Translate this page into:
A mass protruding from the pancreas featuring extensive myxoid change
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 75-year-old woman was found to have an abdominal tumor on abdominal echogram examination during a health checkup. Abdominal imaging showed that a solid tumor was located in the vicinity of the pancreas, spleen, and left kidney. In particular, the tumor was widely adherent to the distal pancreas. Laboratory examination revealed no significant findings, although there was a slightly elevated serum level of carbonic antigen 19-9. The specimen revealed a well-circumscribed, round-to-oval tumor, measuring 80 mm × 62 mm × 52 mm and 136 g in weight. It was an encapsulated, tan-white, solid tumor with a gelatinous appearance on the cut surface. Imprint (direct smear) cytology specimens were obtained from the tumor, although only a small number of cell clusters were observed on the glass slides. With the use of a cell transfer technique, immunocytochemistry (ICC) was performed on these specimens. A diagnosis was put forward after cytologic evaluation accompanied by ICC [Figure 1].
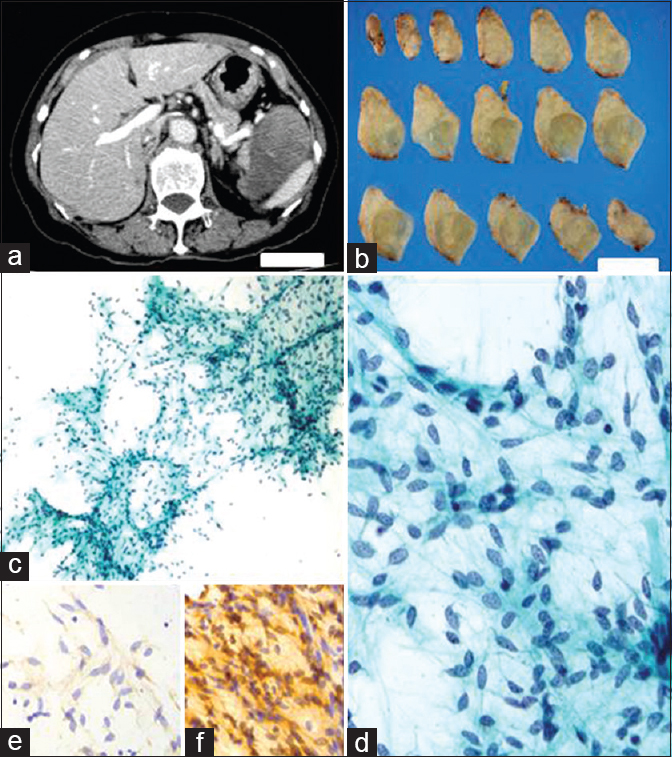
- (a and b) Abdominal computed tomography and cut surfaces of the resected tumor specimen. Scale bars indicate 5 cm; (c and d) The cellularity was sparse. The tumor cells were observed cohesively in a haphazard fashion and had oval-to-elongated, bland nuclei (Papanicolaou; c, ×100; d, ×400); (e and f) The tumor cells were positive for CD34 (e), bcl-2 (f), and vimentin, and negative for desmin, alpha-smooth muscle actin, S-100 protein, c-kit, and pan-cytokeratin (e and f, ×400)
QUESTION
Q1: What is your Interpretation?
-
Neuroendocrine neoplasm
-
Neurogenic neoplasm
-
Gastrointestinal stromal tumor (GIST)
-
Other mesenchymal neoplasm.
ANSWER
A1: Other mesenchymal neoplasm
The correct cytological interpretation is – this is a case of primary myxoid solitary fibrous tumor (SFT) of the pancreas.
Cytology of pancreaticobiliary juices and examination of fine-needle aspiration materials are recognized as effective diagnostic modalities for pancreatic tumors, especially for infiltrating duct adenocarcinoma.[1] However, cytologic diagnosis of pancreatic mesenchymal tumors is considered difficult because these tumors are so rare.[2] During cytologic evaluation of the present case, even greater difficulty was encountered because the cellularity was so sparse. Within the cell clusters, bland spindle tumor cells were arranged haphazardly with a lack of uniform polarity, and thin-walled capillaries were also present. The tumor cells appeared almost uniform, and had oval-to-elongated nuclei with finely granular, evenly distributed chromatin, without evident nucleoli. There was no significant necrosis or mitoses. Thus, the cytologic diagnosis offered was a benign mesenchymal tumor, and this was confirmed by the ICC results for pan-cytokeratin and vimentin. Moreover, diagnoses of neurogenic neoplasm and GIST were not consistent with the negative ICC results for S-100 protein and c-kit, respectively. The diffusely positive ICC results for both CD34 and bcl-2 suggested it being a SFT.
Histological correlation and follow-up of the present case
Histologically, the tumor displayed a patternless pattern of spindle tumor cells along with evidence of marked myxomatous change. The tumor displayed a branching vascular pattern along with perivascular hyalinization. Immunohistochemical studies gave results similar to those obtained by ICC, with the addition of positive results for STAT6 [Figure 2]. The histologic diagnosis was myxoid SFT. Furthermore, the tumor was considered to have arisen from the pancreas because of an involvement of pancreatic parenchyma in the tumor periphery within the resected specimen. The patient's postoperative course was uneventful, and no signs of recurrence have been observed during a 3-year follow-up.
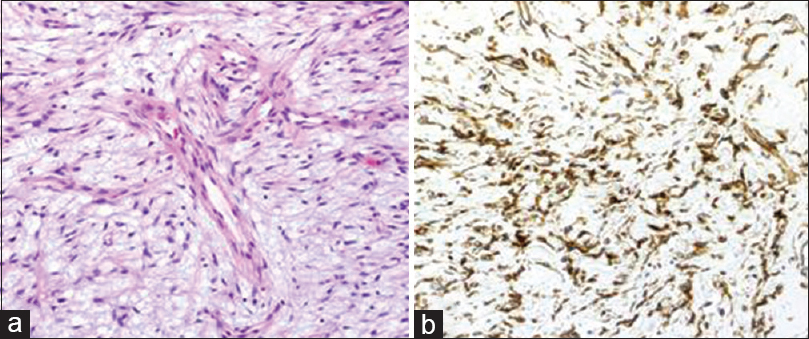
- (a) Histologically, the spindle tumor cells had proliferated haphazardly, and thin-walled capillaries were also evident (H and E, ×200); (b) Immunohistochemical study revealed a positive result for STAT6 (×200)
Brief review of topic
SFTs are uncommon mesenchymal tumors, and although they can occur at any location,[3] more than ten cases with a pancreatic origin have been reported.[456] These previous cases showed tendencies toward: (1) female occurrence, (2) mild clinical symptoms, (3) pancreatic head location, and (4) benign clinical course. The present tumor displayed some unusual features, as follows: (1) located in pancreatic tail, (2) formation of an extrapancreatic protruding, large mass, and (3) histologically extensive myxoid change. The present case is the first case of a myxoid variant of pancreatic SFT. In addition, the present case exhibited histologic features that were also exhibited by the myxoid SFTs at other sites reported by de Saint Aubain Somerhausen et al.[7] and Lau et al.,[8] namely, obscuring of the typical histological characteristics of SFT such as alternating areas of high and low cellularity, a patternless pattern of spindle cells, and blood vessels displaying a typical staghorn formation.
A preoperative cytomorphologic diagnosis by endoscopic ultrasound-guided fine-needle aspiration cytology may be requested in the routine clinical situation, although in the present case it was not. In typical benign SFT cases, the cytology usually exhibits: (1) variable cellularity, (2) a bloody background, (3) oval-to-spindle-shaped cells, with a uniformity of cell shape, (4) bland nuclei without nucleoli, (5) no mitotic figures or necrosis, and (6) intercellular collagen.[9] The cytologic findings in our case matched features 3, 4, and 5 mentioned above. Based on situations in which fine-needle aspiration is employed, Shidham et al. summarized the cytology findings in benign SFT as follows.[10]
-
Greater degree of cohesion
-
Cohesive clusters with haphazard overlap and lack of uniform polarity
-
Some clusters with fascicular pattern with focal suggestion of palisading
-
Bland spindle cells
-
Scant cytoplasm
-
Many naked nuclei
-
Oval-to-elongated nuclei
-
Finely granular, evenly distributed chromatin
-
Inconspicuous nucleoli
-
Insignificant mitotic activity
-
Bloody background
-
Pink, amorphous substance, consistent with collagen
-
No polygonal or epithelioid cells
-
Frequent paucicellular smears.
The cytologic findings in our case matched features 1, 2, 4, 5, 7–10, 13, and 14 mentioned above. In contrast, in malignant SFT findings of few cohesive clusters, larger number of single cells, moderate degree of nuclear pleomorphism, prominent nucleoli, mitotic figures, and high cellular smears have been reported.[10]
For the purposes of differential diagnosis, benign neurogenic neoplasm, benign lipomatous neoplasms, and low-grade GISTs were considered. In neurogenic neoplasms, such as neurilemmoma (schwannoma) or neurofibroma, spindle cell morphology with moderate cellularity and a fibrillary background are common, and nuclei or tumor cells are elongated with pointed ends and have a wavy or twisted appearance.[11] In lipomatous neoplasms, the presence of vacuolated fat cells with nuclei pushed to the periphery and delicate vessels without much fibrous tissue may be a hint for adequate diagnosis.[1112] GIST can show a wide spectrum of cytologic features, such as variable cellularity, cohesive clusters, or dispersed cells. The spindle tumor cells have oval-to-elongated or irregular-shaped nuclei, and chromatin is finely to coarsely granular. There may have fascicles in which tumor cells are orientated or organized in one direction.[13]
In the present case, SFT needs to be considered a candidate in the list for differential diagnosis by cytomorphologic findings because of the nuclear shape and a haphazard arrangement of tumor cells. However, a definitive diagnosis of SFT might be difficult. Indeed, in a cytomorphometric analysis of six SFTs reported by Gupta et al.,[14] no definitive cytologic features for diagnosis could be identified because of morphologic overlap with other tumors. In the present case, the sparse cellularity due to extensive myxoid change increased the difficulty of cytologic diagnosis. However, application of an ICC panel is useful for diagnosing SFT,[10] and a positive result for STAT6 has recently been reported to be effective.[15] Especially when paucicellular specimens are encountered, as in the present case, we consider the application of ICC to be an essential addition to adequate cytologic evaluation to narrow the field of candidates for differential diagnosis.
ADDITIONAL QUIZ QUESTIONS
Q2: Which of the following cytomorphologic features is characteristic for typical benign SFT?
-
High cellularity
-
Ambiguous nuclear appearance
-
Mitotic figures
-
Intercellular collagen.
Q3: Which of the following cytomorphologic features is characteristic for typical malignant SFT in fine-needle aspiration?
-
Bloody background
-
Paucicellular smears
-
Nuclear pleomorphism
-
Intercellular collagen.
Q4: Which of the following antibodies is the best diagnostic marker for SFT?
-
STAT6
-
Pan-cytokeratin
-
Vimentin
-
CD34.
ANSWERS
A2: d. Intercellular collagen; A3: c. Nuclear pleomorphism; A4: a. STAT6.
SUMMARY
Although extremely rare, pancreatic SFT does occur.
ICC is effective, and indeed essential, for diagnosing such a mesenchymal tumor, especially when there is extensive myxoid change.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
HT carried out the cytologic evaluation and also drafted the manuscript. HD and KK carried out the pathologic evaluation. TO and SK carried out the cytologic evaluation. YT carried out the radiologic evaluation. SO conceived the study and also participated in pathologic evaluation and helped to draft the manuscript. KN participated in pathologic evaluation and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board of National Defense Medical College, Japan.
LIST OF ABBREVIATIONS (In alphabetic order)
GIST - Gastrointestinal stromal tumor
ICC - Immunocytochemistry
SFT - Solitary fibrous tumor.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Pancreatic cytopathology: A practical approach and review. Arch Pathol Lab Med. 2009;133:388-404.
- [Google Scholar]
- Howard J, Idezuki Y, Ihse I, Prinz R, eds. Surgical Diseases of the Pancreas (3rd ed). Baltimore: Williams & Wilkins; 1998. p. :633-6.
- World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon; 2002. p. :86-8.
- [Google Scholar]
- Solitary fibrous tumour of the pancreas: A new member of the small group of mesenchymal pancreatic tumours. Virchows Arch. 1999;435:37-42.
- [Google Scholar]
- Solitary fibrous tumour of the pancreas: A case report. An Sist Sanit Navar. 2012;35:133-6.
- [Google Scholar]
- Myxoid solitary fibrous tumor: A study of seven cases with emphasis on differential diagnosis. Mod Pathol. 1999;12:463-71.
- [Google Scholar]
- Myxoid solitary fibrous tumor: A clinicopathologic study of three cases. Virchows Arch. 2009;454:189-94.
- [Google Scholar]
- Solitary fibrous tumor. A cytologic-histologic study with clinical, radiologic, and immunohistochemical correlations. Cancer. 1997;81:116-21.
- [Google Scholar]
- Fine needle aspiration cytology of gastric solitary fibrous tumor: A case report. Acta Cytol. 1998;42:1159-66.
- [Google Scholar]
- Cytology of soft tissue tumors: Benign soft tissue tumors including reactive, nonneoplastic lesions. J Cytol. 2008;25:81-6.
- [Google Scholar]
- Aspiration cytology of lipomatous tumors: A 10-year experience at an orthopedic oncology center. Diagn Cytopathol. 1987;3:295-302.
- [Google Scholar]
- Cytomorphology of gastrointestinal stromal tumors and extra-gastrointestinal stromal tumors: A comprehensive morphologic study. J Cytol. 2013;30:8-12.
- [Google Scholar]
- Solitary fibrous tumour: A diagnostic challenge for the cytopathologist. Cytopathology. 2012;23:250-5.
- [Google Scholar]
- STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552-9.
- [Google Scholar]







