Translate this page into:
Utility of SHOX2 and RASSF1A gene methylation detection on the residual cytology material from endobronchial ultrasound-guided transbronchial needle aspiration

*Corresponding author: Fang Yang, Department of Anorectal Surgery in Traditional Chinese Medicine, the First Affiliated Hospital of Hengyang Medical School, Hengyang, Hunan, China yangfangsina@163.com
-
Received: ,
Accepted: ,
How to cite this article: Lan Z, Zhang J, Yang F, Ma X, He R. Utility of SHOX2 and RASSF1A gene methylation detection on the residual cytology materia l from endobronchial ultrasound-guided transbronchial needle aspiration. CytoJournal. 2024;21:19. doi: 10.25259/Cytojournal_114_2023
Abstract
Objective:
This study aims to assess the effectiveness of Short Stature Homeobox 2 (SHOX2) and RAS Association Domain Family 1 Isoform A (RASSF1A) gene methylation detection in residual liquid-based cytology (LBC) materials from Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (EBUS-TBNA) and investigate the diagnostic accuracy of a comprehensive diagnostic approach.
Material and Methods:
Between June 2022 and May 2023, a total of 110 cases that underwent EBUS-TBNA were enrolled in the study. SHOX2 and RASSF1A genes methylation detection using the residual cytological material, LBC, and cell block (CB) were conducted for each EBUS-TBNA case. The sensitivity and specificity of cytology, CB histopathology, SHOX2, and RASSF1A methylation in diagnosing EBUS-TBNA samples were determined based on follow-up data.
Results:
Among the 72 cases confirmed as pulmonary carcinomas, the methylation test yielded positive results in 24 adenocarcinoma cases, 10 squamous cell carcinoma cases, and 14 small cell carcinoma cases. The sensitivity of the comprehensive diagnosis (combining LBC, CB, and methylation detection) in distinguishing metastatic pulmonary epithelial malignancies in mediastinal and hilar lymph nodes or masses from benign lesions was higher (97.22%, 70/72) than that of morphological diagnosis alone (LBC and CB) (88.89%, 64/72; P < 0.05).
Conclusion:
SHOX2 and RASSF1A methylation detection demonstrates a high sensitivity and negative predictive value in the identification of pulmonary epithelial malignancies and holds promise as a valuable ancillary approach to enhance morphological diagnosis of EBUS-TBNA.
Keywords
Endobronchial ultrasound-guided transbronchial needle aspiration
Cytology
Short stature homeobox 2
RAS association domain family 1
Isoform A
Methylation
INTRODUCTION
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a common minimally invasive procedure that combines bronchoscopy with ultrasound to both diagnose and stage lung cancer, infections, and other conditions affecting the lungs, mediastinum or lymph nodes. [1,2] It offers the advantage of real-time imaging and concurrent sampling. By visualizing the specific target area with ultrasound guidance, the operator can obtain samples using a fine needle for cytology (conventional smear and liquid-based cytology [LBC]) and cell block (CB).[1] This integrated approach minimizes the potential risks of complications and ensures a more efficient diagnostic process.[2]
As a first-line approach for mediastinal lymph node staging of pulmonary cancer, EBUS-TBNA has high sensitivity (>90%) and excellent specificity.[3] LBC provides a superior resolution in revealing the morphological characteristics of neoplastic cells compared to CB analysis.[4] Conversely, CBs can harness the capabilities of immunohistochemistry to address the limitations of LBC morphology.[3] Nevertheless, it is essential to acknowledge that the accuracy of diagnosis can be influenced by the sampling process, particularly in institutions where rapid on-site evaluation (ROSE) is unavailable.[5] Furthermore, the effectiveness of EBUS-FNAC evaluation is greatly contingent on the expertise of the cytopathologist.
Efforts to maximize the utility of collected specimens for enhancing the sensitivity of EBUS-TBNA in detecting pulmonary malignant lesions are clinically important. The CB material, which is specifically prepared for immunohistochemistry and targeted genetic testing to meet the increasing therapeutic demands, offers an established resource for such a purpose. However, it is noteworthy that the discarded supernatant and residual cytology material from LBC procedures may hold valuable material for molecular detection.[6]
The promoter hypermethylation of two genes, namely, Short Stature Homeobox 2 (SHOX2) and RAS Association Domain Family 1, and Isoform A (RASSF1A), has been extensively recognized as diagnostic biomarkers for lung cancer.[7,8] In our institution, we have implemented SHOX2 and RASSF1A methylation detection as an adjunctive technique, utilizing discarded and residual materials from the LBC procedures, which have been found to significantly enhance diagnostic accuracy. In this retrospective study, we aimed to assess the sensitivity and specificity of SHOX2 and RASSF1A methylation detection in distinguishing metastatic pulmonary carcinoma from other conditions and confirm the superiority of combining methylation detection with morphological interpretation (LBC and CB) compared to relying solely on morphological criteria.
MATERIAL AND METHODS
Patients
This was a retrospective study comprising 115 consecutive patients with significant mediastinal lymph node (LN) enlargement (short-axis diameter >1 cm) who underwent EBUS-TBNA at our institution from June 2022 to May 2023. We evaluated cytological and histopathological diagnoses and the methylation detection of SHOX2 and RASSF1A. The study inclusion criteria were as follows: (1) enrollment of benign lesions or reactive hyperplasia in lymph nodes required confirmation of their diagnoses through regression, either with or without medical treatment (excluding antitumor therapy), or by remaining stable in size for at least 6 months; (2) the inclusion of malignant lesions in lymph nodes depended on their confirmation through immunohistochemistry and/or regression following specific etiological treatment; and (3) had repeated pathological examination. The exclusion criteria were as follows: (1) lack of follow-up information and (2) history of malignant pulmonary lesions. Therefore, 110 patients were included in this study.
Samples collection and processing
An experienced pulmonologist conducted the EBUS- TBNA procedure. The process involved advancing the bronchoscope with an ultrasound probe at its tip to identify LNs meeting the sampling criteria. Once suitable LNs were identified, the TBNA needle was guided through the working channel of the bronchoscope and positioned in the tracheal or bronchial wall, targeting the suspicious area of the lymph nodes. Aspirations were obtained using negative pressure generated by a syringe attached to the needle. Most of the collected tissue coagulum was transferred onto two or three pieces of filter paper, which were subsequently placed into a 10% neutral formaldehyde solution.[9] Other aspirations were preserved in 15 mL of a cell storage solution (Anbiping Medical Company Technology Co., Ltd., Guangzhou, Guangdong, China) for LBC.
After dehydration, the tissue coagulum was embedded in paraffin to create CBs, which were then sectioned into slices of 3 μm thickness and subsequently stained using hematoxylin and eosin (H&E). The LBC specimen underwent initial agitation and centrifugation, and the resulting supernatant was collected and mixed with the residual fluid in the specimen container for subsequent methylation detection. Using the Sedimentation Cell Prep Plus LBC Processor in a liquid-based preparation system (LBP-2601, Guangzhou Anbiping Medical Company Technology Co., Ltd.), cells present in the deposits were automatically transferred onto a glass slide to form a diagnostic area measuring 13 mm in diameter, which was then stained using the Papanicolaou stain.[4]
The DNA methylation levels of SHOX2 and RASSF1A in residual cytology material were assessed using the commercial SHOX2 and RASSF1A Methylation Detection Kit (Tellgen, Shanghai, China) following the manufacturer’s instructions. Methylated SHOX2 and RASSF1A DNA plasmids were employed as controls. Quantitative real- time polymerase chain reaction (PCR) was carried out using an ABI 7500 Real-time PCR instrument (4351106, Applied Biosystems, Foster City, CA, USA). A positive outcome for RASSF1A methylation was indicated by the presence of a smooth “S”-shaped amplification curve in the FAM fluorescence signal and a threshold cycle (CT) value <35. Conversely, a CT ≥ 35 indicated a negative result for RASSF1A methylation. Similarly, a positive result for SHOX2 methylation was characterized by a smooth “S”-shaped amplification curve in the VIC fluorescence signal and a CT value <32. A CT ≥ 32 was indicative of a negative result for SHOX2 methylation. The cutoff values of △Ct for RASSF1A and SHOX2 methylation (△CtSHOX2 = CtSHOX2– CtBeta-actin (β-ACTB); △CtRASSF1A = C tRASSF1A–Ctβ-ACTB) were set to 12 and 9, respectively. In addition, a range of 18 ≤ Ctb-ACTB ≤ 32 was considered as an eligible internal reference, ensuring the reliability of the results.
Data analysis
Cytological assessments were performed by two experienced cytopathologists who utilized a five-tier system (non- diagnostic, benign, atypical, suspicious, or positive for malignancy).[10] In cases where both cytopathologists provided concordant diagnoses, those interpretations were adopted. For instances where discordant interpretations arose, resolution was achieved through discussion between the two cytopathologists, involving a senior cytopathologist if necessary. The cytological results were subsequently categorized into three groups: (1) positive (indicating malignancy), (2) uncertain (reflecting atypical or suspicious findings for malignancy), and (3) negative (comprising non-diagnostic or benign results).[4]
H&E stained slices from CBs were examined by two pathologists specialized in pulmonary pathology. Immunohistochemistry and/or special stains for these cases were also reviewed. Pathological outcomes were classified into three groups: (1) positive (indicative of malignancy or favoring malignancy), (2) uncertain (involving a few cells that could not be definitively categorized as benign or malignant), and (3) negative (with no identification of malignant cells).
In cases where methylation tests for the SHOX2 gene, RASSF1A gene, or both genes yielded a positive result, the overall methylation test was considered positive. Likewise, if any of the three testing methods produced a positive outcome, it was considered a positive result for the triple test.
The calculation of sensitivity, specificity, accuracy and false- negative rates for CB, cytology, methylation test and the combination of CB detection, cytology, and methylation test for diagnosing malignancy were based on the retrieved follow-up data.
Statistical analysis was conducted using the χ2 test to assess differences between CB and LBC, as well as between morphology (CB and LBC) and the combined approach of morphology with methylation testing. Data analysis was conducted using Microsoft Excel software (Version 2020, Microsoft Corporation, Redmond, WA, USA) and R software (Version 4.1.2, R Foundation, Vienna, Austria) with a significance level set at P < 0.05 to determine statistical significance.
RESULTS
A total of 110 patients aged between 19 and 90 years (average age, 64.03 years) who EBUS-TBNA were identified as eligible for study analysis. Among these patients, there were 30 females and 80 males, resulting in a male-to-female ratio of 1:2.33 (P < 0.05). Nodal station included in the analyses is shown in Table 1.
| Nodal station | Cases (n=110) |
|---|---|
| 2R | 2 |
| 4L | 9 |
| 4R | 28 |
| 7 | 31 |
| 10L | 2 |
| 10R | 13 |
| 11L | 17 |
| 11R | 8 |
R: Right, L: Left, n: number of cases
As indicated in Table 2, a total of 72 patients were diagnosed with pulmonary carcinomas, constituting 66.36% of the overall cohort. Among these 72 cases, there were 14 diagnoses of small cell carcinoma (SCC), 14 of squamous cell carcinoma (SQC), and 44 of adenocarcinoma (ADC). The remaining 38 cases were distributed as follows: Two were identified as inflammatory pseudotumor, one as solid fibrous tumor, one as mesothelioma, one as metastatic adenocarcinoma, one as malignant perivascular epithelioid cell tumor (PECOMA), one as T-cell lymphoblastic lymphoma (T-LBL), one as diffuse large B-cell lymphoma, eight as tuberculosis, one as fungal infection, one as sarcoidosis, and 20 as reactive lymphadenopathy.
| Pathological types | Cases (n) | LBC (%) | CB (%) | LBC+CB (%) | Methylation (%) | LBC+CB+Methylation (%) |
|---|---|---|---|---|---|---|
| SCC | 14 | 9 (64.29) | 11 (78.57) | 11 (78.57) | 14 (100.00) | 14 (100.00) |
| SQC | 14 | 4 (28.57) | 9 (64.29) | 11 (78.57) | 10 (71.43) | 13 (92.86) |
| ADC | 44 | 29 (65.91) | 40 (90.91) | 42 (95.45) | 24 (54.55) | 43 (97.73) |
| Total | 72 | 42 | 60 | 64 | 48 | 70 |
LBC: Liquid-based cytology, CB: Cell block, SQC: Squamous cell carcinoma, ADC: Adenocarcinoma, SCC: Small cell carcinoma, n: Number of cases
Among the SCC (n = 14), SQC (n = 14) and ADC (n = 44) cases, cytological examination identified 9, 4 and 29 cases as positive, correspondingly [Figure 1a-c]. Conversely, CB analysis recognized 11, 9, and 40 cases as positive for SCC, SQC, and ADC, respectively [Table 2]. The sensitivity of CBs at 83.33% surpassed that of LBC at 58.33% (P < 0.001).
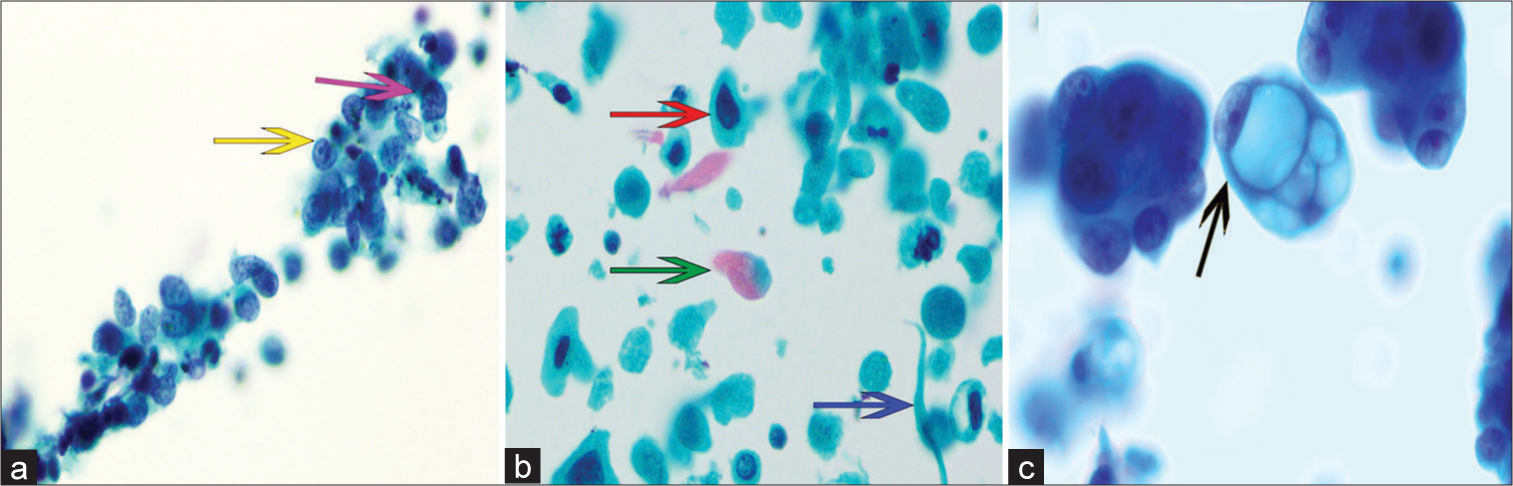
- Cytomorphological characteristics of pulmonary epithelial malignancies in EBUS-TBNA LBC specimen. Papanicolaou stain, ×400. (a) Small-cell carcinoma displaying tumor cells with nuclear indentation (yellow arrow), scant cytoplasm, and hyperchromatic nuclei (purple arrow). (b) Squamous cell carcinoma characterized by coarse chromatin fibers (red arrow), bichromatic cytoplasm (green arrow), and an elongated pattern (blue arrow). (c) Adenocarcinoma exhibiting prominent nucleoli and mucous vacuoles (black arrow). (EBUS-TBNA: Endobronchial ultrasound- guided transbronchial needle aspiration, LBC: Liquid-based cytology.)
However, LBC produced positive results in 2 ADC cases with negative histology and in 2 SQC cases where positive CB results were absent.
Among the 72 cases of pulmonary malignancies, 48 cases exhibited positive methylation results, comprising 24 ADC, 10 SQC, and 14 SCC. Notably, SCC displayed the highest sensitivity at 100%, with SQC ranking second at 71.43% (10/14), followed by ADC at 54.54% (24/44). Of the 48 cases with positive methylation, 38 exhibited positive SHOX2 promoter methylation, while the remaining ten cases only demonstrated RASS1FA promoter methylation. Table 3 illustrated the distribution of positive methylation results into three categories: SHOX2 only, RASS1FA only, and both positive. This distribution varied significantly among ADC (54.17%, 25.00%, and 20.83%), SQC (60.00%, 30.00%, and 10.00%), and SCC (7.14%, 7.14%, and 85.72%) (P < 0.001). However, there was no statistically significant difference in the distribution between ADC and SQC (P = 0.389). Among the 48 patients with positive methylation, the methylation positivity rate of the RASS1FA gene in SCC (92.86%) was significantly higher than that in ADC (45.83%, P = 0.011) and SQC (40.00%, P = 0.0018).
| Pathological types | SHOX2 positive only (%) | RASSF1A positive only (%) | Positive for SHOX2 and RASSF1A (%) | Total |
|---|---|---|---|---|
| ADC | 13 (54.17) | 6 (25.00) | 5 (20.83) | 24 |
| SQC | 6 (60.00) | 3 (30.00) | 1 (10.00) | 10 |
| SCC | 1 (7.14) | 1 (7.14) | 12 (85.72) | 14 |
| Total | 20 | 10 | 18 | 48 |
SHOX2: Short Stature Homeobox 2, RASSF1A: RAS Association Domain Family 1 Isoform A, SQC: Squamous cell carcinoma, ADC: Adenocarcinoma, SCC: Small cell carcinoma
Promoter methylation of the SHOX2 gene was also identified in three cases: one case of T-LBL, one case of metastatic adenocarcinoma, and one case of malignant PECOMA. In contrast, no positive methylation results were observed in any of the benign lesions. This study demonstrated a 100.00% specificity in methylation detection, effectively distinguishing between benign and malignant lesions. The average DNA concentration of the samples was 2.24 ng/μL, ranging from a high of 15.4 ng/μL to a low below 0.1 ng/μL. Notably, among the samples that tested positive for methylation, the lowest DNA concentration measured was below 0.1 ng/μL. In addition, seven samples did not exhibit an internal reference curve and were, therefore, considered invalid tests, consequently classified as negative for methylation detection. △Ct of lung carcinoma cases with positive methylation results is shown in Figure 2.
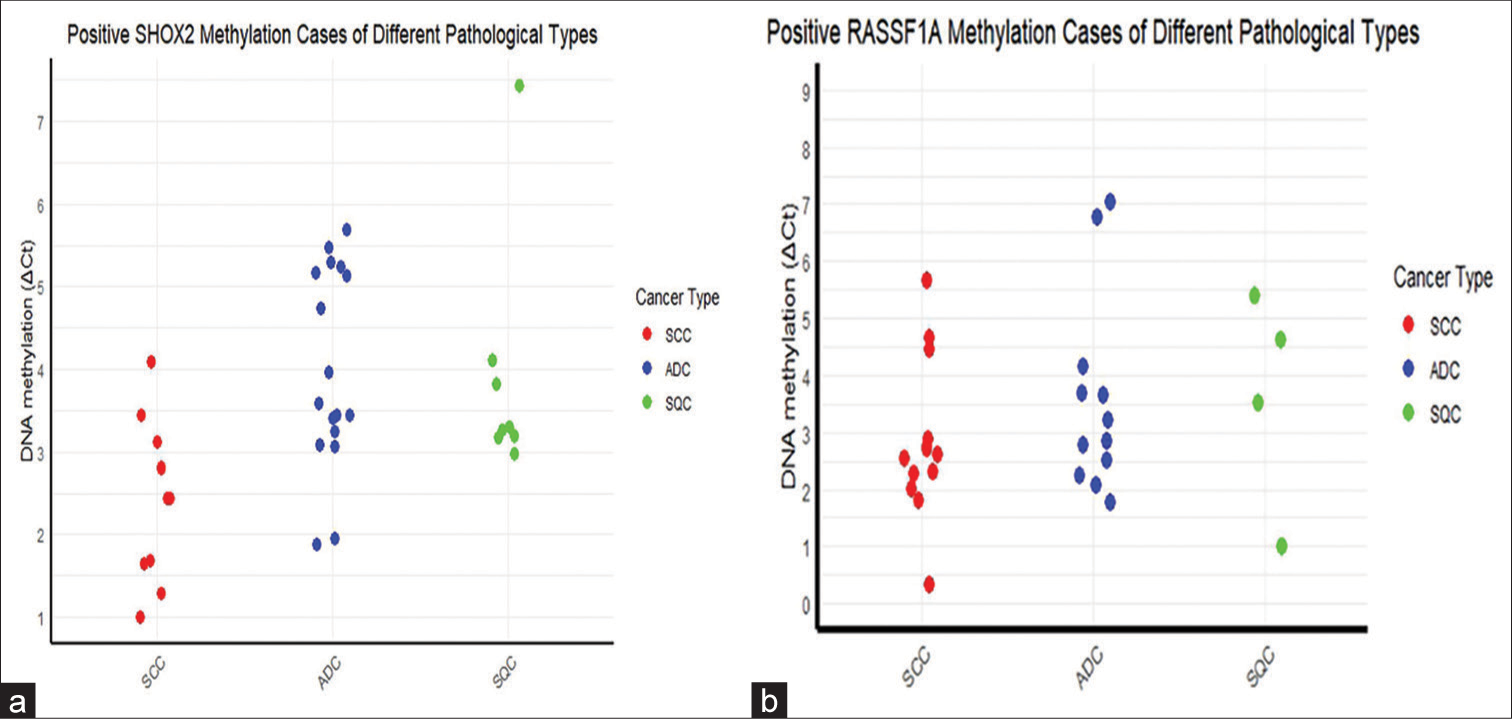
- Delta Ct of lung carcinoma cases with positive methylation results. (a) SHOX2 methylation positive cases of different types of lung carcinoma. (b) RASSF1A methylation positive cases of different types of lung carcinoma. (SHOX2: Short Stature Homeobox 2, RASSF1A: RAS Association Domain Family 1 Isoform A, Ct: Cycle threshold, SQC: Squamous cell carcinoma, ADC: Adenocarcinoma, SCC: Small cell carcinoma.)
As illustrated in Table 4, uncertain results were observed in six cases of lung carcinomas using both LBC and CBs. Among these cases, inadequate sampling [Figure 3a and b] accounted for five instances, while technical artifacts were responsible for one case [Figure 4a]. Notably, among the six cases, apart from one instance displaying negative methylation outcomes due to inadequate sampling, positive methylation results supported the diagnosis in the remaining five cases. Furthermore, one case with uncertain histology and negative LBC was also confirmed by a molecular test. In addition, three cases were reported with uncertain cytology or histology alone [Figure 4b]; yet, all exhibited negative methylation test results, leading to the ultimate exclusion of a malignant diagnosis. Of the 28 cases that tested negative for all three diagnostic tests, only one case was finally diagnosed as ADC. Therefore, the sensitivity of comprehensive diagnosis (LBC, CBs, and methylation detection) in distinguishing metastatic pulmonary epithelial malignancies in nodes or masses of mediastinal and hilar lymph from benign lesions was higher than that of morphological diagnosis (LBC and CB) (70/72, 97.22% vs. 64/72, 88.89%, P < 0.05) [Figure 5].
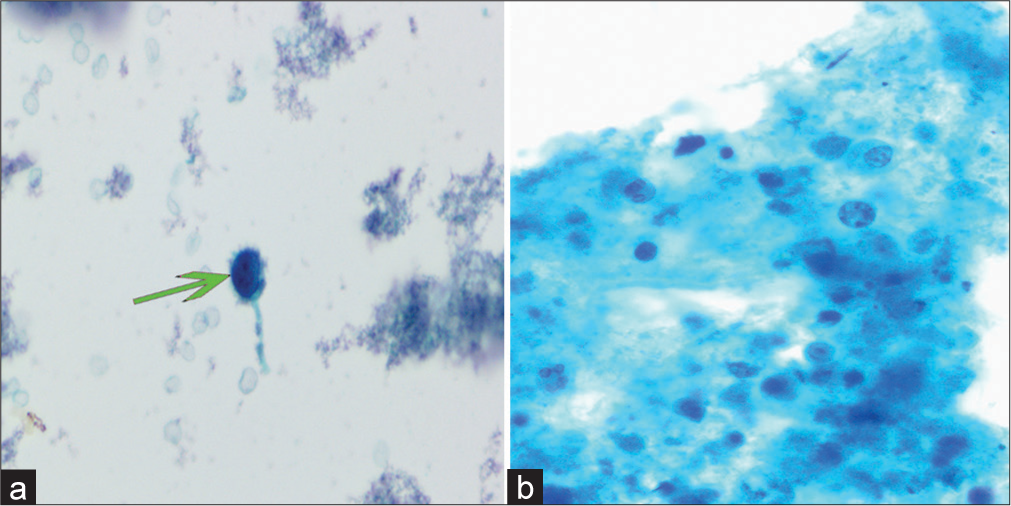
- Illustration of undetermined LBC features. Papanicolaou stain, ×400. (a) Cases with inadequate sampling displaying single atypical cells in the observation field (green arrow). (b) Absence of definite atypical cells in the presence of necrotic background. (LBC: Liquid-based cytology.)
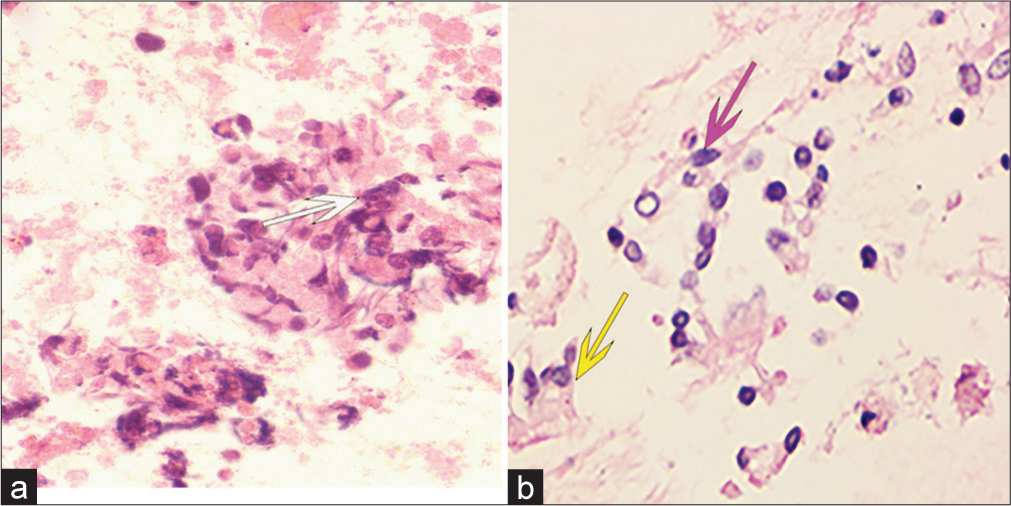
- Examples of undefined cell block diagnoses. Hematoxylin and eosin (stain, ×400). (a) Distorted cells with technical artifacts, hindering correct morphological identification despite the presence of a necrotic background (white arrow). (b) Degenerated ciliated columnar epithelial cells (purple arrow) raising suspicion, although cells with distinct cilia (yellow arrow) are occasionally found in the vicinity.
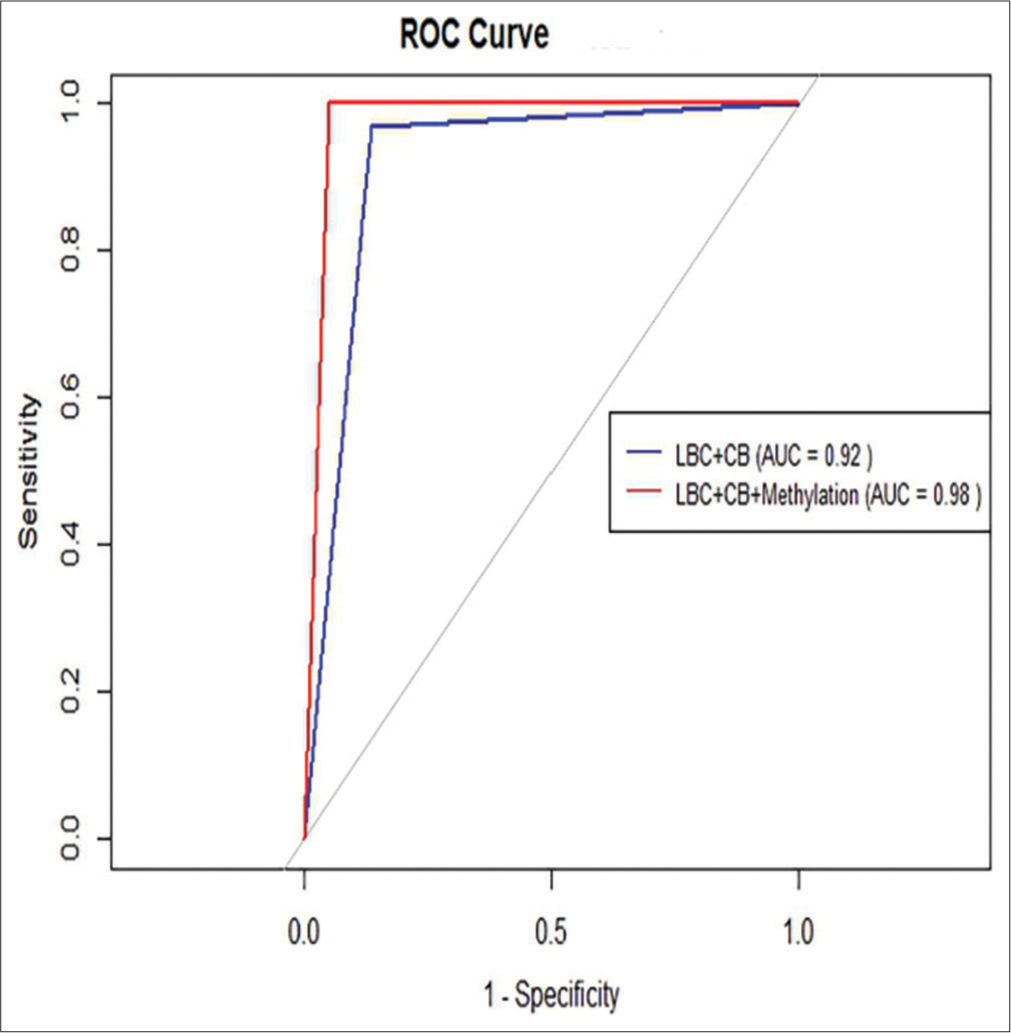
- Receiver operating characteristic (ROC) curves for the combination of LBC with CB and for the combination of methylation detection with LBC and CB in the diagnosis of lung carcinoma. The red dashed line indicates the effectiveness of the combination of methylation detection with LBC and CB (AUC = 0.98). The blue line denotes the effectiveness of the combination of LBC and CB (AUC = 0.92). (LBC: Liquid-based cytology, CB: Cell block, AUC: Area under the curve.) (1) Sensitivity or True Positive Rate (TPR): This measures the proportion of actual positives that are correctly identified as such (e.g., the percentage of sick people who are correctly diagnosed as having the condition). (2) 1-Specificity or False Positive Rate (FPR): This measures the proportion of actual negatives that are incorrectly identified as positives (e.g., the percentage of healthy people who are incorrectly diagnosed as having the condition).
| Diagnoses | Malignant (Cases with positive/negative methylation results) | Benign | Total |
|---|---|---|---|
| LBC/CB | |||
| Uncertain/negative | 0 | 1 | 1 |
| Uncertain/uncertain | 6 (5/1) | 0 | 6 |
| Negative/negative | 1 (0/1) | 27 | 28 |
| Negative/uncertain | 1 (1/0) | 2 | 3 |
LBC: Liquid-based cytology, CB: Cell block
DISCUSSION
ROSE involves specific criteria for assessing specimen adequacy to ensure an adequate amount of diagnostic material for subsequent diagnosis.[11,12] However, a small percentage of cases may be erroneously classified as “adequate” during ROSE, primarily due to the inherently challenging nature of the procedure.[13] Previous research has demonstrated the feasibility of conducting ancillary techniques using supernatant or residual stored LBC material.[6,14,15]
The SHOX2 gene is a member of the SHOX gene family and is located on the 3q25.32 locus in humans.[16] This gene encodes a protein with a DNA-binding structural domain comprising 60 amino acids and has been extensively recognized and investigated as a transcription regulatory factor. RASSF1A, located at 3p21.3, is part of the Ras signaling pathways and plays a pivotal role in regulating both cell proliferation and apoptosis.[17] DNA methylation alterations have emerged as one of the most promising biomarkers for cancer detection. Consistent with prior studies, the analysis of SHOX2 and RASSF1A methylation in this research yielded positive results in 66.67% of metastatic pulmonary malignancies.[18-20] Among these malignancies, SCC displayed the highest positivity rate in the methylation test, followed by SQC, while ADC exhibited the lowest positivity rate.[20] The relative lower sensitivity of ADC impair diagnostic efficacy of the kit due to a considerate subset of ADCs may present hypomethylation within the SHOX2 gene body.[21] Notably, although one case each of PECOMA, lymphoma, and metastatic adenocarcinoma showed positive methylation results in this study, the excellent specificity (100%) of methylation detection in distinguishing pulmonary carcinomas from other benign lesions remained consistent with previous studies.[18-20,22] However, it is worth mentioning that the DNA concentration of the samples in this study was significantly lower compared to that of other types of pathological specimens reported in the previous research.[18-2,22] In fact, four cases of non-SCC produced invalid negative results due to Ctb-ACTB values exceeding 32. The notable efficiency in detection might be attributed to prior conventional PCR amplification, which increased the concentration of the relevant DNA fragments before methylation-specific PCR. Furthermore, the process of cell preservation solutions appeared to slow down cell disruption and DNA degradation, resulting in smaller quantities of extracted DNA that remained of high quality.[4]
Thus, the remarkable sensitivity of the detection method was able to compensate for the limitations associated with inadequate sampling, technical artifacts, and diagnostic challenges based solely on morphology. Furthermore, the use of residual LBC material conserved valuable CB material that could have otherwise been utilized for molecular testing. In addition, the methylation status could serve as a predictive factor for guiding subsequent treatments because the presence of methylated RASSF1A is an unfavorable prognostic indicator for patients undergoing pemetrexed doublets therapy.[23]
Morphological evidence remains a fundamental component of EBUS-TBNA diagnosis.[24] Both detection methods can complement each other due to their ability to address distinct morphological challenges. LBC provides superior nuclear and cytoplasmic resolution of tumor cells, facilitated by rapid fixation and the absence of artificial artifacts.[3,25,26] Nevertheless, LBC demonstrates limited effectiveness in diagnosing benign lesions because the sedimentation-based cell collection process tends to eliminate necrotic material, carbon particles, and histiocytes.[27] In addition, LBC may lead to suspicions of atypical cells in cases involving myofibroblasts, macrophages, epithelioid cells, benign neoplastic cells, and especially malignant cells due to morphological similarities and the absence of adjacent stromal cells.[4] In fragmented CB tissues, contaminated ciliated columnar epithelial cells exhibit fewer characteristic cilia compared to their counterparts in LBC.[5] The primary advantage of CB lies in its ability to enhance immunohistochemistry detection.[28,29] Consequently, despite LBC and CB being interpreted by different subspecialist units, in cases where conflicting results arise, collaborative discussion remains essential.
At present, the morphological diagnosis relies significantly on the expertise of pathologists. It is imperative not only to consolidate diagnostic insights and experiences gained from morphological assessments in EBUS-TBNA but also to investigate cases with indeterminate morphological interpretations guided by methylation test results.[30] In this present study, no cases exhibited negative morphology while demonstrating methylation positivity. The retrospective review of cases with positive methylation results but uncertain morphology played a crucial role in identifying atypical tumor cells within suboptimal samples and detecting atypical tumor cells potentially obscured by other components or technical artifacts, thereby enhancing diagnostic accuracy. Furthermore, when morphology suggested pulmonary epithelial malignancies in cases with valid negative methylation results, it was essential to carefully seek diagnostic evidence to avoid misdiagnosis. Thus, cross- referencing morphological diagnosis with methylation detection results is crucial. When both indicate positivity or negativity, the diagnosis becomes more definitive. In instances where morphological diagnosis suggests malignancy but the methylation test yields negative results, it could be due to inadequate DNA concentration or the absence of methylation in specific gene promoter regions associated with malignancy. Conversely, when the morphological diagnosis suggests a benign condition but the methylation test shows positive results, it may be attributed to insufficient sampling or a potential misinterpretation of the morphology.
This study was mainly limited by the small sample size. There was also a selection bias, as some patients with missing follow-up information could not be included in the study. Moreover, the presence of invalid tests and other negative results in malignant cases may not accurately represent the true methylation status of the two genes. Finally, the relationship between the methylation levels of SHOX2 and RASSF1A and patient clinical characteristics was not investigated, due to concerns about the non-negligible false negatives using residual cytological samples.
SUMMARY
Despite the lower average DNA concentration of the remaining EBUS-TBNA cytology material compared to other specimens, the utilization of this material for SHOX2 and RASSF1A gene methylation detection has proven to be highly efficient. It not only makes optimal use of available resources but also provides strong support for morphological diagnosis. The integration of morphology and methylation detection in patient evaluations holds great promise, particularly in institutions that do not employ ROSE.
AVAILABILITY OF DATA AND MATERIALS
The data analyzed in this study can be accessed through the corresponding author upon reasonable request.
ABBREVIATIONS
ADC – Adenocarcinoma
β-ACTB – Beta-actin
CB – Cell block
CT – Cycle threshold
EBUS-TBNA – Endobronchial ultrasound-guided transbronchial needle aspiration
H&E – Hematoxylin and eosin
LBC – Liquid-based cytology
LN – Lymph node
PCR – Polymerase chain reaction
PECOMA – Perivascular epithelioid cell tumor
RASSF1A – RAS Association Domain Family 1, Isoform A
ROSE – Rapid on-site evaluation
SCC – Small cell carcinoma
SHOX2 – Short Stature Homeobox 2SQC – Squamous cell carcinoma
SCC – Squamous cell carcinoma
T-LBL – T-cell lymphoblastic lymphoma
ACKNOWLEDGMENT
We appreciate the technical support provided by Dr. She Bin and Technician Zhang Yan from Shanghai Methyldia Technology Co. Ltd, Tellgen Corporation. We also thank Zhiyun Edits Limited for the linguistic editing and proof reading of the manuscript.
AUTHOR CONTRIBUTIONS
ZL: Study design, data collection and analysis, and manuscript drafting; JZ: Data collection and cytology review; FY: PCR detection, statistical analysis, and manuscript review; XM: Cytology review and CB evaluation; RH: CB evaluation and manuscript review. All authors have read and approved the final version of the manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The research/study approved by the Institutional Review Board at the Research Ethics Board at the First Affiliated Hospital of Hengyang Medical School, University of South China., number 2022II0512004, dated May 12, 2022. The need for informed consent was waived by the Research Ethics Board.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
FUNDING
This study was supported by the Natural Science Foundation of Hunan Province (Grant No.2022JJ40395).
References
- Endobronchial ultrasound-guided intranodal forceps biopsy (EBUS-IFB)-technical review. J Thorac Dis. 2019;11:4049-58.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical applications of endobronchial ultrasound (EBUS) scope: Challenges and opportunities. Diagnostics (Basel). 2023;13:2565.
- [CrossRef] [PubMed] [Google Scholar]
- EBUS-TBNA: A 2-year experience from a tertiary pathology centre with cyto-histological correlation. Acta Cytol. 2022;66:396-408.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of liquid-based cytology on residual needle rinses collected from core needle biopsy for lung nodule diagnosis. Cancer Med. 2021;10:3919-27.
- [CrossRef] [PubMed] [Google Scholar]
- Endobronchial ultrasound-guided transbronchial fine-needle aspiration: The University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. Am J Clin Pathol. 2008;130:434-43.
- [CrossRef] [PubMed] [Google Scholar]
- To obtain more with less: Cytologic samples with ancillary molecular techniques-the useful role of liquid-based cytology. Arch Pathol Lab Med. 2018;142:299-307.
- [CrossRef] [PubMed] [Google Scholar]
- Methylation analysis of SHOX2 and RASSF1A in bronchoalveolar lavage fluid for early lung cancer diagnosis. Ann Diagn Pathol. 2017;27:57-61.
- [CrossRef] [PubMed] [Google Scholar]
- Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann Oncol. 2013;24:2866-70.
- [CrossRef] [PubMed] [Google Scholar]
- Tissue-clot-coagulum cell block in EBUS/TBNA specimen adequacy: A real world experience. Diagn Cytopathol. 2022;50:583-5.
- [CrossRef] [PubMed] [Google Scholar]
- Processing and reporting of cytology specimens from mediastinal lymph nodes collected using endobronchial ultrasound-guided transbronchial needle aspiration: A state-of-the-art review. J Cytol. 2020;37:72-81.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing ROSE for adequacy of EBUS-TBNA compared with a direct-to-cell block approach as a response to the COVID-19 pandemic. J Am Soc Cytopathol. 2022;11:368-74.
- [CrossRef] [PubMed] [Google Scholar]
- Adequacy of cytology and small biopsy samples obtained with rapid onsite evaluation (ROSE) for predictive biomarker testing in non-small cell lung cancer. Pathology. 2023;55:917-21.
- [CrossRef] [PubMed] [Google Scholar]
- Adequate at rapid on-site evaluation (ROSE), but inadequate on final cytologic diagnosis: Analysis of 606 cases of endobronchial ultrasound-guided trans bronchial needle aspirations (EBUS-TBNA) Diagn Cytopathol. 2019;47:367-73.
- [CrossRef] [PubMed] [Google Scholar]
- Liquid biopsy assay for lung carcinoma using centrifuged supernatants from fine-needle aspiration specimens. Ann Oncol. 2019;30:963-9.
- [CrossRef] [PubMed] [Google Scholar]
- Liquid biopsy of fine-needle aspiration supernatant for lung cancer genotyping. Lung Cancer. 2018;122:72-5.
- [CrossRef] [PubMed] [Google Scholar]
- Signaling pathways and clinical application of RASSF1A and SHOX2 in lung cancer. J Cancer Res Clin Oncol. 2020;146:1379-93.
- [CrossRef] [PubMed] [Google Scholar]
- Quantitative analysis of mRNA expression levels and DNA methylation profiles of three neighboring genes: FUS1, NPRL2/G21 and RASSF1A in non-small cell lung cancer patients. Respir Res. 2015;16:76.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic performance of RASSF1A and SHOX2 methylation combined with EGFR mutations for differentiation between small pulmonary nodules. J Cancer Res Clin Oncol. 2023;149:8557-71.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of exfoliated tumor cells combined with DNA methylation in bronchoalveolar lavage fluid for lung cancer. Medicine (Baltimore). 2023;102:e34955.
- [CrossRef] [PubMed] [Google Scholar]
- Performance evaluation of SHOX2 and RASSF1A methylation for the aid in diagnosis of lung cancer based on the analysis of FFPE specimen. Front Oncol. 2020;10:565780.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of the prognostic value and gene expression mechanism of SHOX2 in lung adenocarcinoma. Front Mol Biosci. 2021;8:688274.
- [CrossRef] [PubMed] [Google Scholar]
- The diagnostic potential of SHOX2 and RASSF1A DNA methylation in early lung adenocarcinoma. Front Oncol. 2022;12:849024.
- [CrossRef] [PubMed] [Google Scholar]
- Predictive value of unmethylated RASSF1A on disease progression in non-small cell lung cancer patients receiving pemetrexed-based chemotherapy. Cancer Biomark. 2020;27:313-23.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular testing in EBUS-TBNA specimens of lung adenocarcinoma: A study of concordance between cell block method and liquid-based cytology in appraising sample cellularity and EGFR mutations. Mol Diagn Ther. 2018;22:723-28.
- [CrossRef] [PubMed] [Google Scholar]
- The role of endobronchial ultrasound-guided transbronchial needle aspiration liquid-based cytology in the diagnosis of mediastinal lymphadenopathy. Diagn Cytopathol. 2020;48:316-21.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of liquid-based cytology test in intrathoracic lymph nodes and lung lesions sampled by endobronchial ultrasonography-transbronchial needle aspiration. Diagn Cytopathol. 2021;49:1251-6.
- [CrossRef] [PubMed] [Google Scholar]
- Usefulness and problems of cytological examination by endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of mediastinal lymphadenopathy: A retrospective single-centre study. Mol Clin Oncol. 2021;15:138.
- [CrossRef] [PubMed] [Google Scholar]
- Handling and standardization of EBUS needle aspiration in NSCLC patients: The value of the cell block, a monoinstitutional experience. Thorac Cancer. 2022;13:2480-8.
- [CrossRef] [PubMed] [Google Scholar]
- The contribution of cell blocks in the diagnosis of mediastinal and hilar lymphadenopathy samples from endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) Cureus. 2023;15:e39673.
- [CrossRef] [PubMed] [Google Scholar]
- Methylation assessment for the prediction of malignancy in mediastinal adenopathies obtained by endobronchial ultrasound-guided transbronchial needle aspiration in patients with lung cancer. Cancers (Basel). 2019;11:1408.
- [CrossRef] [PubMed] [Google Scholar]









