Translate this page into:
Cytologic diagnosis of a rare soft-tissue lesion: Think beyond the usual

*Corresponding author: Sanjay Gupta, Division of Cytopathology, ICMR-National Institute of Cancer Prevention and Research, Noida, Uttar Pradesh, India. sanjaydr17@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Kumar N, Gupta R, Gupta S. Cytologic diagnosis of a rare soft-tissue lesion: Think beyond the usual. CytoJournal 2020;17:24.
A 52-year-old man presented with a 2 cm non-tender swelling on the left arm for the past 4 years with a rapid increase in size over the past 3 months. There was no history of prior trauma, pain in the swelling, or restricted movements of the arm. The swelling was not fixed to the underlying bone or overlying skin. Plain radiograph showed an irregular soft-tissue opacity at mid-arm level with few specks of calcification and well-maintained bony cortices. With a clinical diagnosis of a soft-tissue tumor, fine-needle aspiration (FNA) was performed using 22G needle and smears were stained using Giemsa stain. The cytomorphological features are shown in Figure 1.
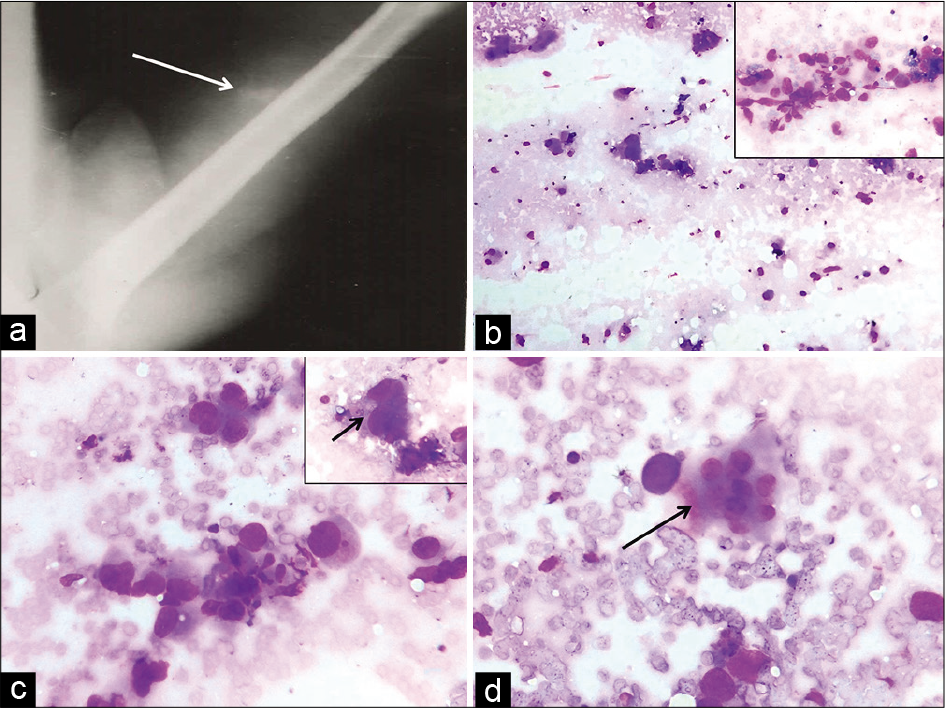
- Plain radiograph demonstrates a soft-tissue lesion with specks of calcification (arrow) and intact underlying cortices of the humerus (a). Fine-needle aspiration smears showing low cellularity (b, Giemsa, ×10) and few groups of round to polygonal cells in the inset (Giemsa, ×40). Prominent nuclear pleomorphism (c, Giemsa, ×40), better seen in inset (arrow) and occasional multinucleated osteoclast-like giant cell marked by arrow (d, Giemsa, ×40) are seen.
QUESTION
What is your cytological interpretation?
Pleomorphic liposarcoma
Pleomorphic leiomyosarcoma
Myositis ossificans
Osteosarcoma of soft tissue
The correct cytological interpretation is:
d. Osteosarcoma of soft tissue.
EXPLANATION
Cytological smears were paucicellular showing groups of pleomorphic round to polygonal cells with anisonucleosis and nuclear hyperchromasia. Occasional multinucleated giant cells were also seen. The cytological features were indicative of a pleomorphic sarcomatous tumor. However, in conjunction with the radiological features of a soft-tissue lesion with internal specks of calcification, osteosarcoma appeared to be the likely possibility. A diagnosis of pleomorphic liposarcoma requires identification of characteristic lipoblasts with hyperchromatic central nucleus indented by multiple cytoplasmic vacuoles. In pleomorphic leiomyosarcoma, fascicles of spindle cells with elongated blunt-ended nuclei are seen admixed with stripped nuclei and pleomorphic cells. Although myositis ossificans may mimic a sarcomatous tumor, it usually displays milder nuclear pleomorphism and many more multinucleated giant cells.
The lump was excised in toto and submitted for histopathological examination. We received a soft-tissue specimen with attached fibro-fatty tissue measuring 2.5 cm in diameter. The specimen gave focal gritty sensation on sectioning through it. Cut section of the specimen showed a gray-white circumscribed lesion, 2 cm in diameter. There were no areas of hemorrhage or necrosis within the lesion.
Histopathologic examination of the lesion showed a tumor composed of pleomorphic round to polygonal cells with bizarre nuclear shapes and frequent mitotic figures [Figure 2a]. Abundant neoplastic osteoid was seen surrounding the cells without any interposition of cartilaginous component between the cells and osteoid [Figure 2b]. Focal necrosis was also seen in the tumor. The histomorphological features in conjunction with the radiological findings were consistent with the diagnosis of an extraskeletal osteosarcoma (EOS) of the left arm. Since the detailed workup of the patient could not be carried out at our center due to the paucity of resources, the patient was referred to an oncology center for further investigations and management. The diagnosis was confirmed to be an EOS and the patient was managed with aggressive chemotherapy but was subsequently lost to follow up.
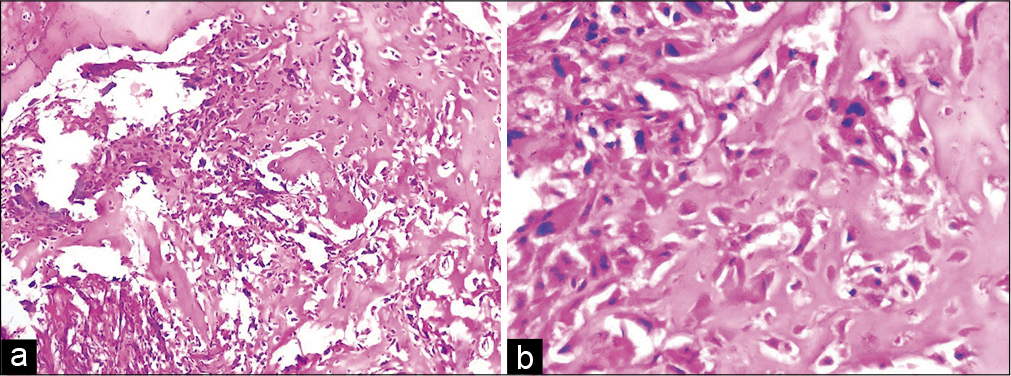
- Histological photomicrographs showing a cellular tumor (a, H&E, ×10) with prominent osteoid production (b, H&E, ×40). The osteoid is directly in contact with the pleomorphic tumor cells.
ADDITIONAL QUIZ QUESTIONS
Q1. What proportion of soft-tissue sarcomas is constituted by EOS?
3–4%
5–6%
<1%
8–10%
Q2. What is the most common age group affected by EOS?
2nd–4th decade
3rd–5th decade
5th–7th decade
6th–8th decade
Q3. The cytologic differential diagnoses for EOS include the following except:
Malignant fibrous histiocytoma
Pleomorphic leiomyosarcoma
Embryonal rhabdomyosarcoma
Myositis ossificans
Answers to the quiz questions:
Q1. The correct answer is C (<1%)
Q2. The correct answer is D (6th–8th decade)
Q3. The correct answer is C (embryonal rhabdomyosarcoma).
The explanations of these questions follow in the next section.
DISCUSSION
EOS, a rare soft-tissue tumor, accounts for about 1% of all malignant lesions of the soft tissues.[1] Similar to the skeletal osteosarcomas, EOS occurs more frequently in males as compared to females, with most common location being lower extremity followed by upper limbs, retroperitoneum, trunk, and head and neck.[2] However, in contrast to the skeletal variant, EOS is detected in the sixth to eight decades of life.[2] Our patient also was a male in the sixth decade of his life with a lesion in the left arm.
In addition to the rarity of EOS, the non-specific nature of clinical and radiological features of this lesion usually precludes the optimum utility of FNA in its pre-operative diagnosis.[3] Although occasional cases of EOS can display spotty to massive calcification or ossification on radiographs, the radiologic diagnosis in extraskeletal location like soft tissue is usually not straightforward due to overlapping features in other sarcomas.[4] In the present case, the soft-tissue location of the mass prompted a radiologic impression of soft-tissue tumor despite the presence of specks of calcification.
The cytologic features of EOS have been described relatively infrequently as single cases or small case series. Nicol et al. reported four cases of EOS, none of which could be reported as osteosarcoma in the primary location on FNA. The authors stressed the lack of matrix material in FNA smears from EOS, as also the difficulty in characterizing the material as dense collagen, osteoid, or chondroid in smears.[5] Nagira et al. described the cytological findings of scrape smears from the excised primary tumor as well as FNA of the recurrent tumor in a patient diagnosed as EOS. In their case, FNA smears revealed moderate cellularity with pleomorphic sarcoma-like picture. In addition, abundant fragments of acellular matrix material were seen closely associated with the atypical cells. However, the authors categorically emphasized the inability to identify this material as osteoid or dense collagen.[1] A few other cytologic reports of this entity have been on scrape or imprint cytology.[6] Similar to the report by Nicol et al., FNA diagnosis of EOS could not be rendered in our case due to multiple reasons: (1) low cellularity; (2) presence of pleomorphic cells and osteoclast-type giant cells suggesting a soft-tissue tumor; (3) absence of matrix material; and (4) the purely soft-tissue location of the tumor.
The various cytologic differential diagnoses to be considered in such a pleomorphic soft-tissue tumor would include malignant fibrous histiocytoma, pleomorphic variants of liposarcoma, leiomyosarcoma, or rhabdomyosarcoma.[5] Cytological differentiation of these tumors from each other depends largely on the identification of the characteristic features like lipoblasts in liposarcoma or cross-striations in rhabdomyosarcoma, which may be present very focally in the aspiration smears. Cases of EOS that shows matrix material admixed with atypical cells on aspiration cytology should be differentiated from myositis ossificans, extraskeletal chondrosarcoma, and synovial sarcoma. Smears from myositis ossificans usually display benign spindle cells along with multinucleated giant cells and matrix material.[7] Myxoid and mesenchymal chondrosarcoma show uniform round cells in either myxoid matrix or compact clusters with hyaline cartilage.[8,9] Radiologic investigations such as magnetic resonance imaging or computed tomography scan are often helpful in the diagnosis of mesenchymal chondrosarcoma.[1]
The histological features of EOS are similar to their skeletal counterparts with subtypes such as osteoblastic, chondroblastic, fibroblastic, telangiectatic, and small cell variants.[5] Although the clinical behavior of EOS is not well defined due to the rarity of the lesion, a recent study reported that patients with chondroblastic variants had a more favorable prognosis than those with osteoblastic differentiation.[2] In view of the rarity of EOS, there is a lack of consensus regarding the appropriate management algorithm for this tumor. However, there is growing evidence of the benefit of an aggressive chemotherapeutic regime.[10]
SUMMARY
EOS, a rare entity, should be considered in the cytologic differential diagnosis of subcutaneous or soft-tissue masses with calcifications apparent radiologically and cytology smears displaying pleomorphic sarcoma-like picture, with or without matrix material. Close clinical, radiologic, and cytohistologic correlation assists in arriving at a precise diagnosis and allowing appropriate management.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare no conflict of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors have contributed significantly and agree with the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
The authors have taken permission from institutional review board for publication of this manuscript.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
EOS – Extraskeletal osteosarcoma
FNAC – Fine needle aspiration cytology.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
References
- Scrape and Fine-needle aspiration cytology of extraskeletal osteosarcoma. Diagn Cytopathol. 2002;27:177-80.
- [CrossRef] [PubMed] [Google Scholar]
- A review of 40 patients with extraskeletal osteosarcoma. Cancer. 1995;76:2253-9.
- [CrossRef] [Google Scholar]
- Radiologic evaluation of soft tissue tumors In: Enzinger FM, Weiss SW, eds. Soft Tissue Tumors (3rd ed). St. Louis: C. V. Mosby; 1995. p. :39-88.
- [Google Scholar]
- Fine-needle aspiration biopsy of skeletal versus extraskeletal osteosarcoma. Cancer. 1998;84:176-85.
- [CrossRef] [Google Scholar]
- Extraskeletal osteogenic sarcoma of the mediastinum. Arch Pathol Lab Med. 1989;113:430-3.
- [Google Scholar]
- Fine-needle aspiration biopsy cytology of myositis ossificans. Mod Pathol. 1994;7:23-5.
- [Google Scholar]
- The diagnosis of extraskeletal myxoid chondrosarcoma by fine needle aspiration. Cytopathology. 1995;6:273-6.
- [CrossRef] [PubMed] [Google Scholar]
- Mesenchymal chondrosarcoma of the retroperitoneum, Report of a case diagnosed by fine needle aspiration biopsy with immunohistochemical, electron microscopic demonstration of S-100 protein in undifferentiated cells. Acta Cytol. 1995;39:1237-43.
- [Google Scholar]
- Extraskeletal osteosarcoma has a favourable prognosis when treated like conventional osteosarcoma. J Cancer Res Clin Oncol. 2005;131:520-6.
- [CrossRef] [PubMed] [Google Scholar]







