Translate this page into:
Atypical epithelioid cells in pleural effusion as foreign second population: A diagnostic cytopathology dilemma

-
Received: ,
Accepted: ,
How to cite this article: Zhao S, Hou L, Wu W, Wu C. Atypical epithelioid cells in pleural effusion as foreign second population: A diagnostic cytopathology dilemma. CytoJournal. 2024;21:3. doi: 10.25259/Cytojournal_23_2023
A 56-year-old female presented with a right pleural effusion. The chest computed tomography (CT) showed a round mass shadow of the right middle and lower lobe of the lung. The pleural fluid showed atypical epithelioid cells [Figure 1].
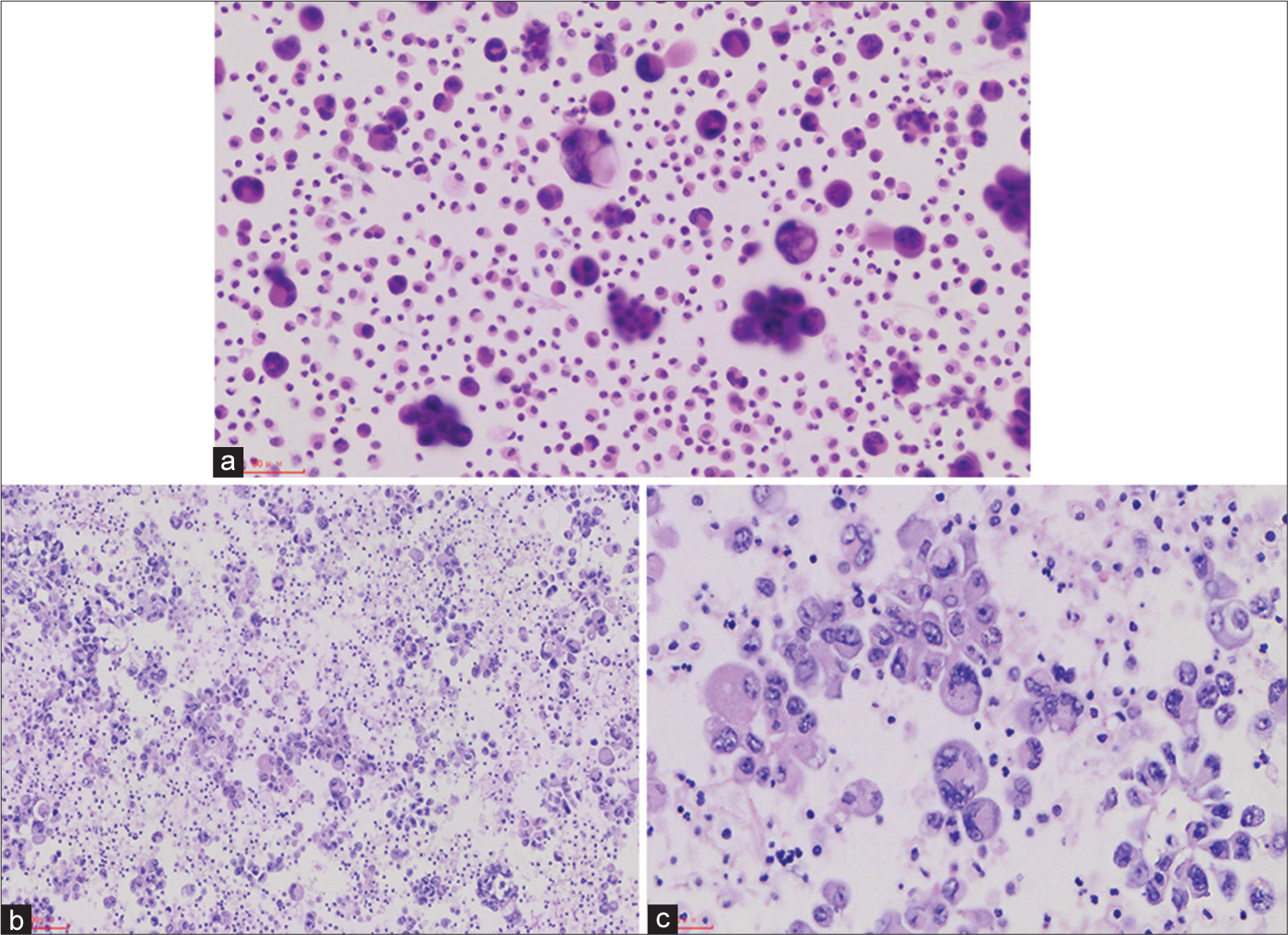
- (a) Pleural effusion smear showed many atypical epithelioid cells as second foreign population (high magnification of hematoxylin eosin (Hematoxylin and Eosin 200×)). (b) The embedded pleural fluid section showed that the tumor cells were scattered or clustered (medium magnification of Hematoxylin and Eosin 200×). (c) The embedded pleural fluid section showed significant atypia, nuclear disorientation and nucleolus (high magnification of Hematoxylin and Eosin 400×).
Q1. What is your interpretation of the pleural effusion smear?
Proliferated mesothelial cells
Mesothelioma
Metastatic adenocarcinoma
Malignant cells, pending immune characterization
ANSWER
The correct cytological interpretation is d.
EXPLANATION
A diagnosis of the pleural effusion cell block is a “malignant tumor.” The pleural effusion smear cells block showed tumor cells epithelioid, with significant atypical, prominent nucleoli and giant tumor cells, and nuclear deviation. In this case, the touch prep shows variably sized loose clusters and single cells with acinar formation. The cells show abundant eosinophilic cytoplasm. The nuclei have moderate-to-severe heteroplasia with occasional prominent nucleoli. At the time, “malignant tumor” was the most appropriate diagnosis. The differential diagnosis would include suspicion for carcinoma and other sarcomas.
ADDITIONAL QUIZ QUESTIONS
Q2. Hematoxylin-eosin(HE) stained sections are prepared from the tissue cores [Figure 2] and show a solid, cellular neoplasm. What is your interpretation?
Non-small cell lung cancer (NSCLC)
Epithelioid Sarcoma
Sarcomatoid carcinoma
Metastatic epithelioid hemangioendothelioma (EHE)
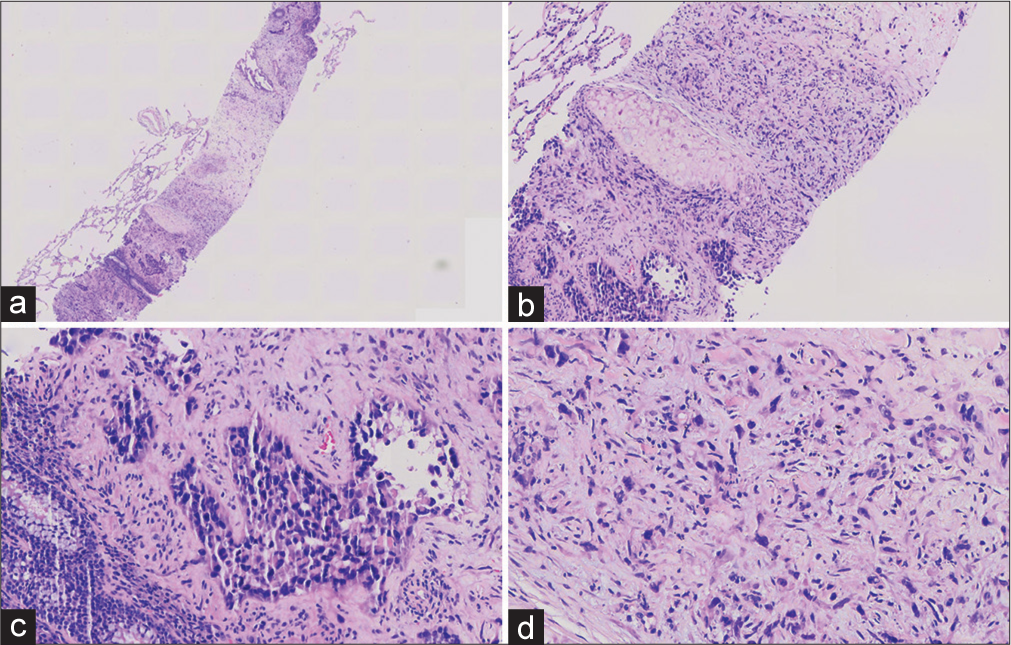
- (a) Tumor cells were seen in the fibrous stroma as epithelioid, with the low magnification of Hematoxylin and Eosin, 40×. (b) The tumor cells were arranged in two ways. The glandular and the solid area are patchy, with a medium magnification of Hematoxylin and Eosin, 100×. (c) A small amount of red blood cells can be seen in the vascular lumen. The cells had significant atypia and high magnification of Hematoxylin and Eosin, 200×. (d) The cells in the solid area were epithelioid or short spindle-shaped, with nuclear deviation, with high magnification of Hematoxylin and Eosin, 400×.
ANSWER
The correct cytological interpretation is d.
Immunohistochemistry and molecular results
Immunohistochemical (IHC) staining was performed by the envisioned method. The results of IHC staining were as follows:
Tumor cells did not express CKpan, TTF1, P40, GATA3, CD10, calretinin, smooth muscle actin, or desmin; tumor cells expressed vascular markers such as CAMTA1, CD31, and ERG [Figure 3a-c], and vimentin was positive; the positive index of Ki-67 was approximately 5%. T (1p36) (CAMTA1) gene translocation (+) was detected by fluorescence in situ hybridization (FISH) (Note: red and green separation signals can be seen in > 50% of tumor cells) [Figure 3d].
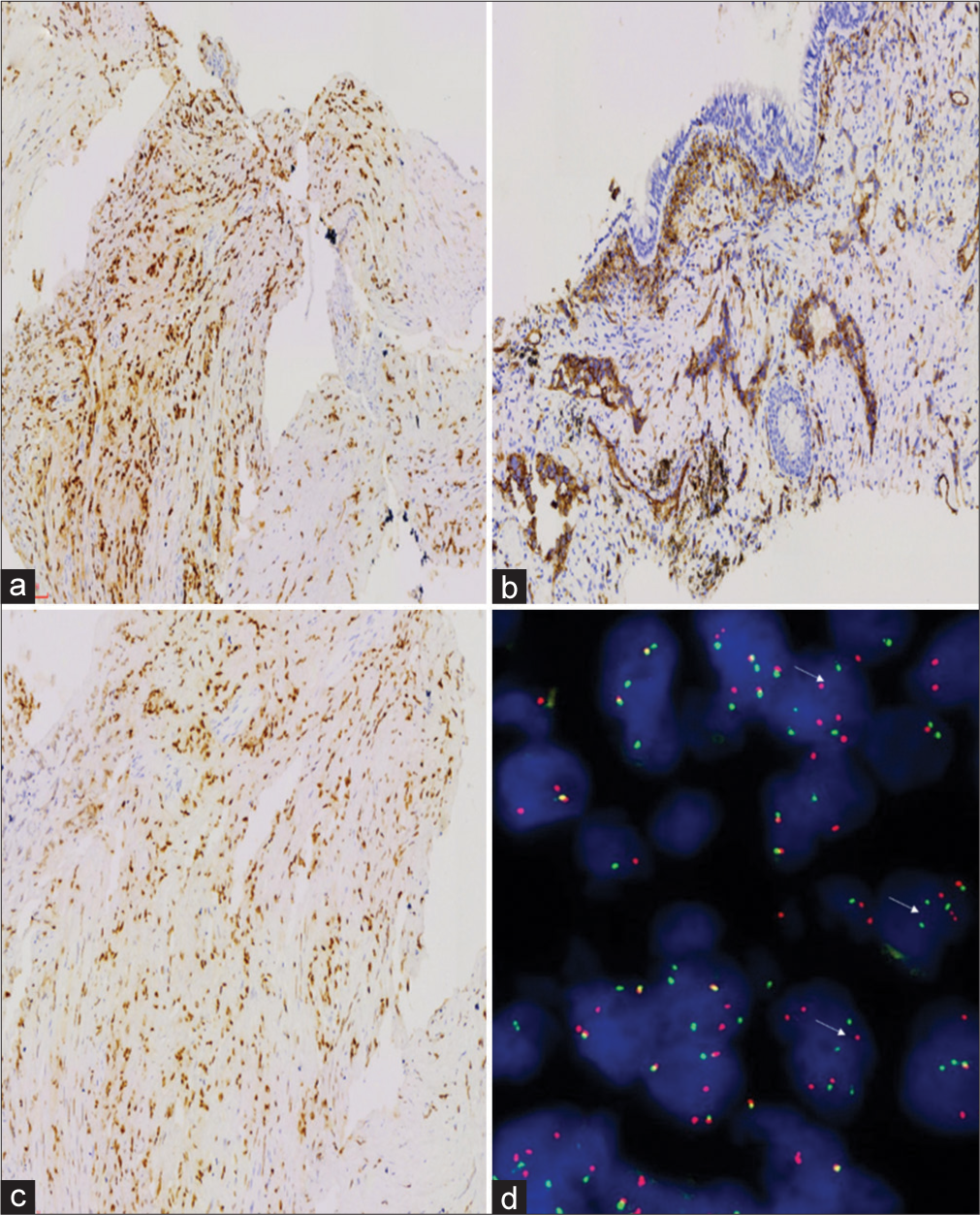
- (a) Immunohistochemical (IHC) CAMTA1 test showed that the tumor nucleus was positive, amplified by the envision method, 100×. (b) IHC CD31 test showed that the tumor nucleus was positive, amplified by the envision method, 100×. (c) IHC ERG test showed that the tumor nucleus was positive, amplified by the envision method, 100×. (d) Fluorescence in situ hybridization (FISH) detection showed that WWTR1-CAMTA1 fusion signal appeared in more than 50% of cells-the FISH method ×1000 (400×).
EXPLANATION FOR ADDITIONAL QUIZ QUESTIONS
Microscopic appearance: Tumor cells in the biopsy tissue were arranged in two ways: Adenoid structural area and solid area. A small amount of red blood cells could be seen.
In the cavity of the adenoid area, epithelioid tumor cells, conspicuous small nucleoli could be seen, and tumor cells had significant atypia. Short spindle cells could be seen in the solid area, some nuclei were large and biased. Moreover, some cells had cytoplasmic vacuoles, and a single red blood cell could be seen in individual cytoplasmic vacuoles.
No clear myxochondroid or no clear myxochondroid or hyaline matrix was found. IHC staining was as follows: Tumor cells did not express CKpan, TTF1, P40, and so on; so, epithelial cancer was not considered. Tumor cells expressed vascular markers such as CD31, and ERG positive; so, EHE specific antibody CAMTA1 was added and positive. T (1p36) (CAMTA1) gene translocation (+) was detected by FISH (Note: Red and green separation signals can be seen in >50% of tumor cells). Hence, we considered it as EHE, angiosarcoma differentiated in some areas with blood lakes.
The final diagnosis was EHE of the lung with angiosarcoma-like components.
DISCUSSION
EHE is a rare low-grade malignant angiogenic tumor that is most common in the lung and liver, always with multiple nodules.
EHE was first proposed by Weiss and Enzinger[1] in 1982 and was easily misdiagnosed morphologically as cancer. Its biological behavior was between epithelioid hemangioma and epithelioid angiosarcoma, which was defined as intermediate tumors. Along with the in-depth understanding of the disease, more studies[2,3] found that the tumor had a relatively high local recurrence rate (10–15%), metastasis rate (20–30%), and mortality (10–20%), which far exceeded the definition of rare recurrence and rare metastasis of intermediate tumors. Therefore, EHE was classified as a low-grade malignant tumor.[4] EHE often occurs in the lung and, or liver, typically as multiple nodules; however, it could occur in any body part, including bone, soft tissue, and heart.[5]
Morphologically, mucinous cartilage-like matrix or hyaline degeneration, the tumor cells were arranged in a cord or nest shape. The tumor cells were relatively uniform in size, round, and oval. The nuclear chromatin was consistent, and the nucleolus was not prominent. The cytoplasm was light eosinophilic, or the nucleus was biased. Moreover, vacuoles could be seen in some cytoplasm containing single or multiple red blood cells, which are called vesicular cells. It suggested the formation of a vascular lumen. Nuclear mitosis and necrosis were rare in classic EHE tumors.
Many studies have reported the morphological characteristics and diagnostic criteria of atypical EHE. The morphological criteria of atypical EHE vary in different studies, including tumor size, necrosis, nuclear grade, and threshold of the mitotic count. Anderson et al. reported a clinicopathological study of 52 cases of thoracic epithelioid malignant vascular tumors.[6] This showed epithelioid vascular tumors involving the chest divided into low-grade EHE, moderate EHE, and highly malignant epithelioid angiosarcoma with 4-year survival rates of 83%, 22%, and 9%, respectively. Studies have shown that the prognosis of moderate EHE was significantly worse than that of low-grade EHEs. In addition, survival analysis also showed that pleural involvement was a poor prognostic indicator. Similarly, the EHE of the pleura previously reported in our research group had a poor prognosis.[7] This patient had pleural involvement and pleural effusion. The examination report showed that the prognosis was poor. Deyrup et al.[2] performed a morphological risk assessment on the biological behavior of 49 cases of soft-tissue EHE. The risk model showed that a maximum tumor diameter >3 cm and mitosis >3/50 HPF were significantly correlated with poor patient prognosis. Therefore, the EHE could be divided into a high-risk group and a low-risk group. Shibayama et al.[3] scored 61 patients with EHE based on tumor size (i30 vs. >30 mm) and histological characteristics (typical vs. atypical). Survival analysis showed that the 5-year overall survival rates of the low (24 cases), medium (28 cases), and high (nine cases) risk groups were 100%, 81.8%, and 16.9%, respectively. Gong et al.[8] discussed the clinicopathological features of EHE, diagnosis, and differential diagnosis of eight cases of atypical EHE. They found that there were high-grade nuclei, active mitosis, solid flake growth mode, and tumor necrosis in the morphology of atypical EHE, and the biological behavior was more invasive. The follow-up results showed that there were six cases of metastasis, of which three cases died, and the prognosis was worse than that of classical EHE. Certain lineage changes in the morphology of EHE are significantly related to the prediction of patients. Therefore, how to accurately grade and guide the clinical treatment of EHEs will be the direction of future research.
EHE tumor cells express the vascular markers CD31, CD34, ERG, and FLI1. Cytokeratin can be expressed in 25% ~ 30% of cases, especially in biopsy specimens, which may be misdiagnosed as poorly differentiated carcinoma.[5] In addition, EHE highly expresses CAMTA1. EHE with vascular lacuna generally does not express CAMTA1 but is TFE3 positive. However, it should be noted that TFE3 immunohistochemistry is not completely specific. Both CAMTA1 and TFE3 were nuclear positive in this case, but CAMTA1 gene translocation was detected.
Atypical EHE and epithelioid angiosarcoma can cross and migrate morphologically. The application of IHC markers and the detection of specific fusion genes are very essential for differential diagnosis. Approximately 90% of EHE can produce t (1; 3) (p36.3; q23-25) ectopically, resulting in the WWTR1-CAMTA1 fusion gene, while about 5% of EHE contains the YAP1-TFE3 gene fusion. Studies have shown that EHE of ectopic fusion of the WWTR1 gene is rarely reported and mainly occurs in the heart.[3] The previous literature reported that epithelioid angiosarcoma could express the CAMTA1 marker, but the above fusion genes were not found.[6,8] Yang et al. compared the detection of CAMTA1 expression in cases of EHE and other vascular tumors using FISH and IHC. The sensitivity and specificity of IHC were 85.7% and 100%, respectively, whereas the sensitivity and specificity of FISH were both 100%.[9] Therefore, the detection of fusion genes is very important to distinguish EHE and epithelioid angiosarcoma.
DIFFERENTIAL DIAGNOSIS
Pulmonary EHE intersects with poorly differentiated adenocarcinoma, epithelioid angiosarcoma, and epithelioid hemangioma, which need to be differentiated according to microscopic morphology, IHC, and molecular detection.
Poorly differentiated adenocarcinoma
Epithelial cells or scattered vacuolar cells with nest-like arrangement in the lung EHE are easily misdiagnosed as poorly differentiated adenocarcinoma. In contrast to EHE, tumor cells have obvious atypia and obvious mitotic images. IHC shows CK pan positivity and vascular marker negativity. Therefore, if CK pan is negative in poorly differentiated tumors, it is suggested to add antigenic markers for identification.
Epithelioid angiosarcoma
In contrast to EHE, epithelioid angiosarcoma is more prone to interstitial hemorrhage, blood lake formation, papillary or fissure growth of tumor cells, obvious large nucleoli, active mitotic imaging, and so on. In addition to classic EHE, vascular lacunar-like structures were found in some areas, with significant atypia and mitotic images. However, both CAMTA1 IHC staining and FISH probes were positive. Therefore, this case was diagnosed as EHE with epithelioid angiosarcoma transformation in some areas.[10-13]
Epithelioid hemangioma
There are few cells in the nest of epithelioid hemangioma with vacuolar cytoplasmic vacuoles and red blood cells in the nest of epithelioid hemangioma, which suggests microvascular formation. Eosinophil infiltration can be seen around erythrocytes. These conditions are easily misdiagnosed as EHE. However, most EHE had no clear formation of vascular lacuna, the stromal was often mucinous cartilage-like, and most of them had no eosinophil infiltration.[14-20]
Epithelioid sarcoma
It often occurs in the limbs with the growth of tumor cells in multiple nodules under the microscope, and necrosis often occurs in the center of the nodule. In contrast to EHE, epithelioid sarcoma cells can express epithelial markers such as AE1/AE3 and epithelial membrane antigen (EMA), and the expression of INI1 is often absent.[21-24]
TREATMENT AND PROGNOSIS
EHE has limited experience in treatment, and more radical treatment may be needed. Therefore, surgical resection should ensure that the cutting edge is negative, supplemented by radiotherapy, chemotherapy, and targeted therapy if necessary.[25] This patient is currently undergoing the third PC chemotherapy regimen. EHE in the lung will indicate a poor prognosis if accompanied by pleural effusion or spindle tumor cell components. In this case, pleural effusion was obvious, and an angiosarcoma area appeared, which may be related to the poor prognosis of the patient.
A rare case of epithelioid hemangioendothelioma transforming from some areas to epithelioid angiosarcoma was discussed. Under a microscope, except for the classic EHE area, vascular lacuna formation was seen in some areas. Tumor cell atypia, mitotic imaging, and proliferative activity were significantly higher than those in the EHE area. IHC techniques and FISH detection confirmed that CAMTA1 gene translocation occurred in this case. Vascular lacunae and tumor cell atypia were found in some areas, suggesting that they may be transformed into epithelioid angiosarcoma.
A case of EHE with epithelioid angiosarcoma of the spine is reported in the literature,[26] but there was no genetic confirmation. From atypical morphology to final accurate diagnosis benefitting from immunohistochemistry and molecular diagnosis, we learned the development process of disease progression. We should not only recognize the classic morphology but also find clues regarding the classic morphology in atypical patients to provide a basis for accurate clinical diagnosis and treatment.
FOLLOW-UP AND PROGNOSIS
The patient received chemotherapy (albumin paclitaxel 420 mg/dL, carboplatin 680 mg/dL) combined with bevacizumab (400 mg/dL) injection intravenously on January 26, 2022. At present, the fourth course of treatment has been carried out. The current situation was stable.
SUMMARY
From atypical morphology to final accurate diagnosis benefitting from immunohistochemistry and molecular diagnosis, we learned the development process of disease progression. We should not only recognize the classic morphology but also find clues regarding the classic morphology in atypical patients to provide a basis for accurate clinical diagnosis and treatment.
ABBREVIATIONS
CT - Computed Tomography
EHE - Epithelioid Hemangioendothelioma
FISH - Fluorescence in situ Hybridization
HE - Hematoxylin-Eosin staining
IHC - Immunohistochemical
NSCLC - Non-small Cell Lung Cancer.
AUTHOR CONTRIBUTIONS
The manuscript was approved by all authors for publication. Substantial contributions to the conception, drafting the work and the acquisition, analysis of data for the work: S Z; Reviewing it critically for important intellectual content, and interpretation of data for the work: C W; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy, complete the Fish for the work, and interpretation of data for the work: W W;Design of the work, reviewing it critically for important intellectual content, and resolved any part of the work appropriately: L H. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript. All authors have participated sufficiently in the work and agreed to be accountable for all aspects of the work.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The Institutional Review Board of Shanghai Pulmonary Hospital approved this retrospective study (IRB NO. K22-081Y).
Written informed consent was obtained from all the participants prior to the publication of this study.
ACKNOWLEDGMENT
The authors would like special thanks to Professor Wang Jian of the Cancer Hospital Affiliated to Fudan University for his guidance and help in diagnosis and to Dr. Bai Qianming, Dr. Xue Tian, and Dr. Ren Min of the molecular laboratory of the Cancer Hospital Affiliated to Fudan University.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of cytojournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through the automatic online system.
FUNDING
Supported by the National Natural Science Foundation of China (82272766); Clinical Research Foundation of Shanghai Pulmonary Hospital; Shanghai Municipal Health Commission (20194Y0129).
References
- Epithelioid hemangioendothelioma: A vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970-81.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelioid hemangioendothelioma of soft tissue: A proposal for risk stratification based on 49 cases. Am J Surg Pathol. 2008;32:924-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinicopathologic characterization of epithelioid hemangioendothelioma in a series of 62 cases: A proposal of risk stratification and identification of a synaptophysin-positive aggressive subset. Am J Surg Pathol. 2021;45:616-26.
- [CrossRef] [PubMed] [Google Scholar]
- Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240-2.
- [CrossRef] [Google Scholar]
- Atypical primary epithelioid hemangioendothelioma of the heart. J Thorac Oncol. 2006;1:188-9.
- [CrossRef] [PubMed] [Google Scholar]
- Thoracic epithelioid malignant vascular tumors: A clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol. 2015;39:132-9.
- [CrossRef] [PubMed] [Google Scholar]
- Often misdiagnosed primary pleural epithelioid hemangioendothelioma: A clinicopathological analysis of five cases. Zhonghua Bing Li Xue Za Zhi. 2020;49:1288-93.
- [Google Scholar]
- Atypical epithelioid hemangioendothelioma: A clinicopathological analysis of eight cases. Zhonghua Bing Li Xue Za Zhi (Chin J Pathol). 2019;48:620-5.
- [Google Scholar]
- Fluorescence in situ hybridization for WWTR1-CAMTA1 has higher sensitivity and specificity for epithelioid hemangioendothelioma diagnosis. Am J Transl Res. 2020;12:4561-8.
- [Google Scholar]
- A case of primary epithelioid angiosarcoma of the tonsil in a patient with recurrent hepatocellular carcinoma with pulmonary metastasis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (Chin J Otorhinolaryngol Head Neck Surg). 2021;56:869-70.
- [Google Scholar]
- Primary pleural epithelioid angiosarcoma manifesting as a loculated hemothorax: A case report and literature review focusing on CT findings. Radiol Case Rep. 2021;16:3072-5.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelioid angiosarcoma Causing spinal cord compression. Cureus. 2021;13:e14325.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelioid angiosarcoma arising after an endovascular aneurysm repair: Case report and review of the literature. Acta Clin Belg. 2021;76:397-401.
- [CrossRef] [PubMed] [Google Scholar]
- The clinicopathologic features and differential diagnosis of ocular Kimura disease and epithelioid hemangioma. Zhonghua Yan Ke Za Zhi. 2021;57:689-95.
- [Google Scholar]
- Epithelioid hemangioma involving large arteries in the skin. J Cutan Pathol. 2022;49:377-80.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelioid hemangioendothelioma and epithelioid hemangioma: Pazopanib as a potential salvage therapy. Case Rep Oncol. 2021;14:309-17.
- [CrossRef] [PubMed] [Google Scholar]
- Previously unreported permanent tattoo-associated angiolymphoid hyperplasia with eosinophilia/ epithelioid hemangioma. Pathol Int. 2021;71:219-21.
- [CrossRef] [PubMed] [Google Scholar]
- Concurrent epithelioid hemangioma and diffuse cavernous hemangioma in the rectum clinically mimicking a malignant tumor: A case report. Virchows Arch. 2021;479:847-51.
- [CrossRef] [PubMed] [Google Scholar]
- Transarticular epithelioid hemangioma of the ankle-a case of a rare vascular neoplasm. Skeletal Radiol. 2021;50:1263-70.
- [CrossRef] [PubMed] [Google Scholar]
- primary epithelioid hemangioma of the central nervous system: A case report and review of the literature. J Neuropathol Exp Neurol. 2021;80:717-9.
- [CrossRef] [PubMed] [Google Scholar]
- Sclerosing epithelioid fibrosarcoma: A distinct sarcoma with aggressive features. Am J Surg Pathol. 2021;45:317-28.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelioid sarcoma with retained INI1 (SMARCB1) expression. Histopathology. 2021;78:464-6.
- [CrossRef] [PubMed] [Google Scholar]
- Real-world outcomes of patients with locally advanced or metastatic epithelioid sarcoma. Cancer. 2021;127:1311-7.
- [CrossRef] [PubMed] [Google Scholar]
- Epithelioid sarcoma arising in a long-term survivor of an atypical teratoid/rhabdoid tumor in a patient with rhabdoid tumor predisposition syndrome. Pediatr Dev Pathol. 2021;24:164-8.
- [CrossRef] [PubMed] [Google Scholar]
- A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosome Cancer. 2011;50:644-53.
- [CrossRef] [PubMed] [Google Scholar]
- Spinal epithelioid hemangioendothelioma with epithelioid angiosarcomatous areas. Skeletal Radiol. 2005;34:745-9.
- [CrossRef] [PubMed] [Google Scholar]







