Translate this page into:
Voided urine test to diagnose prostate cancer: Preliminary report

*Corresponding author: R.B. Nerli, Department of Urology, Division of Urologic-Oncology, JN Medical College, KLE Academy of Higher Education and Research, Belagavi, Karnataka, India. rbnerli@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Nerli RB, Ghagane SC, Bidi SR, Thakur ML, Gomella L. Voided urine test to diagnose prostate cancer: Preliminary report. CytoJournal 2021;18:26.
Abstract
Objectives:
Prostate cancer (PCa) is a common malignancy affecting elderly male. At present, PCa is estimated using serum prostate-specific antigen (PSA). Prostate biopsy remains the gold standard to confirm the diagnosis of PCa. In this preliminary study, we have assessed the feasibility of detecting PCa using voided urine by targeting the genomic vasoactive intestinal peptide receptor (VPAC) expressed on malignant PCa cells.
Material and Methods:
Patients ≥40 years old, with no lower urinary tract symptoms (LUTS) and serum PSA levels of <1.6 ng/mL formed the control group and patients ≥40 years old, with LUTS and serum PSA >2.6 ng/ mL formed the study group. Patients were advised to give the first 50 mL of voided urine sample for the detection of malignant markers by targeting the VPAC. The results of histopathological studies were then compared to the results of urine biomarker.
Results:
The study revealed absence of malignant markers in 75 patients (control group). In the study group, all the 33 patients with adenocarcinoma were positive for malignant markers in the biomarker study and absence of malignant markers in the 32 patients with benign histology. The results of the biomarker studies and histopathology were consistent with each other.
Conclusion:
This preliminary study validates our belief that patients with PCa do shed malignant cells in the urine which can be identified by targeting the VPAC. The investigation is easy and our data appear to be highly encouraging and further serve as a simple, reliable, and a non-invasive tool in the detection of PCa.
Keywords
Prostate cancer
Biomarker
Voided urine
Non-invasive
INTRODUCTION
Prostate cancer (PCa) is a common non-cutaneous malignancy affecting men all over the world. In the United States alone, it accounts for 29% of all diagnosed cancers in the male and remains the second most common cause of mortality due to cancer. It accounts for 13% of all cancer deaths.[1,2] In the year 2021, ~248,530 men in the United States were diagnosed with cancer of the prostate and ~34,130 men died due to prostate malignancy.[3] Many centers in India have reported a rising incidence of PCa.[4] Estimation of serum prostate-specific antigen (PSA) test is commonly used both to detect and follow-up patients with PCa. This test of estimation of serum PSA is quick, cheap, and easily performed at all laboratories.
Clinical uro-oncologists across the globe have been using this test and can easily analyze the results and formulate risk levels of the disease and risk of cancer progression. Disease-specific mortality has reduced using serum PSA test for screening.[5-7] However, this gain has come at a considerable cost leading to unnecessary prostatic biopsies in 70–80% of the suspected populations based on the cutoff values for serum PSA.[5] PSA-based diagnosis/screening leads to overdiagnosis of cancer, especially the detection of “non-life-threatening” disease, which often results in overtreatment in such patients.[8]
As of today, PCa is suspected whenever digital rectal examination (DRE) findings are abnormal or serum PSA levels are elevated. Confirmation of PCa is based on performing biopsies in all patients having either an elevated serum PSA value and/or abnormal DRE. Prostate biopsy be it transrectal or transperineal is associated with risks, including local infection, systemic sepsis, and hemorrhage.[9] Prostate biopsy as of today is the most commonly used procedure to detect the disease, grade the lesion, and estimate the volume of the disease. At times, cancers can also be missed due to inadequacies associated with the sampling of the prostate tissue and that may result in poor sensitivity (false negatives).
A non-invasive and definitive test for PCa would be most welcome to patients and clinicians, whether by imaging or molecular biofluid analysis. New biomarkers for the detection and staging of PCa have become an absolute must. Urinary biomarkers for PCa are subject of ongoing research and represent a promising alternative or addition to serum-based biomarkers.[10] Urine-based tests being non-invasive might be suitable for both clinical and (mass) screening purposes, and also for prediction and to gain prognostic information.
Prostatic cancer cells are known to get shed into the prostatic urethra and hence get collected through voided urine sample. These cells could then be imaged optically, by specifically targeting the vasoactive intestinal peptide receptor (VPAC)1 (combined vasoactive intestinal peptide and pituitary adenylate cyclase activating peptide family of cell surface receptors) with the same peptide labeled fluorophore. Trabulsi et al.[11] reported their preliminary study on detection of PCa noninvasively, by a simple and reliable assay by targeting genomic VPAC expressed on malignant PCa cells shed in voided urine. The assay detected VPAC positive cells in 98.6% of the patients having a PCa diagnosis, (n = 141), and none (0%) of the males with benign prostatic hyperplasia (BPH) (n = 10). In this preliminary prospective study, we have assessed the feasibility of detecting cancer of the prostate using voided urine sample and targeting the genomic VPAC expressed on malignant PCa cells.
MATERIAL AND METHODS
This study was conducted with the consent obtained from the university/institutional ethical committee. Patients attending urological services ≥40 years old, with no lower urinary tract symptoms and serum PSA levels of <1.6 ng/ml formed the control group and patients ≥40 years old, with lower urinary tract symptoms and serum PSA >2.6 ng/ml formed the study group. Patients with urinary tract infection, hematuria, history of urothelial carcinoma, and radiotherapy were excluded from the study. All the patients in the control and study groups were advised to give the first 50 ml of voided urine sample for testing. The urine samples were stored and processed at 22°C for up to 4 h. However, if the processing was likely to be delayed due to some reason for more than 4 h of urine collection, then the samples could be stored at −10°C for up to 72 h.
Processing of urine samples[11]
These samples were labeled with the hospital identification number, date of collection, and the clinical diagnosis. The samples of urine were centrifuges using a cytocentrifuge 2000 ×g for 10 min. Except for 250 μL of supernatant, the rest was discarded. The cells were then resuspended, cytocentrifuged, and fixed in 97% ethanol. A cell area of approximately 1 cm in diameter was covered with TP4303 solution (0.5 μg). The slide was then incubated in dark, at 22°C for 20 min and then thoroughly rinsed with deionized water and the slide was air dried. The dried slide was then added with 20 μL of 4,6-dimidino-2-phenylindole, dihydrochloride (DAPI) (Fisher Scientific, PA) which strongly binds to A-T rich region of DNA in the cell nucleus.
The slide was covered with a coverslip and observed using a fluorescent microscope [Figure 1a]. Cells stained with TP4303 interaction appeared with dark orange fluorescence around the nucleus and thereby indicated the presence of VPAC molecules around the cell surface [Figure 2]. In the absence of VPAC, the cell nucleus that was bound only with DAPI appeared dark blue [Figure 3]. However, normal epithelial cells that may only have minimal or no expression of VPAC do not interact with TP4303 and therefore show only cell nucleus.

- (a) Fluorescence microscope instrument. (b) Hitachi™ HI VISION Avius® ultrasound scanner ((Hitachi Ltd., Tokyo, Japan). (c) Schematic diagram showing a template of 12-core biopsy labeled as zones (Z1 to Z12).

- Fluorescence imaging of cells prepared from voided urine. Each image is presented in four subsections. (a) Cell morphology. (b) Subsections are the cell nucleus in blue, (c) The bottom left is the orange fluorescence of TP4303 bound to vasoactive intestinal peptide receptor expressed the cell membrane. (d) The bottom right fusion of the two filters (DAPI-Cy5) showing the orange fluorescence around the malignant cells.
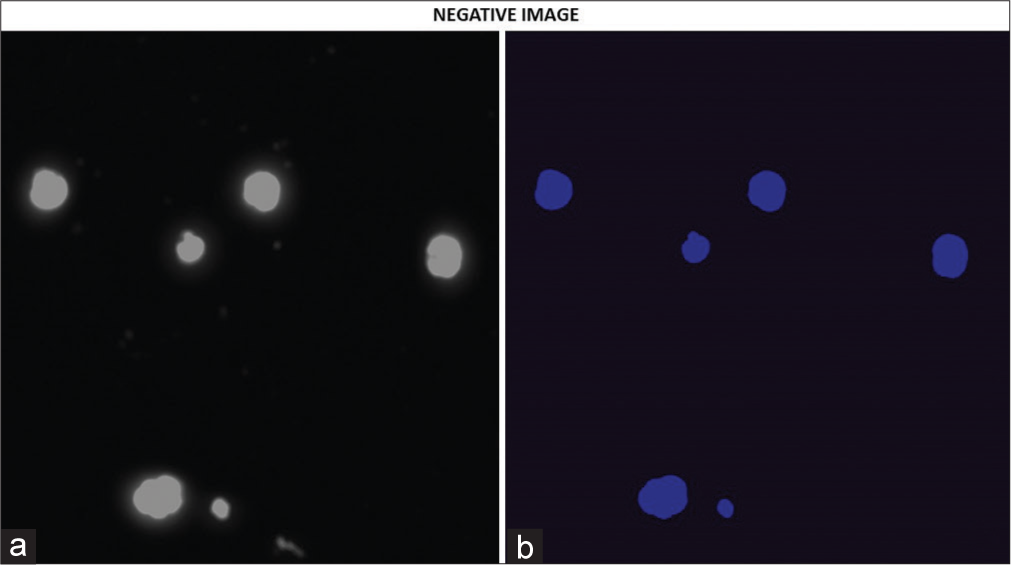
- (a and b) Fluorescence imaging of cells prepared from voided urine. Again each image is presented in two subsections as described. This subject had a negative biopsy and was prostate cancer free. The absence of the orange fluorescence indicates the absence of vasoactive intestinal peptide receptor.
All patients in the study group underwent a standard 12 core prostate biopsy using a transrectal ultrasonography (TRUS) guidance [Figure 1b]. The specimens were labeled properly denoting the zone of the prostate [Figure 1c] and sent for histopathological estimation. The results of the histopathological studies were then compared to the results of urine biomarker.
RESULTS
During the study period November 1, 2019–May 31, 2020, a total of 75 males were included into the control group. The mean age was 57.37±9.83 (range 41–76) years and mean serum PSA was 0.87±0.34 (range 0.41–1.59) ng/mL. All these patients were admitted for the management of either renal and/or ureteric calculi.
During the same period, 65 patients were included in the study group. The mean age of these patients was 70.1±6.36 (range 43–85) years and mean serum PSA was 9.41±6.55 (range 2.68–1200) ng/mL t [Table 1]. Twelve core TRUS-guided prostate biopsy revealed adenocarcinoma in 33 patients and BPH in the remaining 32 patients. The biomarker study revealed absence of malignant markers in all the 75 patients in the control group. In the study group, all the 33 patients with adenocarcinoma were positive for malignant markers in the biomarker study and absence of malignant markers in the 32 patients with benign histology. The results of the biomarker studies and histopathology were consistent with each other [Table 2].
| Mean age yrs. | Mean PSA ng/mL | Benign histology | Adenocarcinoma | Total | |
|---|---|---|---|---|---|
| Control group (75) | 60.66±9.5 | 1.08±0.66 | – | – | – |
| Study group (65) | 72.6±8.15 | 23.10±28.75 | 32 | 33 | 65 |
PSA: Prostate-specific antigen
| Malignant markers positive | Malignant markers negative | False positive (%) | False negative (%) | Total | |
|---|---|---|---|---|---|
| Control group (75) | - | 75 | 0 | - | 75 |
| Study group (65) | 33 | 32 | 0 | 0 | 65 |
DISCUSSION
Urine is a naturally available fluid medium, containing salts, acids, and substances that are filtered by the glomerulus, as well as proteins (<20 kDa) that are secreted by cells, and cells that originate from urinary tract downstream of glomerular filtration.[12] Solid components of urine can be easily separated from the liquid portion using low-speed centrifugation. The resulting solid pellet consists of cells, casts, mucin, and debris, whereas the liquid portion contains the soluble components including proteins, exosomes, biochemicals, and cell-free nucleic acids. The composition of urine is highly variable across individuals due to numerous factors such as age, diet, gender, and physical activity.[13,14]
Using urine for clinical assays has its own advantages. Collection of urine is non-invasive and carries no risk or harm to the patient. Urinary samples can be collected in large volumes at frequent intervals, making it possible to examine the urine sample repeatedly. Urine also contains secretions from the prostate and exfoliated prostate epithelial cells due to its close proximity with the rest of the urinary tract.[14,15] A number of prostatic biomarkers are also released into the urine, including cell-associated markers and secreted cell-free markers. DRE of the prostate or prostatic massage can enrich the presence of prostatic biomarkers in urine. Urine is better suited than blood as a source of biomarker for localized and early stage PCa, as blood contains markers from virtually all body tissues, leading to high background interference that can hinder detection ability, whereas urine is enriched in material coming directly from the prostate gland.[15]
Prostate cells that appear in the urine are luminal epithelial cells that have been shed from the gland, however, it is not fully understood the exact nature of urinary prostate cells and as to how these cells are released into urine. Normal cell turnover leads to shedding of prostate cell. There is limited investigation available on the intactness of the prostate cells. The morphological features of prostate cells have been well defined in several cytological studies.[16-18] Microscopic studies using conventional cytology staining methods revealed that prostate cells found in the urine sediment have a distinct appearance; they generally are round with a high nuclear to cytoplasm ratio, prominent nucleoli, and often present in small clusters. Identification of prostate cells merely based on the morphology is challenging even for the trained cytopathologists because of the overlapping appearance with other cell types found in the urine sediment as well as their scarcity in regularly voided urine specimens.
The study reported by Trabulsi et al.[11] targeted that the VPAC is known to express in elevated density on PCa cells at the beginning of oncogenesis so as to develop a simple, completely non-invasive, inexpensive, and reliable test to screen or detect PCa. It has been reported that 1 g of a growing tumor sheds nearly 0.4% (3.4 × 106 cells) every 24 h.[18] Complete or parts of these cells pass through the prostatic ducts and appear in the urine even without performing DRE. It has been demonstrated that the genomic VPAC is expressed on the cell surface of the PCa cells.[11] TP4303 is known to specifically target the cell surface VPAC and help in identifying PCa cells.[11] It has also been seen that the cells that are targeted by TP4303 were truly PCa cells.[11]
Our preliminary study has shown that the patients with adenocarcinoma of the prostate shed cancer cells in the urine that could be easily identified by targeting the VPAC. The results of the biomarker study and histopathology correlated in 100% of the cases. There were no false-positive or false-negative results. Trabulsi et al.[11] reported detection of VPAC positive cells in 98.6% of the patients having a PCa diagnosis, (n = 141), and none (0%) of the males with BPH (n = 10).
This preliminary study of ours validates our belief that patients with PCa shed malignant cells in the urine that can be identified by targeting the VPAC. Our study is simple and can be feasible in most centers dealing with patients of PCa. Our study is a small preliminary study and the results need to be validated by a multicentric study with a large number of patients. If validated, this study could form the basis in future (i) to indicate a repeat prostate biopsy in patients with an elevated serum PSA and negative TRUS-guided biopsy and (ii) to indicate a reduced number of cores on TRUS-guided biopsy.
CONCLUSION
This preliminary study of ours validates our belief that patients with prostate cancer shed malignant cells in the urine that can be identified by targeting the VPAC receptors. Our study is simple and can be feasible in most centres dealing with patients of prostate cancer. Our study is a small preliminary study and the results need to be validated by a multicentric study with a large number of patients. If validated this study could form the basis in future i) to indicate a repeat prostate biopsy in patients with an elevated serum PSA and negative TRUS guided biopsy, and ii) to indicate a reduced number of cores on TRUS guided biopsy.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no known competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All persons who meet authorship criteria are listed as authors, and all authors certify that they have participated in the concept, design, analysis, writing, or revision of the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
All authors have been personally and actively involved in substantial work leading to the paper, and will take responsibility for its content.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
BPH – Benign prostatic hyperplasia
DAPI – Dimidino-2-phenylindole, Dihydrochloride
DRE – Digital rectal examination
LUTS – Lower urinary tract symptoms
PCa – Prostate cancer
PSA – Prostate specific antigen
VPAC1 – Combined vasoactive intestinal peptide
PACAP – Pituitary adenylate cyclase activating peptide
TRUS – Transrectal ultrasonography
TP4303 – Thakur Peptide 4303.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
References
- Cancer Facts and Figures. 2021. Available from: https://www.cancer.org/cancer/prostate-cancer/about/new-research.html2021 [Last accessed on 2021 May 27]
- [Google Scholar]
- Incidence of prostate cancer at a single tertiary care center in North Karnataka. Indian J Cancer. 2016;53:429-31.
- [Google Scholar]
- Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-8.
- [CrossRef] [PubMed] [Google Scholar]
- Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European randomised study of screening for prostate cancer (ERSPC) Eur Urol. 2009;56:584-91.
- [CrossRef] [PubMed] [Google Scholar]
- Mortality results from the Göteborg randomized population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725-32.
- [CrossRef] [Google Scholar]
- Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br J Cancer. 2009;101:1833-8.
- [CrossRef] [PubMed] [Google Scholar]
- Specific detection of prostate cancer cells in urine by multiplex immunofluorescence cytology. Hum Pathol. 2009;40:924-33.
- [CrossRef] [PubMed] [Google Scholar]
- Tumour markers in prostate cancer III: Biomarkers in urine. Acta Oncol. 2011;50(Suppl 1):85-9.
- [CrossRef] [PubMed] [Google Scholar]
- Development of a voided urine assay for detecting prostate cancer non-invasively: A pilot study. BJU Int. 2017;119:885-95.
- [CrossRef] [PubMed] [Google Scholar]
- Sources of urinary proteins and their analysis by urinary proteomics for the detection of biomarkers of disease. Proteomics Clin Appl. 2009;3:1029-43.
- [CrossRef] [PubMed] [Google Scholar]
- Standardizing the experimental conditions for using urine in NMR-based metabolomic studies with a particular focus on diagnostic studies: A review. Metabolomics. 2015;11:872-94.
- [CrossRef] [PubMed] [Google Scholar]
- Approaches to urinary detection of prostate cancer. Prostate Cancer Prostatic Dis. 2019;22:362-81.
- [CrossRef] [PubMed] [Google Scholar]
- Prostatic adenocarcinoma diagnosed by urinary cytology. Am J Clin Pathol. 2000;113:29-34.
- [CrossRef] [PubMed] [Google Scholar]
- Morphologic features of prostatic adenocarcinoma on Thin Prep (R) urinary cytology. Diagn Cytopathol. 2011;39:101-4.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic features of prostatic adenocarcinoma in urine: A clinicopathologic and immunocytochemical study. Diagn Cytopathol. 1988;4:300-5.
- [CrossRef] [PubMed] [Google Scholar]









