HPV-negative and high-grade finding in Pap cytology

*Corresponding author: Deepti Jain, Department of Pathology, Wayne State University, Detroit, United States. deepti.jain@wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Jain D, Elbashir L, Shidham VB. HPV-negative and high-grade finding in Pap cytology. CytoJournal. 2024;21:2. doi: 10.25259/Cytojournal_57_2023
A 69-year-old post-menopausal female with past medical history of idiopathic thrombocytopenic purpura, status post-total abdominal hysterectomy for uterine tumor, and chemotherapy presented with abnormal vaginal bleeding. Computerized tomography (CT) scan chest/ abdomen/pelvis revealed a 2.9 × 2.0 cm mass in the vaginal cuff. High-risk human papillomavirus (HR-HPV) testing was negative. Papanicolaou (Pap)-stained ThinPrep showed atypical hyperchromatic-crowded groups (HCGs) [Figure 1].

- Pap stain: (a: ×20; b: ×40) Clusters of epithelial cells with high nucleocytoplasmic ratio; hyperchromatic nuclei; prominent nucleoli; nuclear overcrowding; and indistinct cell borders, (c: ×20; d: ×40) mesenchymal element demonstrating cigar-shaped to spindle cells (arrows) with clusters of inflammatory cells.
QUESTION # 1
The findings in Pap smear [Figure 1] may represent all except
Low-grade squamous intraepithelial lesion (LGSIL)
Atypical glandular cells of uncertain significance (AGUS)
Adenocarcinoma
Carcinosarcoma (Malignant-mixed Mullerian tumor)
ANSWER
a. Low-grade squamous intraepithelial lesion (LGSIL)
LGSIL (Option a) is characterized by distinct cytological features. The cells exhibit nuclear enlargement, typically 3 times the normal size without significantly high nuclear to cytoplasmic (N/C) ratio. Koilocytic changes include hyperchromasia along with coarse chromatin and nuclear membrane irregularities with diagnostic empty perinuclear cytoplasmic space sharply demarcated from peripheral cytoplasm. Binucleation and multinucleation are is frequent. Nucleoli are generally inconspicuous or absent. Low-grade squamous intraepithelial lesion may show increased keratinization seen as dense orangeophilic (atypical parakeratosis).[1]
This is a case of recurrent uterine carcinosarcoma (UCS). The patient presented with chief complaints of abnormal vaginal bleeding for the past 1 month. Four years before this consultation, the patient underwent total abdominal hysterectomy, bilateral salpingo-oophorectomy (BSO), and pelvic lymph node sampling. Evaluation of the hysterectomy of the specimen revealed a stage IB UCS. She underwent chemotherapy; however, after 2 cycles, her thrombocytopenia got exacerbated, and chemotherapy was terminated.
During the present consultation, rectovaginal examination revealed a mass on the left vaginal apex. On CT scan, chest/ abdomen/pelvis revealed a 2.9 × 2.0 cm vaginal mass. There was no evidence of metastatic disease in the chest, abdomen, or pelvis. Pap smear and concurrent left vaginal biopsy were done. Pap smear revealed a biphasic neoplasm composed of atypical glandular cells with a spindle cell component. The malignant glandular component was comprised three-dimensional hyperchromatic crowded cell groups with high N/C ratio, coarse clumped chromatin, and occasional prominent nucleoli, whereas spindle cell components demonstrated high N/C ratio, hyperchromasia, irregular chromatin, and nuclear pleomorphism.
Histopathological examination (H&E) of the left vaginal biopsy [Figure 2a and 2b] revealed high-grade carcinoma with sarcomatoid features. Immunohistochemistry demonstrated [Figure 2c] focal immunoreactivity for cytokeratin CAM 5.2 [Figure 2d] with mutant p53 expression [Figure 2e] and nonimmunoreactivity for desmin [Figure 2f].
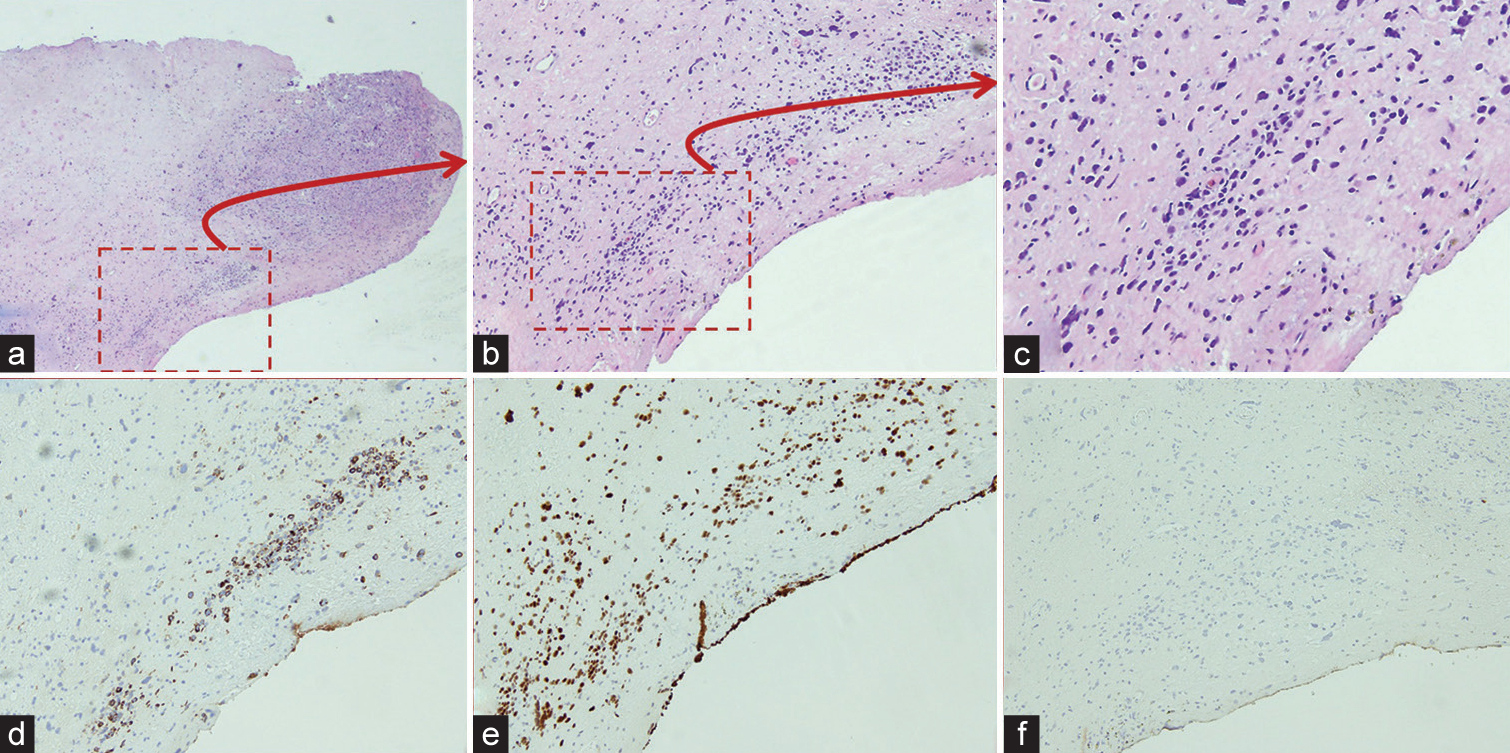
- Left vaginal biopsy; (a: H&E, ×4; b: H&E, ×10; c: H&E, Zoomed) demonstrated sheets of pleomorphic spindle cells with variable cellularity. (d-f: immunohistochemistry, ×10) focal immunoreactivity for CAM5. 2, aberrant p53 expression, and non-immunoreactivity for desmin.
Based on cytology and vaginal biopsy findings, a diagnosis of vaginal cuff recurrence of carcinosarcoma was made, and the patient underwent external beam radiation therapy and vaginal brachytherapy.
Depending on multiple variables from cytomorphological features sampled in the Pap smear to the interpreter experience adenocarcinoma (Option c) and the carcinomatous component of UCS may have to be interpreted as atypical glandular cells of uncertain significance (AGUS) (Option b). However, the presence of spindle cells and the patient’s history of UCS would point to the definitive interpretation as carcinosarcoma (malignant mixed Mullerian tumor).
DISCUSSION
A biphasic neoplasm showing both a malignant epithelial component and a spindle cell/mesenchymal component on a Pap smear should raise the suspicion for the possibility of carcinosarcoma.[2-5] It is reported that the detection rate of malignant cells in cervical samples of histologically proven UCS ranges from 56% to 70%;[2-5] however, UCS usually lacks the sarcomatous component in Pap smears.
The carcinomatous component of UCS is more readily identifiable than the mesenchymal component [Table 1].[2-8]
| S. No. | Author/Year | No. of cases | Pap type | Findings |
|---|---|---|---|---|
| 1. | Tenti et al., 1989[2] | 10 | Conventional |
|
| 2. | Costa et al., 1992[3] | 21 | Conventional |
|
| 3. | Casey et al., 2003[4] | 9 | Conventional |
|
| 4. | Snyder et al., 2004[5] | 25 | Conventional |
|
| 5. | Salman et al., 2006[6] | 1 | SurePath (LBC) |
|
| 6. | Gupta et al., 2014[7] | 8 | SurePath (LBC) |
|
| 7. | Hanley et al., 2015[8] | 40 | 4 conventional, 36 liquid based |
|
AGUS: Atypical glandular cells of uncertain significance, LBC: Liquid-based cytology
This is an important diagnostic pitfall in cytology as most cases of UCS may be reported as AGUS, squamous cell carcinoma, endometrial adenocarcinoma, poorly differentiated carcinoma, or adenocarcinoma not otherwise specified.[5]
Detecting UCS through cytologic examination poses diagnostic challenges due to several factors. These challenges include sampling errors, random exfoliation of cells, cellular degeneration, and sporadic shedding of mesenchymal component.[4] In addition, frequent association with atrophy can also make it difficult to detect UCS cells in the background of numerous HCGs of parabasal cells (PBC) in atrophic samples during cytologic examination of Pap smears.[7]
In the current case, on initial review, the smear was interpreted as a hypercellular smear with an inflammatory background, cellular degeneration, and numerous malignant glandular cells in clusters and three-dimensional balls [Figure 1a and 1b], raising the possibility of AGUS favoring neoplasia, but HR-HPV testing was negative. After re-reviewing the smear, HCGs composed of a few cigar-shaped spindle cells [Figure 1c] suggestive of a mesenchymal component was identified. The final diagnosis of high-grade carcinoma with sarcomatous features were rendered. The cytologic findings were consistent with the vaginal biopsy results.
It is important to consider that, while HPV testing is a helpful tool in cervical cancer screening, a negative high-risk HPV test does not automatically exclude malignancy, because some malignancies arising in the uterine corpus or adnexa can be identified in cervical smears due to exfoliation, drop metastasis, and/or direct extension to cervix, such as UCS endometrial carcinomas, clear cell carcinoma, high-grade serous carcinoma, and mesonephric adenocarcinoma are not HPV related.
Furthermore, there are other aggressive cancers such as endometrial carcinomas, clear cell carcinoma, high-grade serous carcinoma, mesonephric adenocarcinoma, and adenocarcinomas arising from other sites that show high-grade morphology in Pap smears despite testing negative for HPV.[9,10]
One of the potential reasons for the initial omission of the mesenchymal elements is the frequent association of atrophic changes in the elderly patients with abundant inflammatory cells and cellular degeneration with many HCGs of PBCs in Pap smears. Another possibility could be the inability to detect spindle cells/mesenchymal elements in Pap smear on the first look as the mesenchymal element with atypical spindle cells [as in the current case, Figure 1c, and arrows in Figure 1d] is rarely present due to infrequent shedding and sampling artifacts.
It is crucial to understand that a significant percentage of women with AGUS may turn out to be high-grade pre-invasive squamous disease, adenocarcinoma in situ, adenocarcinoma, or invasive cancers from sites other than the cervix. Owing to the aggressive nature of UCS, an accurate and timely diagnosis is indispensable. An algorithmic approach is suggested for interpreting AGUS cells in relation to UCS Pap smear [Figure 3].
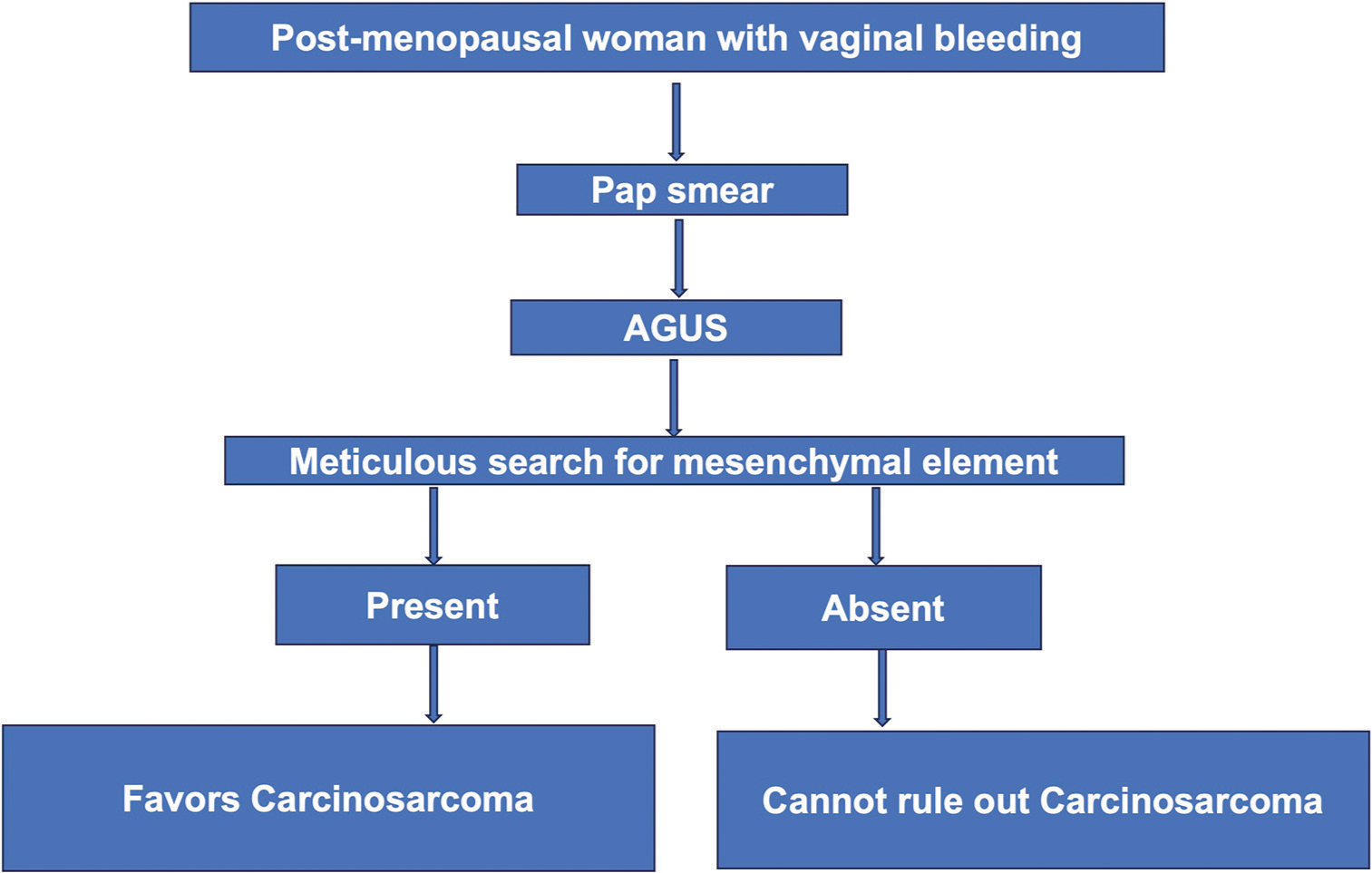
- Algorithmic approach for evaluating atypical glandular cells of uncertain significance in post-menopausal women with reference to uterine carcinosarcoma (UCS). This is not for definitive diagnosis of UCS.
ADDITIONAL QUIZ QUESTION
Question # 2
Most common mutations in uterine carcinosarcoma?
TP53 mutations
HER2
TERT
KRAS.
Question # 3
Most consistent independent predictor of outcome
Age at presentation
Stage
Angioinvasion
Depth of invasion
Number of mitotic figures.
BRIEF REVIEW OF THE TOPIC
UCS, previously known as malignant mixed Müllerian tumor, is a rare and aggressive biphasic tumor in the female reproductive system, accounting for <5% of uterine tumors. It is characterized by the presence of high-grade epithelial and mesenchymal components. UCS is commonly diagnosed in postmenopausal women with a history of bleeding with a median age of presentation of 62 years.[11] The most common locations within the female genital tract include the uterus and ovaries. Cases of UCS originating from the cervix, vagina, ovary, fallopian tube, and peritoneum are also reported.[11]
It is biologically a de-differentiated endometrial carcinoma with its own pathogenesis and molecular profile.[12] Several hypotheses to explain the origination of UCS have been proposed. The collision hypothesis suggested that UCS forms when separate epithelial and mesenchymal tumors collide and merge within the uterus. On the other hand, the combination hypothesis posits that UCS arises from a single stem cell capable of divergent differentiation, giving rise to both epithelial and mesenchymal components within the tumor. Finally, the conversion hypothesis, which is the most widely accepted theory proposed that UCS is a metaplastic carcinoma that carcinomatous cells can undergo a conversion process where they transform into sarcomatous cells through epithelial to mesenchymal transition (EMT).[13,14] This conversion is associated with high scores of EMT gene signatures and is likely influenced by epigenetic alterations at microRNA promoters, histone gene mutations, and gene amplifications.[14]
TP53/p53 abnormalities are present in a majority of the UCS. The proportion of replicative DNA polymerase epsilon (POLE) and hypermutated microsatellite instability-high is related to the epithelial component, being approximately 25% and 3% in endometrioid and non-endometrioid components.[15] The stage at diagnosis follows a bimodal distribution, approximately 40–50% of cases are detected at an early stage (International Federation of Gynecology and Obstetrics [FIGO] Stages I and II), while about 50–60% are detected at an advanced stage (FIGO stages III and IV). At the time of diagnosis, up to 30–40% of patients display lymph node metastases. In addition, about 10% of patients present with distant metastatic spread, with lung as the commonly affected site. These findings highlight the aggressive nature of UCS and the importance of early detection and intervention. The 5-year survival rate of UCS is approximately 30% (Stage I and II: 59%; Stage III: 25%; Stage IV: 9%).[16]
Histologically, the malignant cell in UCS exhibits a biphasic nature; the carcinomatous component is usually endometrioid or serous, but clear and undifferentiated may be seen. The sarcomatous component is usually high-grade sarcoma NOS, but heterologous elements (rhabdo, chondro, osteo, and lipo) might be encountered.[17,18]
Recommended treatment for UCS involves surgical staging with total hysterectomy with BSO, pelvic lymphadenectomy, para-aortic lymph node sampling, and peritoneal washings.[19]
SUMMARY
The Pap smear can serve as both a screening and diagnostic tool.
It is essential to consider UCS as one of the possible potential differentials, particularly in post-menopausal women presenting with vaginal bleeding.
An algorithmic approach in the case of AGUS favoring neoplasia can facilitate correct interpretation of UCS in Pap smears.
Comprehensive evaluation, incorporating clinical history and histological biopsies, should be considered while interpreting the result of Pap smears with AGUS.
It is essential to report on both the carcinomatous and sarcomatous components for proper management.
Answers for Question 2 through 3:
2. a
3. b
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
Given their role as Editorial Board Members, Deepti Jain and Vinod B. Shidham had no involvement in the peer review of this article and has no access to information regarding its peer-review.
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for the appropriate portions of the content of this article. Conceptualization: Vinod B. Shidham, Deepti Jain, Data curation: Deepti Jain, Methodology: Deepti Jain, Leana Elbashir, Writing: Deepti Jain, Leana Elbashir, Writing: review and editing, Vinod B. Shidham. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from the Institutional Review Board (or its equivalent).
LIST OF ABBREVIATIONS (In alphabetic order)
AGUS – Atypical glandular cells of uncertain significance BSO – Bilateral salpingo-oophorectomy CT – Computerized tomography EMT – Epithelial to mesenchymal transition FIGO – International Federation of Gynecology and
Obstetrics H&E – Histopathological examination HCGs – Hyperchromatic crowded groups HPV – Human papillomavirus HR – High risk LBC – Liquid-based cytology LSIL – Low-grade squamous intraepithelial lesion MMMT – Malignant mixed Müllerian tumor N/C – Nuclear-to-cytoplasmic Pap – Papanicolaou PBC – Parabasal cells UCS – Uterine carcinosarcoma
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through an automatic online system.
References
- The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of malignant mixed mesodermal tumor of the uterus: Experience of 10 cases. Eur J Gynaecol Oncol. 1989;10:125-8.
- [Google Scholar]
- Cervicovaginal cytology in carcinosar-coma [malignant mixed mullerian (mesodermal) tumor] of the uterus. Diagn Cytopathol. 1992;8:33-40.
- [CrossRef] [PubMed] [Google Scholar]
- Cervicovaginal (Papanicolaou) smear findings in patients with malignant mixed Mullerian tumors. Diagn Cytopathol. 2003;28:245-9.
- [CrossRef] [PubMed] [Google Scholar]
- An abnormal cervicova-ginal cytology smear in uterine carcinosarcoma is an adverse prognostic sign: Analysis of 25 cases. Am J Clin Pathol. 2004;122:434-9.
- [CrossRef] [PubMed] [Google Scholar]
- Pleomorphic spindle cells and hyperchromatic crowded groups in a cervical smear. Cytopathology. 2006;17:396-8.
- [CrossRef] [PubMed] [Google Scholar]
- Eight cases of malignant mixed Müllerian tumor (carcinosarcoma) of the uterus: Findings in SurePath™ cervical cytology. Diagn Cytopathol. 2014;42:165-9.
- [CrossRef] [PubMed] [Google Scholar]
- Atypical findings on cervicovaginal smears correlate with cervical involvement by malignant mixed Müllerian tumors of the uterus. Acta Cytol. 2015;59:319-24.
- [CrossRef] [PubMed] [Google Scholar]
- HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559-67.
- [CrossRef] [PubMed] [Google Scholar]
- HPV-ISH-negative invasive cervical squamous cell carcinoma: Histologic and Pap test results. Acta Cytol. 2019;63:417-23.
- [CrossRef] [PubMed] [Google Scholar]
- Trends of uterine carcinosarcoma in the United States. J Gynecol Oncol. 2018;29:e22.
- [CrossRef] [PubMed] [Google Scholar]
- Uterine carcinosarcoma: A review of the literature. Gynecol Oncol. 2015;137:581-8.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant biphasic uterine tumours: Carcinosarcomas or metaplastic carcinomas? J Clin Pathol. 2002;55:321-5.
- [CrossRef] [PubMed] [Google Scholar]
- Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell. 2017;31:411-23.
- [CrossRef] [PubMed] [Google Scholar]
- Endometrial carcinosarcoma. Int J Gynecol Cancer. 2023;33:147-74.
- [CrossRef] [PubMed] [Google Scholar]
- The impact of multi-modal therapy on survival for uterine carcinosarcomas. Gynecol Oncol. 2010;116:419-23.
- [CrossRef] [PubMed] [Google Scholar]
- Carcinosarcoma (malignant mixed mesodermal tumor) of the uterus. A Gynecologic Oncology Group pathologic study of 203 cases. Int J Gynecol Pathol. 1990;9:1-19.
- [CrossRef] [PubMed] [Google Scholar]
- Pathologic and molecular features of uterine carcinosarcomas. Semin Diagn Pathol. 2010;27:274-86.
- [CrossRef] [PubMed] [Google Scholar]
- Assessing the effects of lymphadenectomy and radiation therapy in patients with uterine carcinosarcoma: A SEER analysis. Gynecol Oncol. 2008;111:82-8.
- [CrossRef] [PubMed] [Google Scholar]








