Translate this page into:
Cytomorphology of mesenchymal lesions in a tertiary care centre and its correlation with histopathology

*Corresponding author: Sana Ahuja, Department of Pathology, Vardhman Mahavir Medical College and Safdarjung Hospital, New Delhi, India. sanaahuja11@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Shahab J, Maheshwari R, Singh M, Sharma R, Ahluwalia C, Zaheer S, et al. Cytomorphology of mesenchymal lesions in a tertiary care centre and its correlation with histopathology. CytoJournal 2023;20:36.
Abstract
Objectives:
Fine needle aspiration cytology (FNAC) is a minimally invasive method for sampling a heterogenous lesion. It is one of the first-line investigations in the evaluation of soft tissue tumours. However, the heterogeneity of mesenchymal lesions pose a challenge to the cytological diagnosis. The present study aims at evaluation of the cytomorphological findings of mesenchymal lesions on FNAC along with their histopathological correlation.
Material and Methods:
This was a retrospective study conducted over a period of 1 year from January 2021 to January 2022. All the patients of cytologically diagnosed mesenchymal lesions on their FNA aspirates were included in the study. Cytomorphology of the May Grunwald Giemsa and Papanicolaou stained slides were examined and correlated with clinical and histopathological details wherever available.
Results:
Out of the 90 patients, 69 (76.7%) were males and 21 (23.3%) were females. Maximum number of cases were in 4–5th decade of life. The lower limb was found to be the most common site (57.8%) for the mesenchymal lesions. Majority of the cases on FNA in our study were found to be benign – 79 (87.8%), while only 11 cases were malignant (12.2%). On cytological examination, spindle cell lesions were most common followed by adipocytic lesions. Based on the cyto-histological correlation, sensitivity, specificity, negative predictive value, positive predictive value and diagnostic accuracy of FNAC in diagnosis of mesenchymal lesion was 78.6%, 100%, 92.5%, 100% and 94.1% respectively.
Conclusion:
FNAC is a simple and minimally invasive tool that plays an important role in triaging patients with good specificity and sensitivity.
Keywords
Mesenchymal
Fine needle aspiration cytology
Spindle
Neural
Adipocytic
INTRODUCTION
Mesenchymal lesions are a common occurrence in the surgical out patient department. These lesions are categorized based on their differentiation as to which adult tissue they resemble. Malignant mesenchymal lesions are <1% of all malignant neoplasms. Benign mesenchymal lesions are 100 times more common as compared to the malignant mesenchymal lesions.[1] Fine needle aspiration cytology (FNAC) is a minimally invasive method for sampling a heterogenous lesion. FNAC is cost effective and does not compromise the tissue planes in case of a subsequent excision. Although FNAC is not widely accepted as the main diagnostic modality, it plays an important role in triaging of patients i.e. subcategorization of lesions based on their malignant potential.[2] So FNAC can be an important initial step towards narrowing down the possibilities while histopathology remains the gold standard for the final diagnosis.
The present study aims at evaluation of the cytomorphological findings of mesenchymal lesions on FNAC along with their histopathological correlation.
MATERIAL AND METHODS
This was a retrospective study conducted over a period of 1 year from January 2021 to January 2022. The material for the study was collected from the patients being referred to the FNAC outpatient department with a clinical suspicion of a mesenchymal lesion. All the patients of cytologically diagnosed mesenchymal lesions on their FNA aspirates were included in the study. A total of 90 such cases were taken. Cytomorphology of the May Grunwald Giemsa and Papanicolaou stained slides were examined and correlated with clinical and histopathological details wherever available.
RESULTS
Out of the 90 patients, 69 (76.7%) were males and 21 (23.3%) were females with a male to female ratio of 3.3:1. The youngest patient and the oldest patient were of 9 and 70 years respectively. Maximum number of cases were in 4–5th decade of life [Table 1].
| Age (Years) | Benign lesions | Malignant lesions | Total |
|---|---|---|---|
| 0–10 | 1 | 0 | 1 |
| 11–20 | 10 | 0 | 10 |
| 21–30 | 22 | 0 | 22 |
| 31–40 | 19 | 1 | 20 |
| 41–50 | 22 | 12 | 31 |
| 51–60 | 3 | 1 | 4 |
| 61–70 | 2 | 0 | 2 |
| Total | 79 | 11 | 90 |
Extremities were most commonly involved and lower limb was found to be the most common site (57.8%) for the mesenchymal lesions, followed by upper limb [Table 2].
| Site | Benign lesions | Malignant lesions | Total |
|---|---|---|---|
| Lower extremities | 43 | 9 | 52 |
| Upper extremities | 24 | 1 | 25 |
| Trunk | 10 | 1 | 11 |
| Head and neck | 2 | 0 | 2 |
| Total | 79 | 11 | 90 |
FNAC smears of all the 90 cases were adequate. On cytological examination, spindle cell lesions were most common followed by lipomatous lesions [Table 3].
| Type of mesenchymal lesions | Cytological diagnosis |
|---|---|
| Spindle | 43 |
| Pleomorphic | 5 |
| Polygonal | 2 |
| Adipocytic | 29 |
| Round | 11 |
| Total | 90 |
Majority of the cases on FNA in our study were found to be benign – 79 (87.8%), while only 11 cases were malignant (12.2%).
Histology was available in 51 (56.6%) cases.
Out of the 79 benign mesenchymal lesions [Figures 1 and 2], histology was available in 40 cases. Cytological diagnosis in 37 cases (92.5%) was concordant with the histology. Most turned out to be schwannoma and neurofibroma. There was discrepancy observed in 3 cases (7.5%). These cases were reported as benign mesenchymal lesion on cytology, were found to be malignant peripheral nerve sheath tumour and synovial sarcoma on histology [Table 4].
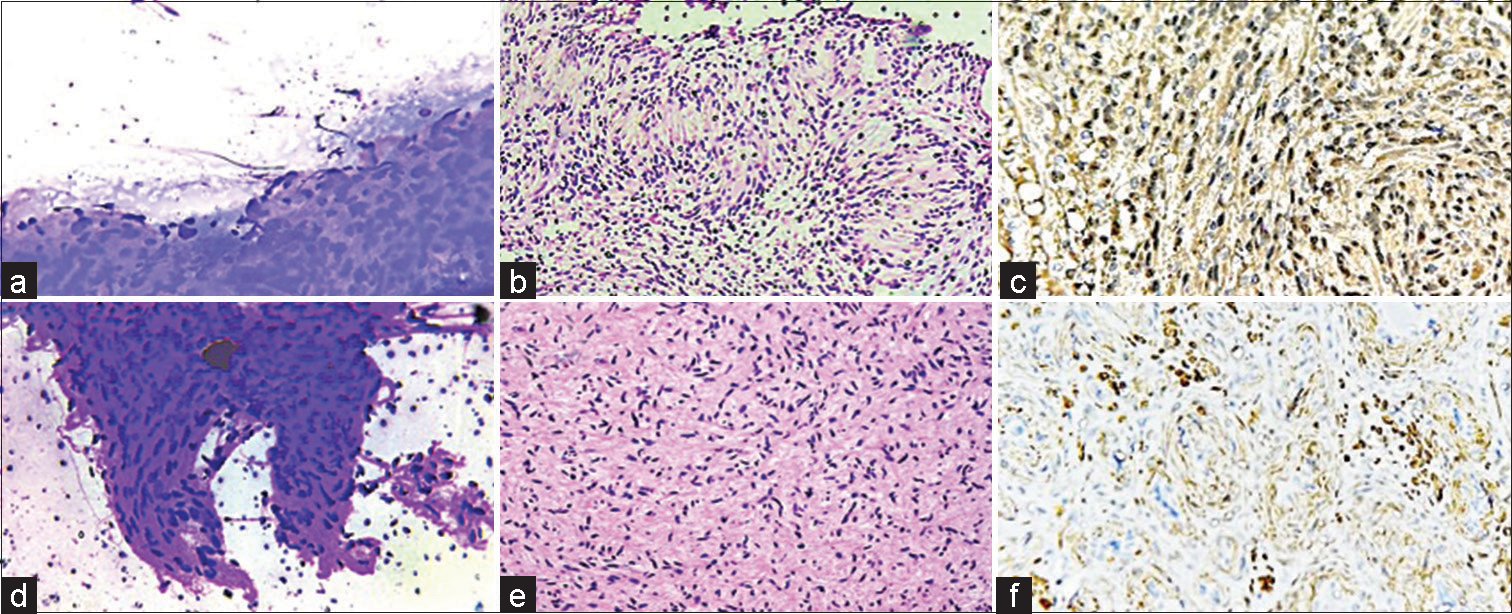
- Benign nerve sheath tumour (a-c) May Grunwald Giemsa stained smears show aggregates of spindled cells with indistinct cytoplasm and elongated nuclei with blunt pointed ends. Hematoxylin and eosin stained sections show Verocay body with alternating hypocellular and hypercellular areas (Schwannoma) confirmed on S-100 immunohistochemistry (×200 magnification). (d-f) May Grunwald Giemsa stained smears show spindled cells with indistinct cytoplasm and elongated nuclei confirmed to be Neurofibroma on histology (Hematoxylin and eosin) with focal S-100 expression (×200 magnification).
- Benign mesenchymal lesion (a-c) May Grunwald Giemsa stained smears show admixture of mature adipocytes and uniform spindle cells. Hematoxylin and eosin stained sections exhibit the triad of mature adipocytes, bland spindle cells and hyalinized rope-like collagen fibres confirming the diagnosis of spindle cell lipoma (×200 magnification). (d-f) May Grunwald Giemsa stained smears show mononuclear stromal cells with oval to spindle shaped nuclei and osteoclast type giant cells confirmed on histology (Hematoxylin and eosin)- Giant cell tumour of tendon sheath (×100, ×200 magnification).
| S. No. | Diagnosis on cytology | Diagnosis on histology | No. of cases |
|---|---|---|---|
| 1. | Lipoma | Conventional lipoma, Spindle cell lipoma | 10 |
| 2. | Benign mesenchymal lesion of neural origin | Neurofibroma | 9 |
| 3. | Benign mesenchymal lesion of neural origin | Schwannoma | 12 |
| 4. | Benign mesenchymal lesion possibly of fibrous origin | Nodular fasciitis | 3 |
| 5. | Benign mesenchymal lesion possibly of fibrous origin | Benign fibrohistiocytic lesion | 1 |
| 6. | GCT of tendon sheath | GCT of tendon sheath | 2 |
| 7. | Benign mesenchymal lesion ? neural ? fibroblastic | Malignant peripheral nerve sheath tumour | 2 |
| 8. | Benign mesenchymal lesion comprising spindle cells | Synovial sarcoma | 1 |
| Total cases | 40 | ||
GCT: Giant cell tumors
Histology was available in all the 11 cytologically diagnosed malignant cases. There was no discrepancy observed. On histopathology, these cases were diagnosed as dermatofibrosarcoma protuberans [Figure 3], synovial sarcoma [Figure 4] and liposarcoma [Figure 5], undifferentiated pleomorphic sarcoma and malignant peripheral nerve sheath tumour [Figure 6] respectively (concordant with cytological diagnosis) [Table 5].

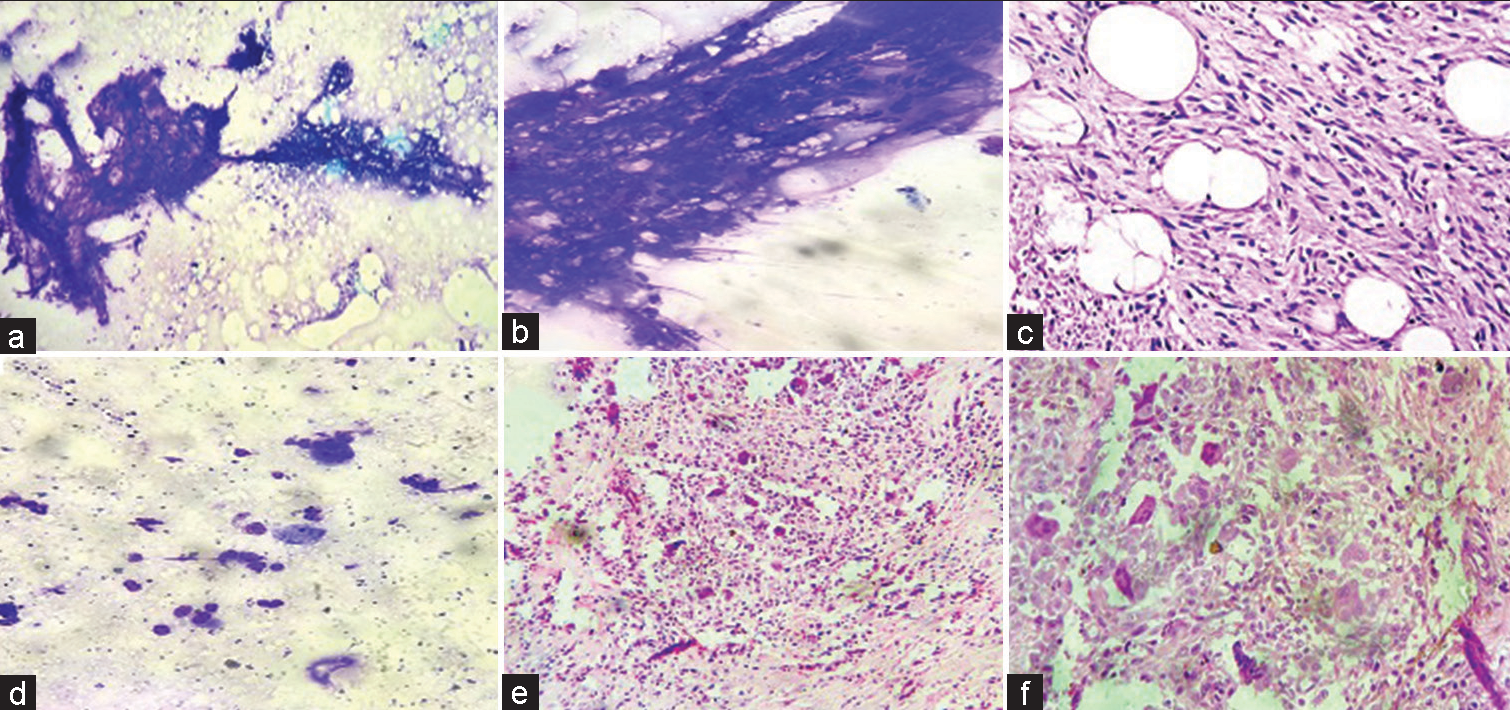
- (a) May Grunwald stained smears exhibiting spindle cells with moderate pleomorphism. (b) Hematoxylin and eosin stained sections show a tumour composed of spindle cells in storiform pattern infiltrating the fat (c and d) Immunohistochemistry exhibiting positivity for vimentin and CD34 in the spindle cells confirming the diagnosis of Dermatofibrosarcoma protuberans (×200 magnification).

- (a) May Grunwald stained smears show groups and numerous isolated, round to oval cells monomorphic cells. (b) Hematoxylin and eosin stained sections show a spindle cell tumour composed of monotonous cells with scant amphophilic cytoplasm, ovoid to spindled vesicular nuclei. (c-f) Immunohistochemistry exhibiting positive expression for vimentin (c), bcl2 (d) and TLE1 (e) in the spindle cells with Ki67 proliferation index of 20% confirming the diagnosis of monophasic synovial sarcoma (×200 magnification).
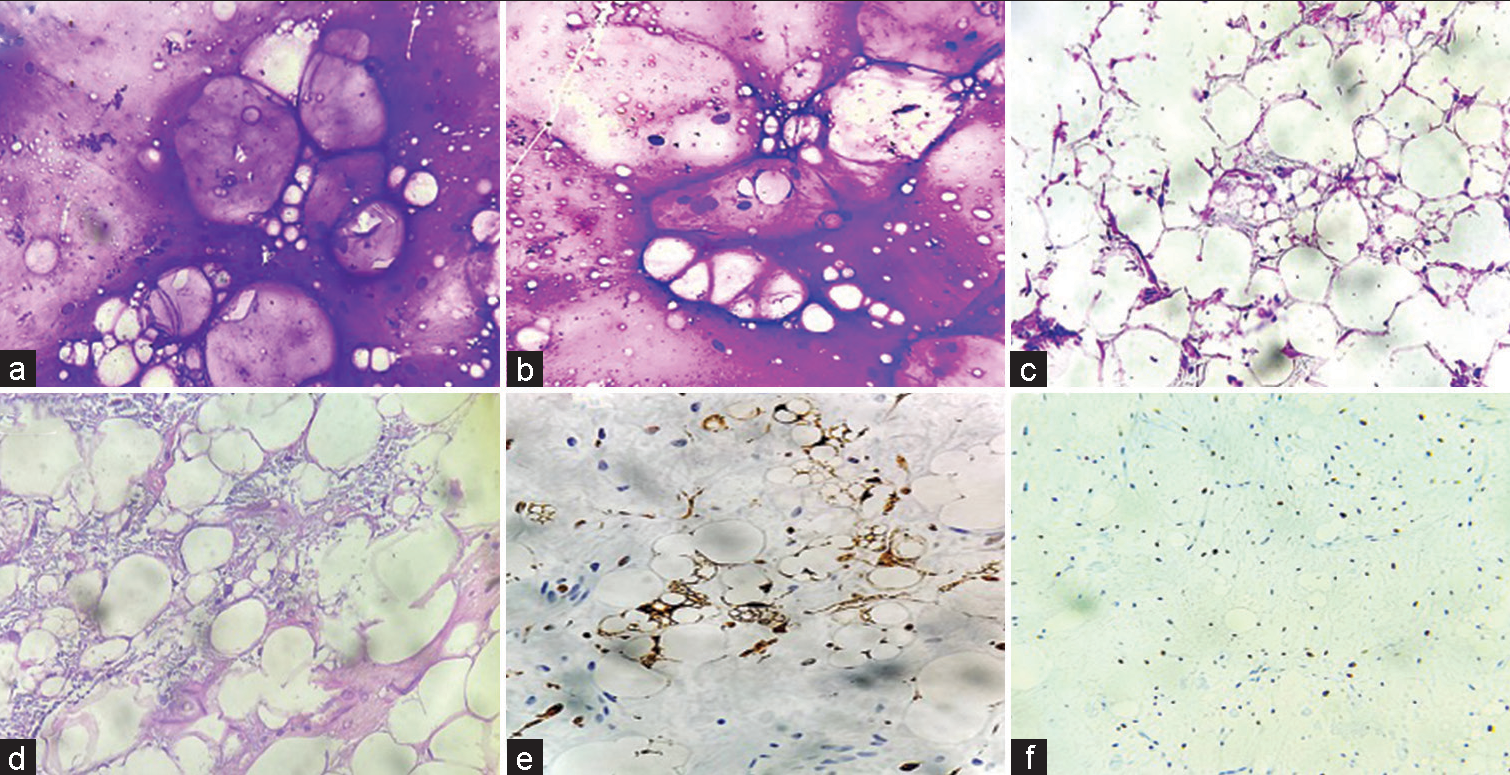
- (a and b) May Grunwald stained smears show abundant metachromatic, myxoid stroma with vacuolated cells. (c and d) Hematoxylin and eosin stained sections show lipoblasts within a myxoid stroma. (e and f) Immunohistochemistry exhibiting positive expression of S-100 in the lipoblasts with Ki67 proliferation index of 30% confirming the diagnosis of Myxoid liposarcoma (×200 magnification).

- (a-c) May Grunwald stained smears show markedly pleomorphic cells with bizzare cells. Hematoxylin and Eosin stained sections show pleomorphic tumour cells with foamy cytoplasm and marked atypia with positive expression for only vimentin- Undifferentiated pleomorphic sarcoma. (d-f) May Grunwald stained smears show spindled cells singly and in clusters with wavy nuclei exhibiting moderate pleomorphism. Hematoxylin and Eosin stained sections exhibits marbled appearance with spindle cells with hyperchromatic focally buckled nuclei with focal positive expression for S-100- Malignant peripheral nerve sheath tumour. (×100, ×200 magnification).
| S. No. | Diagnosis on cytology | Diagnosis on histology | No. of cases |
|---|---|---|---|
| 1. | Malignant mesenchymal lesion, possibly malignant peripheral nerve sheath tumour | Malignant peripheral nerve sheath tumour | 2 |
| 2. | Malignant mesenchymal lesion, possibly liposarcoma |
Myxoid liposarcoma (2), well differentiated liposarcoma (1) | 3 |
| 3. | Malignant mesenchymal lesion | Undifferentiated pleomorphic sarcoma | 2 |
| 4. | Malignant mesenchymal lesion comprising oval to spindle cells in sheets | Synovial sarcoma | 3 |
| 5. | Malignant mesenchymal lesion possibly of fibroblastic origin | Dermatofibrosarcoma protuberans | 1 |
| Total cases | 11 | ||
Based on the cyto-histological correlation, sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV) and diagnostic accuracy of FNAC in diagnosis of mesenchymal lesion was 78.6%, 100%, 92.5%, 100% and 94.1% respectively [Table 6].
| Fine needle aspiration cytology | Histopathological diagnosis | ||
|---|---|---|---|
| Benign | Malignant | Total | |
| Benign | 37 (True negative) | 3 (False negative) | 40 |
| Malignant | 0 (False positive) | 11 (True positive) | 11 |
| 37 | 14 | 51 | |
[Figure 7] depicts the algorithmic approach to cytomorphological diagnosis of mesenchymal tumors.
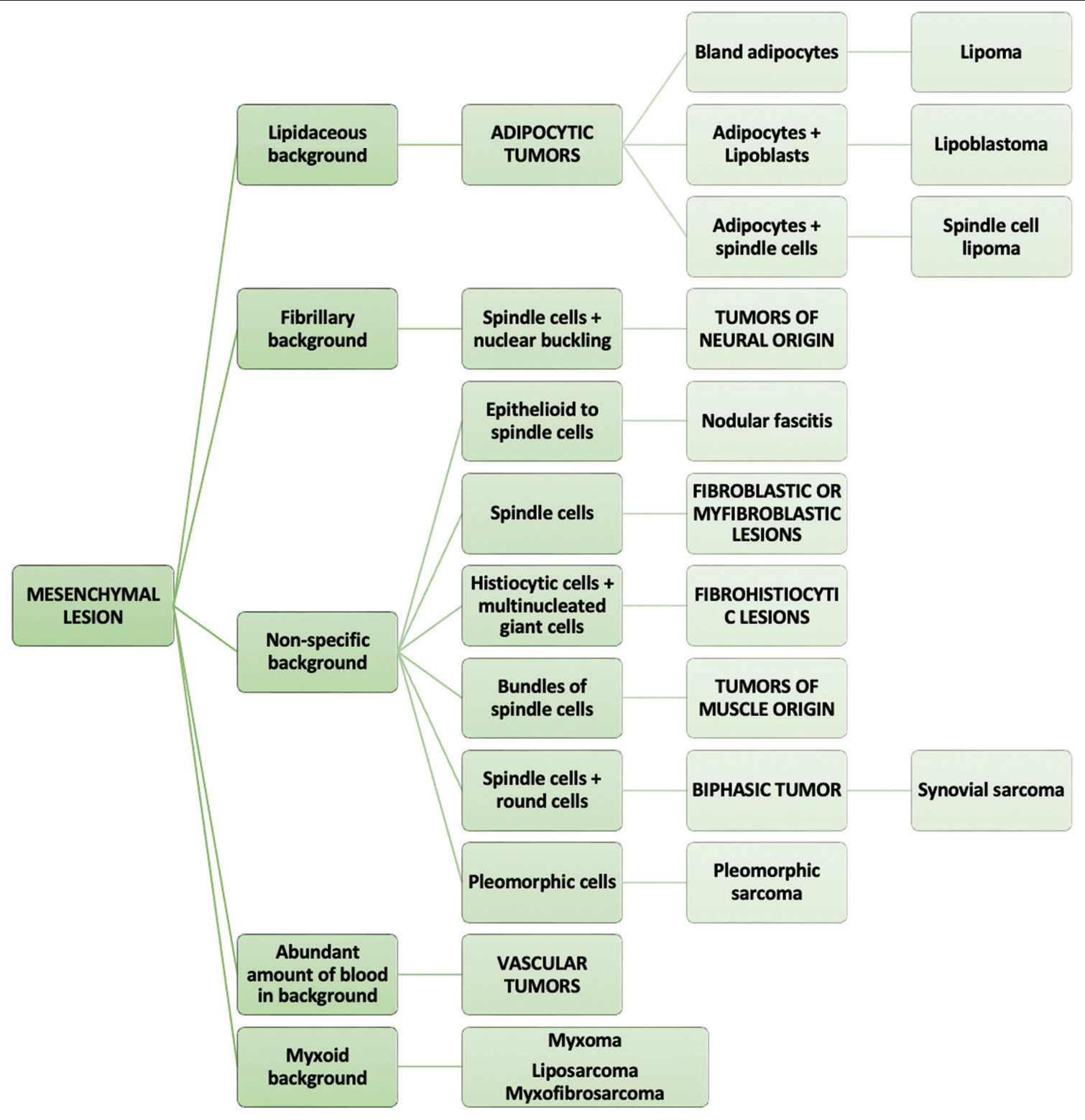
- The algorithmic approach to cytomorphological diagnosis of mesenchymal lesions.
DISCUSSION
The adequacy rate in our study was 100%. Our findings were similar to those of Roy et al., who reported an adequacy rate of 93.4% and by Rani et al. who reported an adequacy rate of 94.5%.[3,4]
In the present study, males outnumbered females by a ratio of 3.3:1, which was in contrast to the findings of Rani et al. (1.4:1), Hirachand et al. (1:1) and Rekhi et al. (1.8:1).[4-6]
Majority of cases in the current study were in 4–5th decade of life with most cases in the age group 21–50 years which was in concordance with the findings of Tailor et al. and Soni et al.[7,8]
Lower limb (57.8%) was found to be the most common site for mesenchymal lesions in our study, followed by upper limb. This was similar to the findings of Kotwal et al., who also reported lower limb as the most common site for soft tissue tumours.[9] Soni et al. observed upper limb and trunk to be the most common site for benign and malignant soft tissue tumors respectively.[8] Rasool et al. had majority of their cases in head and neck.[10] In the series by Tailor et al., upper limb was found to be the commonest site.[7] Trunk was found to the most frequently involved site by Sengupta et al.[11]
In the present study, cyto-histopathological discordance was seen in three cases which reported as benign mesenchymal lesions on cytology and turned out to be malignant nerve sheath tumor and synovial sarcoma respectively. This could be possibly due to deep seated lesions leading to paucicellular smears.
In our study, 87.8% cases were found to be benign, while malignant comprised only 12.2%. which was similar to the data of Tailor et al., Soni et al., Kotwal et al. and Rakheja et al, who found benign tumours to be 93.5%, 95.3%, 82.3% and 94.2% respectively.[7-9,12]
The performance analysis in present study were comparable to the results of Rakheja et al. who observed a sensitivity, specificity, PPV, NPV and diagnostic accuracy of 95.2%, 100%, 100%, 96.4% and 97.9% respectively.[12] Soni et al. observed an accurate characterization in 77.14% cases between the cytological and final histopathological diagnosis. They observed a sensitivity, specificity, PPV, NPV, and accuracy of 70%, 100%, 97.90%, 100%, and 98% respectively in their study.[8]
Rekhi et al. in their study observed the diagnostic accuracy of FNAC for soft tissue tumors to be 98%, with a PPV and NPV of 98% and 100% respectively.[6] A diagnostic accuracy of 90.6% and 91.3% was observed by Roy et al. for benign and malignant soft tissue tumors respectively, with an overall accuracy of 90.8%.[3] Sengupta et al. observed a good cytohistological correlation of 87.2% in diagnosis of soft tissue tumors.[11]
SUMMARY
FNAC is a simple and useful tool that plays an important role in triaging patients. In the current era of minimally invasive procedures, FNAC stands out being extremely helpful in understanding their native nature. With introduction of immunocytochemistry and cell block prepartion, sensitivity and specificity of FNAC is further increased.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
The study was exempt from approval from Institutional Ethics Committee due to its retrospective nature and use of anonymised data.
LIST OF ABBREVIATIONS (In alphabetic order)
FNAC: Fine needle aspiration cytology
NPV: Negative predictive value
OPD: Out patient department
PPV: Positive predictive value.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
References
- World Health Organization, International Academy of Pathology In: Fletcher CD, ed. WHO Classification of Tumours of Soft Tissue and Bone Tumours (5th ed). Lyon: IARC; 2020.
- [Google Scholar]
- Cytologic diagnosis of a rare soft-tissue lesion: Think beyond the usual. Cytojournal. 2020;17:24.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of fine needle aspiration cytology and its correlation with histopathological findings in soft tissue tumours. J Cytol. 2007;24:37-40.
- [CrossRef] [Google Scholar]
- Cytomorphological study of mesenchymal spindle cell lesions of soft tissues by fine needle aspiration cytology. Ind J Pathol Oncol. 2018;5:542-7.
- [CrossRef] [Google Scholar]
- Fine needle aspiration (FNA) of soft tissue tumours (STT) Kathmandu Univ Med J (KUMJ). 2007;5:374-7.
- [Google Scholar]
- Scope of FNAC in the diagnosis of soft tissue tumors-a study from a tertiary cancer referral center in India. Cytojournal. 2007;4:20.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of fine needle aspiration cytology in soft tissue tumors: Our institutional experience. Int J Res Med Sci. 2013;1:443-7.
- [CrossRef] [Google Scholar]
- A prospective study of soft tissue tumors histocytopathology correlation. Patholog Res Int. 2014;2014:678628.
- [CrossRef] [PubMed] [Google Scholar]
- Role of fine needle aspiration cytology in diagnosis of soft tissue tumours; benefits and limitations: A two year retrospective study. J Evid based Med Healthc. 2016;3:1019-24.
- [CrossRef] [Google Scholar]
- Utility of fine needle aspiration cytology in diagnosis of soft tissue lesions with histopathological correlation. Glob J Med Public Health. 2013;2:1-7.
- [Google Scholar]
- Role of cytology in diagnosis of soft tissue sarcomas with special reference to false positive cases. J Cytol. 2009;26:15-9.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology in soft tissue tumors-5-year institutional experience. Diagn Cytopathol. 2022;50:463-70.
- [CrossRef] [PubMed] [Google Scholar]








