Translate this page into:
Perihepatic cystic mass: Zebra or horse?
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
A 67-year-old woman status post a right partial nephrectomy 6 years prior presented with a 3.5 cm fluorine-18-fluorodeoxy-D-glucose (FDG)-avid cystic mass in the right renal fossa and a 2.8 cm non-FDG-avid perihepatic cystic mass. Fine-needle aspiration (FNA) was performed on each lesion, and both had similar cytomorphology [Figure 1].
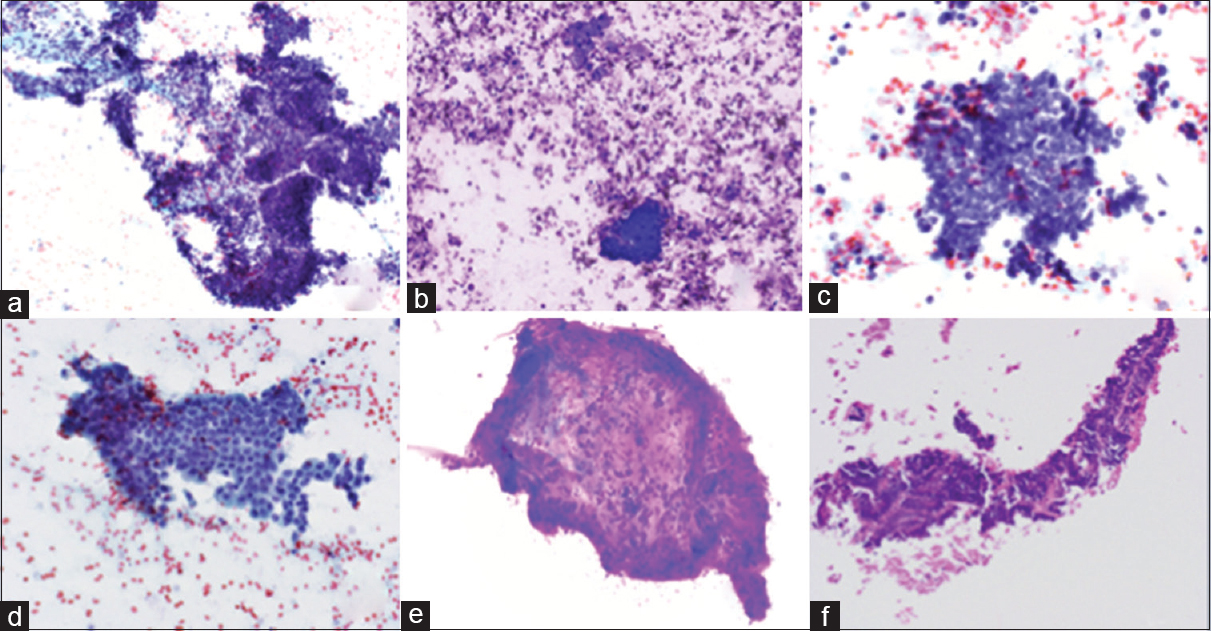
- Photomicrographs of right renal fossa fine-needle aspiration smears. (a) Tissue fragment with stromal spindle cells and cluster of basaloid cells with scant cytoplasm (Papanicolaou, ×200). (b) Cluster of basaloid cells with scant cytoplasm (Diff-Quik, ×400). (c) High power view of cells with scant basaloid cytoplasm (Papanicolaou, ×600) (d) Sheet of bland cells with a moderate amount of cytoplasm (Papanicolaou, ×400). (e) Stromal fragment with spindle cells and metachromatic myxoid stroma (Diff-Quik, ×200). (f) Cell block with epithelial cells with metachromatic stromal material (H and E, ×200)
QUESTION
Q1: What is your interpretation?
-
Metastatic small cell carcinoma
-
Adult Wilms’ tumor (WT)
-
Primitive neuroectodermal tumor
-
Diffuse large B-cell lymphoma
-
Renal cell carcinoma with sarcomatoid change.
ANSWER
The correct interpretation is:
B. Adult WT.
Adult WT is the best answer selection. Among the above answers, only the adult WT and renal cell carcinoma with sarcomatoid change have both an epithelial component and a stromal component. Between these two possibilities, the most appropriate answer is the adult WT because the epithelial component is more consistent with this diagnosis than a renal cell carcinoma. Please see the differential diagnosis section below. In addition, the patient had a partial nephrectomy 6 years prior that was diagnosed as adult WT [Figure 2]. Images of the current computed tomography (CT) scan can be viewed in Figure 3.
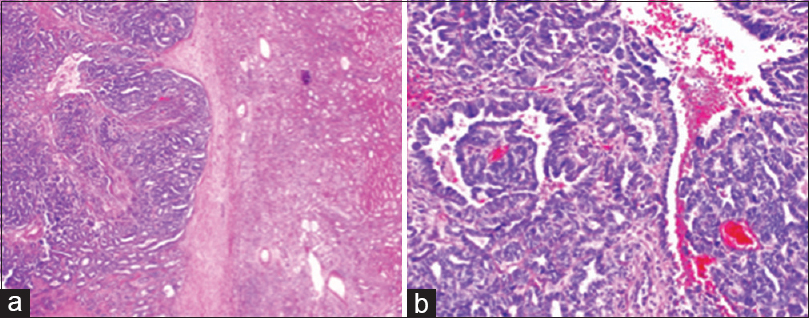
- Photomicrograph of final histopathology from right partial nephrectomy 6 years before fine-needle aspiration. (a) Low power view of the epithelial and stromal components with adjacent uninvolved renal parenchyma (H and E, ×50). (b) High power view of predominately the epithelial component (H and E, ×200)
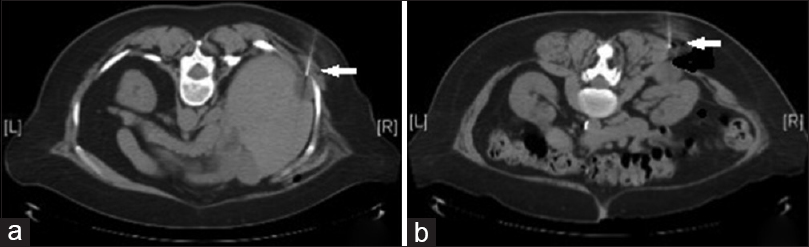
- Computed tomography images (a) showing 2.8 cm perihepatic cystic mass, indicated by the arrow (b) showing 3.5 cm cystic mass in the right renal fossa, indicated by the arrow
The patient underwent surgical resection of both cystic masses with negative margins approximately 1 month after the initial diagnosis by FNA. Radiation therapy was subsequently administered. The patient is doing well 10 months after the surgical procedure, and follow-up CT and positron emission tomography scans performed at 5 and 9 months after the surgical procedure were negative for recurrent disease.
Pediatric WT, also known as nephroblastoma, is the most common pediatric renal malignancy and 98% of WTs occur in children younger than 10 years of age.[12] In contrast, adult WT is a rare tumor with <300 cases reported in the English literature, and it is defined as a WT occurring in a patient older than 15 years of age.[2345]
In the pediatric population, FNA is often used to preoperatively diagnose WT. This allows for neoadjuvant chemotherapy to reduce tumor burden and decrease the likelihood of spillage during subsequent surgical excision. Although there is an emerging role for FNA in the diagnosis of renal cancers, FNA is not often used for the initial diagnosis of renal masses in adults. Instead, renal masses typically are evaluated using imaging modalities, and then, treatment is carried out without prior FNA or core biopsy. Thus, initial FNA diagnosis of adult WT when it presents as only a renal mass is exceptionally rare.[356] However, adult WT typically presents at a more advanced clinical stage than pediatric WT, and thus, FNA has a potential role for initial diagnosis when it has spread beyond the kidney.[3]
WT is composed of blastemal, epithelial, and stromal elements in varying proportions, resulting in monophasic, biphasic, or triphasic tumors.[1]
Blastemal elements are composed of monomorphic small, round cells in high nuclear-cytoplasmic (N: C) ratios, arranged as single cells, sheets, cords, cohesive clusters, and rosettes. Blastemal cells are 1.5–2 times the size of a lymphocyte with scant basophilic cytoplasm and dark blue nuclei with fine chromatin, and nuclear molding.[1378]
The epithelial cells are larger than the blastemal cells with larger nuclei and more cytoplasm. The cytoarchitecture is variable and includes cohesive clusters, sheets, glands, tubules, and glomeruloid bodies. The epithelial component may be associated with metachromatic basement membrane-like material.[18]
The stromal elements are typically composed of spindle cells with a scant amount of cytoplasm within a metachromatic myxoid stroma, but other stromal elements can occur such as smooth and skeletal muscle, cartilage, bone, adipose tissue, and neuroendocrine tissue.[18]
In addition, the FNA biopsy of a WT may be bloody due to high tumor vascularity.[1]
ADDITIONAL QUESTIONS
Q2: Which of the following renal tumors are positive for the immunocytochemical stain WT1 (select all that apply)?
-
WT
-
Clear cell renal cell carcinoma
-
Renal medullary carcinoma
-
Metanephric adenoma.
Q3: Which of the following genetic abnormalities are most commonly seen in adult WT?
-
Complex genetic alterations
-
No known genetic alterations
-
Translocations involving the gene WT1
-
Epigenetic alterations of chromosome 11p15.
Q4: What are the morphologic features of anaplasia in WT?
-
Nuclei three times larger than other nuclei of the same cell type
-
Hyperchromatic nuclei
-
Abnormal mitoses
-
All of the above.
ANSWERS TO ADDITIONAL QUIZ QUESTIONS
Q2: A, D; Q3: A; Q4: D.
BRIEF REVIEW OF THE TOPIC
The clinical presentation of adult WT can include hematuria, malaise, flank pain, anorexia, and weight loss.[3]
Adult WT has a worse prognosis than pediatric WT. Children have a 10% rate of metastasis and 30% present with stage III or IV disease. In contrast, adults have a 29% rate of metastasis and 50% present with stage III or IV disease.[25]
Anaplasia is rare in adult WT. It is defined by three criteria: (1) The nuclei are three times larger than the other nuclei of the same cell type; (2) The nuclei are hyperchromatic; (3) Abnormal mitoses are present. In addition, anaplasia can have intracytoplasmic hyaline globules, but this is a nonspecific finding. Anaplasia in WT can be diffuse or focal, and diffuse anaplasia is associated with a poor prognosis.[1]
Cell block preparation is vital for the interpretation of FNA because it allows immunocytochemical, cytogenetic, and molecular assays to be performed on aspirated material. In addition, FNA rapid on-site evaluation can select cases that might benefit from these studies, and in these cases, a more robust cell block can be obtained by obtaining additional FNA passes. Lesional cells in WT stain with vimentin, cytokeratin (focal), epithelial membrane antigen (EMA), desmin, smooth muscle actin, PAX8, and WT1 are negative for CD45, S100, and FLI-1.[1359]
Pediatric and adult WTs are different at the genetic level. The genetic alterations of pediatric WT include WT1, CTNNB1, and WTX genes or epigenetic alterations of 11p15. Adult WTs typically have complex genetic alterations, and they are similar to those seen in anaplastic WT.[348] The only recurrent cytogenetic alteration in adult WT is a 7q isochromosome, i(7)(q10).[4]
The differential diagnosis of WT includes other small, round, blue cell tumors. In the adult population, this includes small cell carcinoma, lymphoma, renal cell carcinoma with a sarcomatoid change, Ewing sarcoma/primitive neuroectodermal tumor, synovial sarcoma, and metanephric adenoma.[17]
The treatment of adult WT depends on the stage of the tumor and can include surgery, radiation, and chemotherapy.[5]
DIFFERENTIAL DIAGNOSIS
Small cell carcinoma:
-
Hyperchromatic nuclei with fine chromatin
-
No prominent nucleoli, clumping, or chromocenters
-
High N: C ratio
-
Scant delicate cytoplasm
-
Nuclear streaming/crush artifact
-
Degenerative changes and necrosis
-
Mitoses
-
Paranuclear blue blobs
-
Immunocytochemically positive for chromogranin, synaptophysin, CD56, cytokeratin, and thyroid transcription factor-1.
Lymphoma:
-
Cellular specimen
-
Discohesive cells
-
Cells are monomorphic or have a limited spectrum of differentiation
-
Lymphoglandular bodies
-
Immunocytochemical reactions vary with the type of lymphoma.
Renal cell carcinoma with sarcomatoid change:
-
Renal cell carcinoma cytomorphology varies depending on the subtype (the description below is for clear cell renal cell carcinoma, the most common subtype)
-
Short papillary groups or floral groups
-
Epithelial groups with attached metachromatic basement membrane
-
Low N:C ratio
-
Abundant wispy blue cytoplasm on Papanicolaou stain
-
-
Abundant dense cytoplasm with numerous fine well-defined vacuoles on Diff-Quik stain
-
Higher grade tumors may have coarse granular cytoplasm and higher N:C ratios
-
Nuclei vary depending on the grade
-
Low grade: Small, uniform, bland nuclei with smooth nuclear borders, fine chromatin, and inconspicuous nucleoli
-
High grade: Large, bizarre nuclei with irregular nuclear borders, coarse chromatin, cherry-red macronucleoli.
-
-
Sarcomatoid component is rare
-
Dimorphic population (epithelial and sarcomatoid)
-
Three patterns: Spindle, strap (rhabdomyosarcoma-like), and pleomorphic cells
-
Clear cell renal cell carcinoma is the most commonly associated epithelial component associated with sarcomatoid change; however, some series have reported chromophobe renal cell carcinoma as the most common epithelial component.[10]
-
-
Immunocytochemical expression varies depending on the subtype; however, most renal cell carcinomas, including sarcomatoid renal cell carcinomas, express PAX8.[11]
Metanephric adenoma:[12]
-
Cellular aspirates
-
Small uniform oval to round cells with high N: C ratio
-
Fine delicate chromatin and minute or absent nucleoli
-
Small to large tightly packed clusters of cells and short papillae
-
Rare tubules, rosettes, and glomeruloid-like structures
-
Rare psammoma bodies
-
No atypia, pleomorphism, necrosis, and mitoses
-
Immunocytochemically positive for WTl and CD57.
Ewing sarcoma/primitive neuroectodermal tumor:
-
Single cells, clusters of cells, or rosettes
-
Small cells with a high N: C ratio
-
Nuclear molding
-
Wispy cytoplasmic tags
-
Immunocytochemically positive for NSE, FLI-1, and CD99.
Synovial sarcoma:
-
Biphasic pattern (epithelial and spindle cells)
-
Spindle cells usually predominate
-
Spindle cells:
-
Uniform, short spindle cells with irregular nuclear outlines and longitudinal folds
-
Scant cytoplasm
-
Nucleoli can be conspicuous and multiple
-
Significant atypia is absent.
-
-
Epithelial cells:
-
Gland-like acinar spaces, solid cords, and nests
-
Round nuclei with smooth membranes, fine even chromatin, minimal hyperchromasia, and conspicuous nucleoli
-
Abundant finely vacuolated cytoplasm that may contain mucin.
-
-
Immunocytochemically positive for cytokeratin, EMA, vimentin (>80% spindle, <30% epithelial), BerEp4 (90%), calretinin (70%), CD99 (60%), and S100 (30%).
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Alan Marcus: Drafted the article and gave final approval; June Koizumi: Contributed to conception and design and gave final approval; Brian Robinson: Revised the article and gave final approval; Tamara Giorgadze: Contributed to conception and design and gave final approval.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of the institution associated with this study.
LIST OF ABBREVIATIONS (In alphabetic order)
FNA - Fine needle aspiration
WT - Wilms’ tumor.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- DeMay RM, ed. The Art and Science of Cytopathology (2nd ed). Chicago, IL: ASCP Press; 2012.
- Adult Wilms’ tumor metastatic to the lung: Endobronchial ultrasound-guided fine needle aspiration biopsy. Diagn Cytopathol. 2014;42:950-5.
- [Google Scholar]
- High-resolution genomic profiling of an adult Wilms’ tumor: Evidence for a pathogenesis distinct from corresponding pediatric tumors. Virchows Arch. 2011;459:547-53.
- [Google Scholar]
- Adult Wilms’ tumor – Diagnosis and current therapy. Cent European J Urol. 2013;66:39-44.
- [Google Scholar]
- Metanephric adenoma vs. Wilms’ tumor: A report of 2 cases with diagnosis by fine needle aspiration and cytologic comparisons. Acta Cytol. 2007;51:464-7.
- [Google Scholar]
- Wilms’ tumor in adults: Aspiration cytology and cytogenetics. Diagn Cytopathol. 2002;26:99-103.
- [Google Scholar]
- Undifferentiated tumor: True identity by immunohistochemistry. Arch Pathol Lab Med. 2008;132:326-48.
- [Google Scholar]
- Sarcomatoid renal cell carcinoma: A comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17:46-54.
- [Google Scholar]
- Immunohistochemical diagnosis of renal neoplasms. Arch Pathol Lab Med. 2011;135:92-109.
- [Google Scholar]
- Cytologic and cytogenetic analysis of metanephric adenoma of the kidney: A report of two cases. Am J Clin Pathol. 1997;108:544-9.
- [Google Scholar]







