Translate this page into:
Pleural effusion in a 7-year-old child - with unique cells
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 7-year-old male presented with 2-month history of fatigue, pallor, and weight loss and 1-month history of fevers and mucositis. He then developed rash in the form of small papules on his face, hands, and feet, diagnosed to be viral infection. He was started on acyclovir. He continued to be ill with an erythematous rash on his face and hands with intermittent cough. Computed tomography scan of the chest revealed multilobar bilateral foci of pulmonary consolidation and diffuse adenopathy. Later, he acutely developed respiratory distress and was found to have a moderate-to-large right-sided pleural effusion. Thoracocentesis yielded yellow cloudy fluid with elevated cell count and neutrophils, consistent with an exudate. Culture of the fluid grew streptococcus pneumoniae bacteria. Blood culture was negative.
Our laboratory received 7 ml of yellowish pleural fluid. Papanicolaou-stained slide and cell block showed numerous neutrophils, macrophages, and apoptotic debris. Numerous bacteria, lymphocytes, and plasma cells were also present in the background [Figure 1].
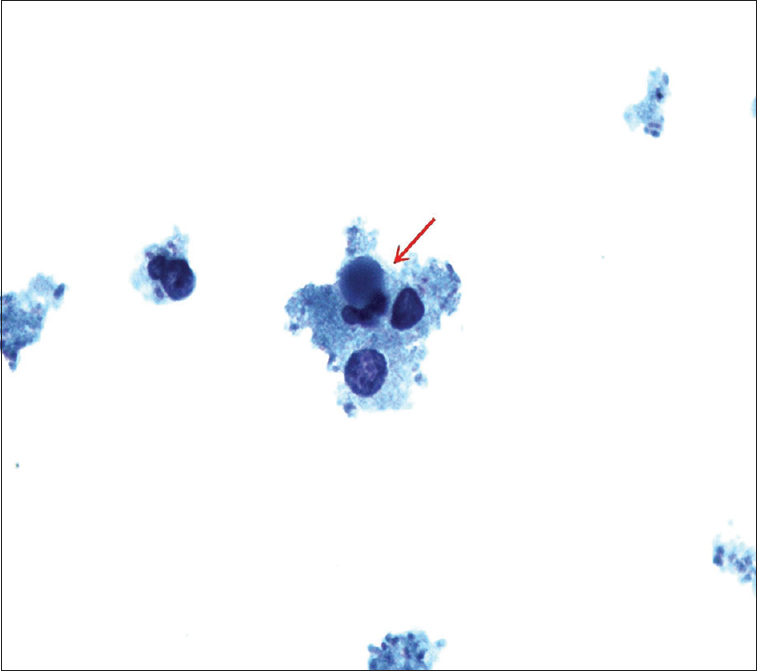
- ThinPrep slide of pleural fluid showing three-lobed neutrophil containing intracytoplasmic round basophilic homogeneous body with smooth borders forming lupus erythematosus cell (red arrow) (Papanicolaou stain, ×100)
WHAT IS YOUR INTERPRETATION?
-
Consistent with bacterial infection
-
Consistent with viral infection
-
Consistent with an autoimmune disorder
-
Nondiagnostic.
ANSWER
The correct answer is c:
Cytology preparation showed many lymphocytes and bacteria along with numerous neutrophils and macrophages. Some of these neutrophils were seen containing intracytoplasmic round basophilic homogeneous bodies with smooth borders pushing the nucleus to the periphery [Figure 1 - red arrow]. The morphology of these round homogeneous bodies in neutrophils was most compatible with hematoxylin bodies with overall features consistent with lupus erythematosus (LE) cells. These homogeneous bodies were also present in macrophages in cell block sections as tart cells [Figure 2 - red arrow]. Some of these round bodies were also seen in the background in clusters forming conglomerates. All these features were diagnostic of systemic lupus erythematosus (SLE), an autoimmune disorder.
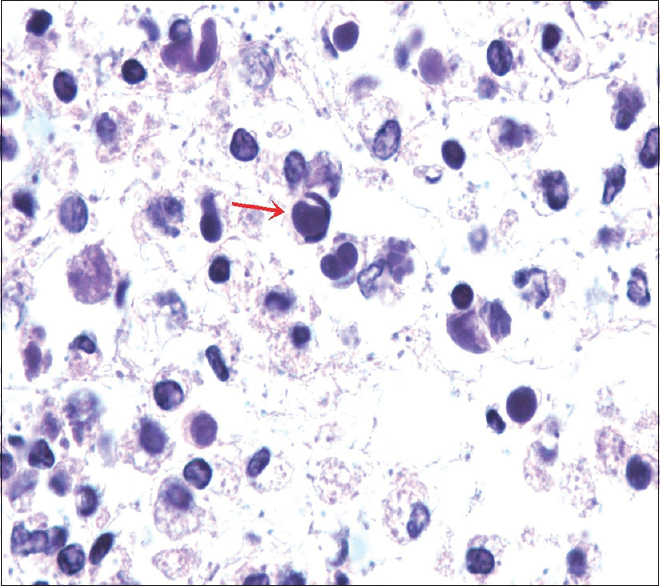
- Tart cell. Cell block showing round bodies within the macrophage pushing the nucleus to periphery forming tart cell (red arrow). These homogeneous bodies can be seen in clusters forming conglomerates along with many bacteria in the background (H and E, ×100)
ADDITIONAL QUIZ QUESTIONS
Q1. What is a LE cell?
-
A neutrophil containing intracytoplasmic homogeneous body of denatured nuclear material of another cell
-
A macrophage which has engulfed the denatured nuclear material of another cell
-
A macrophage which has phagocytosed the lymphocytes
-
A macrophage which has phagocytosed the erythrocytes.
Q2. The most specific finding of SLE in body fluids is
-
Both LE cells and tart cells
-
Both tart cells and histiocytes
-
Both LE cells and erythrocytes
-
LE cells only.
Q3. The diagnosis of SLE requires the following
-
Clinical features and serologic criteria
-
In vitro LE cell assay
-
Both serologic criteria and in vitro LE cell assay
-
Cytological and/or histopathological features.
ANSWERS
Q1. a; Q2. d; Q3. a.
ADDITIONAL LABORATORY INVESTIGATIONS AND FOLLOW-UP OF THE CURRENT CASE
The patient had been seen multiple times at primary care and had been diagnosed with acute otitis media and viral infection. However, he responded poorly to antibiotics and antiviral therapy. Concurrent immunologic work-up revealed increase in inflammatory markers, C-reactive protein 127.1 mg/L (normal range, 0.3–5.0 mg/L), erythrocyte sedimentation rate 60 mm/h (normal range, 0–20 mm/h), positive extractable nuclear antigen Smith antibodies 5 AI (normal ≤0.9 AI), ribonuclear protein antibodies 1.9 AI (normal ≤0.9 AI), positive antinuclear antibodies (ANA) 1:1280 (normal, 1:80) of homogeneous pattern, and anti-double-stranded deoxyribonucleic acid (dsDNA) antibodies >1000 IU/mL (normal, ≤30 IU/mL). Serology for anti-Ro/La antibodies was negative. Complement C3 was low at 41 mg/dL (normal range, 80–180 mg/dL) with normal complement C4 of 12.6 mg/dL (normal range, 10–45 mg/dL).
The cytological findings combined with the physical presentation and positive serology for SLE were diagnostic for SLE. The child was started on prednisone and showed a rapid clinical response.
DISCUSSION
The occurrence of pleural effusions is less frequent in children than in adults and can be caused by various infectious, inflammatory, and neoplastic diseases. Community-acquired bacterial pneumonia remains the most common infectious cause of childhood pleural effusion.[1] Other causes include renal disease, trauma, viral diseases, congenital heart diseases, liver diseases, and tuberculosis. Pleural effusion can be transudate or exudate although these findings are not reliably evaluated in pleural effusions in children. Chylothorax is another form of pleural effusion characterized by milky white fluid and is caused by trauma, medications, lymphomas, infections, or lymphatic diseases.[1]
SLE is an autoimmune chronic inflammatory disorder, primarily affecting women. SLE has varied symptomatology and can involve any organ of the body. Most often patients present with “butterfly” malar rash and arthralgias.[2] Childhood-onset SLE includes about 15%–20% of all patients with an aggressive clinical course and more common systemic presentations such as renal, neuropsychiatric, and hematologic involvements.[345]
Pleuropulmonary involvement in the form of pleural effusion is an infrequent early presentation of SLE. Pleural effusion mostly occurs late during the course of the disease but can be seen in 1% patients as an initial presentation.[2] This case emphasizes the importance of pleural fluid examination in children for the diagnosis of nonneoplastic and noninfectious causes.
Our patient presented with rash, fever, and developed bilateral pleural effusions during the hospital course. Other differential diagnosis includes bacterial infections, tuberculosis, nephrotic syndrome, viral infections, congestive cardiac failure, and neoplasms. Although serology was positive for the markers of SLE, infection was the most common differential of pleural effusion. The patient responded poorly to antibiotics and antiviral drug therapy.
Lupus pleuritic effusion is typically an exudate as was present in our case. Cytology revealed the in vivo finding of LE cells, which is a rare but an important specific diagnostic finding of SLE. LE cells were first described in 1948 in the bone marrow.[6] These cells have been described in the abnormal fluid collection in the body cavities such as pleural, pericardial, and peritoneal cavities and in synovial fluid and CSF.[678] Among these sites, pleural fluid is the most common. LE cells are predominantly polymorphonuclear cells or neutrophils with engulfed denatured nuclear material of another cell.[8] This denaturation of injured or dead cells is caused by opsonization of nucleoproteins by ANA in SLE patients, resulting in the formation of homogeneous bodies. These bodies also referred to as hematoxylin bodies on Wright-Giemsa stain are engulfed by polymorphonuclear cells causing the formation of LE cells.[910]
Tart cells are macrophages or histiocytes which have phagocytosed nuclear debris of other cells. The presence of ‘tart cells’ is not specific for SLE. These can also be seen in patients with lymphomas and metastatic carcinoma.[6] The LE cells should always be differentiated from tart cells as well as with other cells such as histiocytes with phagocytic activity and mucus-secreting cells because of specificity of LE cells.
The in vitro LE cell assay was performed on buffy coat preparation after incubation or at room temperature followed by smear preparation and counting LE cells.[11] This LE cell assay was one of the criteria used for the diagnosis of SLE. However, now serological evidence of elevated autoantibodies such as ANA, anti-dsDNA, and clinical presentation is the mainstay for the diagnosis of SLE.[10]
SUMMARY
Our case was a male child who presented with rash and pleural effusion. The predilection for SLE is much less common in males than females. Furthermore, infection being the most common cause for pleural effusion in children, this case emphasizes the importance of examination and recognition of LE cells morphology in pleural fluid as a clue to the cause of pleural effusion in children.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. Neha Gupta designed the case report and drafted the manuscript. Kasturi Das interpreted the cytology smears, arrived at the final cytologic diagnosis, and supervised the design of the case report. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional review Board (IRB).
LIST OF ABBREVIATIONS (In alphabetic order)
ANA - Antinuclear antibodies
CRP - C-reactive protein
CSF - Cerebrospinal fluid
dsDNA - Double-stranded deoxyribonucleic acid
ESR - Erythrocyte sedimentation rate
LE - Lupus erythematosus
PAP - Papanicolaou
RNP - Ribonuclear protein
SLE - Systemic lupus erythematosus.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Clinical manifestations of systemic lupus erythematosus. Computer analysis of 520 cases. JAMA. 1964;190:104-11.
- [Google Scholar]
- Childhood-onset disease as a predictor of mortality in an adult cohort of patients with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2010;62:1152-9.
- [Google Scholar]
- Taxonomy for systemic lupus erythematosus with onset before adulthood. Arthritis Care Res (Hoboken). 2012;64:1787-93.
- [Google Scholar]
- Update on differences between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Res Ther. 2013;15:218.
- [Google Scholar]
- Synovial fluid cells in systemic lupus erythematosus: Light and electron microscopic studies. Lupus. 1995;4:353-64.
- [Google Scholar]
- Pleuritis and pleural effusion as the initial presentation of systemic lupus erythematous in a 23-year-old woman. Rheumatol Int. 2008;28:1257-60.
- [Google Scholar]
- Case report and review of lupus erythematosus cells in cytology fluids. Diagn Cytopathol. 2007;35:806-9.
- [Google Scholar]







