaluation and manuscript review. All authors have read and approved the final version of the manuscript. All authors have contributed significantly to the work and has agreed to be responsible for every aspect of it.
ACKNOWLEDGMENT
We thank Zhiyun Edits Limited for the linguistic editing and proofreading of the manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
This retrospective study was approved by the Research Ethics Board at the First Affiliated Hospital of Hengyang Medical School, University of South China(2023ll0607003). The need for informed consent was waived by the Research Ethics Board.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
This work was supported by Research Startup Fund for New Doctors at The First Affiliated Hospital, Hengyang Medical School, University of South China.
References
- FIGO 1988 versus 2009 staging for endometrial carcinoma: A comparative study on prediction of survival and stage distribution according to histologic subtype. J Gynecol Oncol. 2014;25:30-5.
- [CrossRef] [PubMed] [Google Scholar]
- Revision of FIGO surgical staging in 2009 for endometrial cancer validates to improve risk stratification. Gynecol Oncol. 2012;125:103-8.
- [CrossRef] [PubMed] [Google Scholar]
- Upstaging based solely on positive peritoneal washing does not affect outcome in endometrial cancer. Mod Pathol. 2005;18:673-80.
- [CrossRef] [PubMed] [Google Scholar]
- Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103-4.
- [CrossRef] [PubMed] [Google Scholar]
- Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol. 2013;128:77-82.
- [CrossRef] [PubMed] [Google Scholar]
- The prognostic significance of positive peritoneal cytology and adnexal/serosal metastasis in stage IIIA endometrial cancer. Gynecol Oncol. 2007;104:401-5.
- [CrossRef] [PubMed] [Google Scholar]
- Prognosis and treatment of positive peritoneal cytology in early endometrial cancer: matched cohort analyses from the National Cancer Database. Am J Obstet Gynecol. 2018;218:329.e1-15.
- [CrossRef] [PubMed] [Google Scholar]
- Abdominopelvic washings in gynecologic pathology: A comprehensive review. Diagn Cytopathol. 2016;44:1039-57.
- [CrossRef] [PubMed] [Google Scholar]
- Diverse properties of the mesothelial cells in health and disease. Pleura Peritoneum. 2016;1:79-89.
- [CrossRef] [PubMed] [Google Scholar]
- PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: A comprehensive immunohistochemical study. Mod Pathol. 2011;24:751-64.
- [CrossRef] [PubMed] [Google Scholar]
- Identifying SOX17 as a sensitive and specific marker for ovarian and endometrial carcinomas. Mod Pathol. 2023;36:100038.
- [CrossRef] [PubMed] [Google Scholar]
- SOX17 is a highly sensitive and specific marker for metastatic ovarian and endometrial carcinomas in cytology cell block specimens. Cancer Cytopathol. 2023;131:465-70.
- [CrossRef] [PubMed] [Google Scholar]
- β-catenin, Pax2, and Pten panel identifies precancers among histologically subdiagnostic endometrial lesions. Am J Surg Pathol. 2023;47:618-29.
- [CrossRef] [PubMed] [Google Scholar]
- Moving into the modern era of molecular classification for endometrial cancer. J Surg Oncol. 2024;129:120-5.
- [CrossRef] [PubMed] [Google Scholar]
- Validation of a one-step genomics-based molecular classifier for endometrial carcinoma in a large Chinese population. Pathol Res Pract. 2024;254:155152.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of liquid-based cytology on residual needle rinses collected from core needle biopsy for lung nodule diagnosis. Cancer Med. 2021;10:3919-27.
- [CrossRef] [PubMed] [Google Scholar]
- Time trend of global uterine cancer burden: An age-period-cohort analysis from 1990 to 2019 and predictions in a 25-year period. BMC Womens Health. 2023;23:384.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of pelvic washing specimens in patients with endometrial cancer: Cytomorphological features, diagnostic agreement, and pathologist experience. Cancer Cytopathol. 2021;129:517-25.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic pitfalls of peritoneal washing cytology and the role of cell blocks in their diagnosis. Diagn Cytopathol. 2003;28:335-41.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of benign peritoneal fluids and pelvic washing specimens: Diagnostic cytomorphologic features and pitfalls. Acta Cytol. 2023;67:176-84.
- [CrossRef] [PubMed] [Google Scholar]
- The relationship between endometrial carcinoma and coexistent adenomyosis uteri, endometriosis externa and myoma uteri. Cancer Detect Prev. 2004;28:94-8.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial cancer prognosis in women with endometriosis and adenomyosis: A retrospective nationwide cohort study of 40 840 women. Int J Cancer. 2022;150:1439-46.
- [CrossRef] [PubMed] [Google Scholar]
- Morphologic features of endometriosis in various types of cytologic specimens. Diagn Cytopathol. 2013;41:936-42.
- [CrossRef] [PubMed] [Google Scholar]
- Endosalpingiosis in peritoneal washings in women with benign gynecologic conditions: Thirty-eight cases confirmed with paired box-8 immunohistochemical staining and correlation with surgical biopsy findings. Cancer Cytopathol. 2013;121:582-90.
- [CrossRef] [PubMed] [Google Scholar]
- Histopathologic diagnosis of endometrial precancers: Updates and future directions. Semin Diagn Pathol. 2022;39:137-47.
- [CrossRef] [PubMed] [Google Scholar]
- Adjuvant radiation therapy in early-stage endometrial cancer with abnormal beta-catenin expression is associated with improved local control. Gynecol Oncol. 2023;174:42-8.
- [CrossRef] [PubMed] [Google Scholar]
- p53 immunohistochemistry is an accurate surrogate for TP53 mutational analysis in endometrial carcinoma biopsies. J Pathol. 2020;250:336-45.
- [CrossRef] [PubMed] [Google Scholar]
- Practical approach to the evaluation of malignant peritoneal fluids in the setting of gynecologic neoplasms. Acta Cytol. 2023;67:143-75.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant peritoneal cytology and increased mortality risk in stage I non-endometrioid endometrial cancer. Gynecol Oncol. 2020;159:43-51.
- [CrossRef] [PubMed] [Google Scholar]
- Prognostic value of peritoneal cytology in stage I serous and clear cell carcinoma of the endometrium. J Clin Med. 2023;12:1609.
- [CrossRef] [PubMed] [Google Scholar]








