Translate this page into:
A case of clear cell adenocarcinoma arising from the urethral diverticulum: Utility of urinary cytology and immunohistochemistry
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Carcinomas rarely arise from the urethral diverticulum. In this report, we present a case of clear cell adenocarcinoma arising from the urethral diverticulum. A 42-year-old woman complained of bloody discharge and lower back pain. Imaging studies showed a tumor involving the region surrounding the urethra and cystourethroscopy showed papillary and villous tumors in the urethral diverticula. Cytology of the urine sediment showed papillary or spherical clusters of atypical cells, some of which had clear abundant cytoplasm and formed mirror ball-like clusters, suggesting adenocarcinoma. Although histological diagnosis was indeterminate by biopsy and transurethral resection (TUR) because of absence of stromal invasion, surgically resected specimen via cysturethrectomy revealed that the tumor was clear cell carcinoma. Urinary cytological findings and immunohistochemical analysis for CD15, Ki-67, and p53 might be useful for accurate diagnosis of clear cell adenocarcinoma that arises from the urethral diverticulum when sufficient materials are not available by biopsy and TUR.
Keywords
Clear cell adenocarcinoma
Ki-67
nephrogenic adenoma
p53
urethral diverticulum
INTRODUCTION
Urethral carcinoma is a relatively rare tumor, which predominantly affects females. In very rare cases, carcinomas arise from the urethral diverticulum,[1] and only approximately 100 cases of urethral diverticulum-associated carcinomas have been reported so far.[2] Most of the urethral diverticulum-associated carcinomas are adenocarcinomas, whereas more than half of all urethral carcinomas are squamous cell carcinomas.[34] Some case reports have shown that clear cell adenocarcinoma (CCA) arises from the female urethral diverticulum.[5–9] CCA is typically an invasive tumor, but can sometimes show minimally invasive papillary growth without any solid nests of tumor cells in the limited amount of biopsied samples and transurethral resection (TUR). In such cases, histological differentiation between CCA and nephrogenic adenoma is extremely difficult.[10] In this report, we present a case of CCA arising from the urethral diverticulum, which showed nephrogenic adenoma-like histology in the biopsy and TUR. The cytological and immunohistochemical findings were informative for the accurate histological diagnosis of this tumor.
CASE REPORT
Clinical features
A 42-year-old woman presented with a 3-month history of bloody discharge from the urethra and lower back pain. Physical examination performed showed no remarkable changes on admission. She did not show urinary dysfunction. Laboratory data of the blood were within normal limits. Serum levels of tumor markers were not evaluated. Cytological examination of the urine sediment was highly suggestive of adenocarcinoma. Computed tomography (CT) and magnetic resonance imaging (MRI) showed a tumor (diameter, 47 mm), which involved the urethra entirely [Figure 1]. There were no remarkable changes in the gynecological system and no swelling was observed in the intra-abdominal and intrapelvic lymph nodes. Cystourethroscopy showed two diverticula fenestrated in the urethral wall. Whitish papillary and villous lesions were observed in both diverticula [Figure 2]. Specimens of biopsy and TUR histologically suggested CCA, but were indeterminate for malignancy. The patient underwent total cystourethrectomy and partial resection of the vaginal wall. Grossly, the tumor extended around the entire urethra and anterior vaginal wall. Final diagnosis of the resected tumor was CCA stage III pT3N0M0.
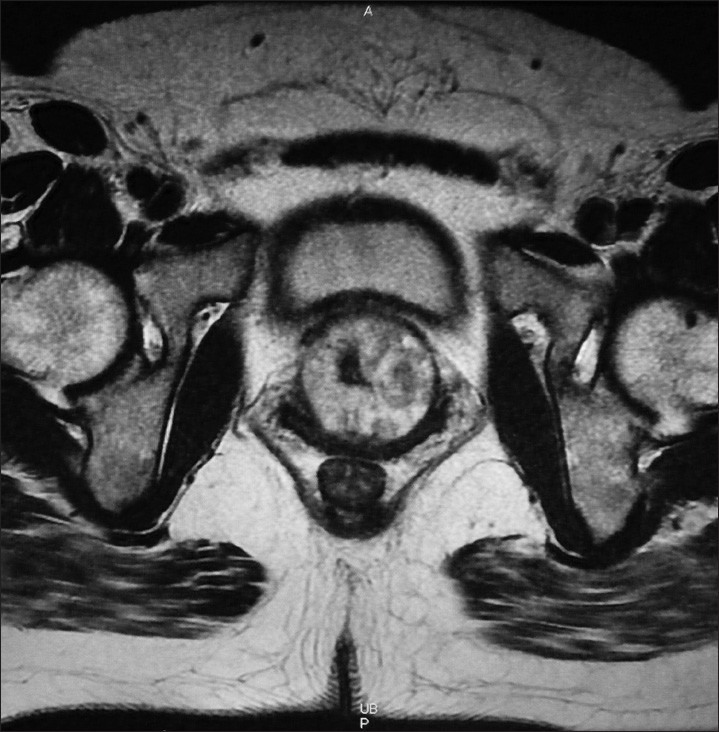
- Magnetic resonance images of the tumor. The horizontal plane of the T1-weighted image shows that the tumor involves the region surrounding the urethra
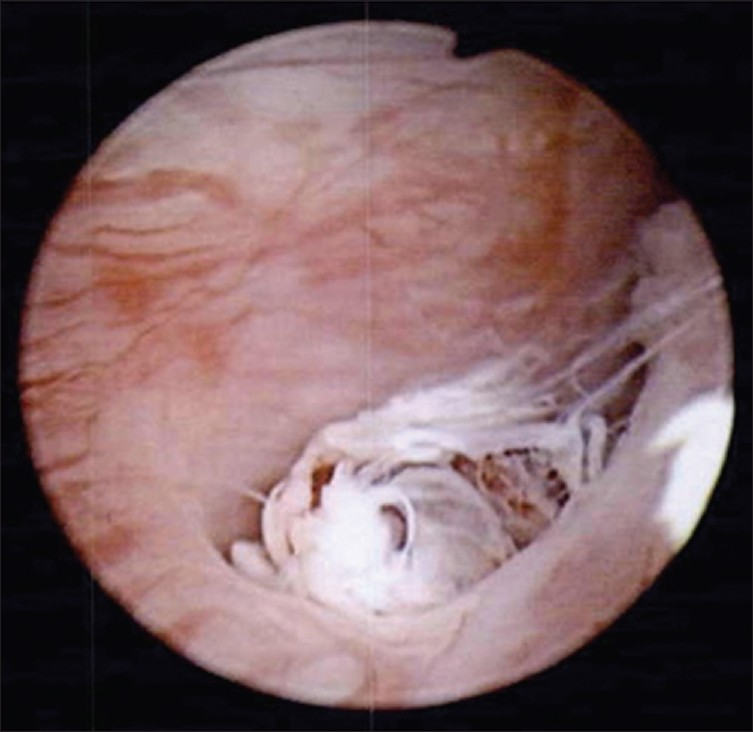
- Cystourethroscopic findings of the urethral lesion. The diverticulum made an orifice into the lumen of the ur ethra and a whitish papillary and villous tumor appeared from the orifice of the diverticulum
Cytopathological features
The Papanicolaou-stained specimens showed a small number of papillary or spherical clusters of atypical cells with many benign urothelial cells and squamous cells in the background [Figure 3a and b]. A few neutrophils and lymphocytes were seen, but no necrotic debris was seen. The nuclei of the atypical cells showed an increase in the chromatin content with fine to granular pattern and irregular contours [Figure 3c]; the nucleoli were prominent. Most of the atypical cells had a moderate amount of cytoplasm that was lightly stained light green; however, some atypical cells showed clear, abundant cytoplasm that formed spherical clusters resembling “mirror balls” [Figure 3b]. These findings suggested a malignant tumor of the urinary system and favored adenocarcinoma.
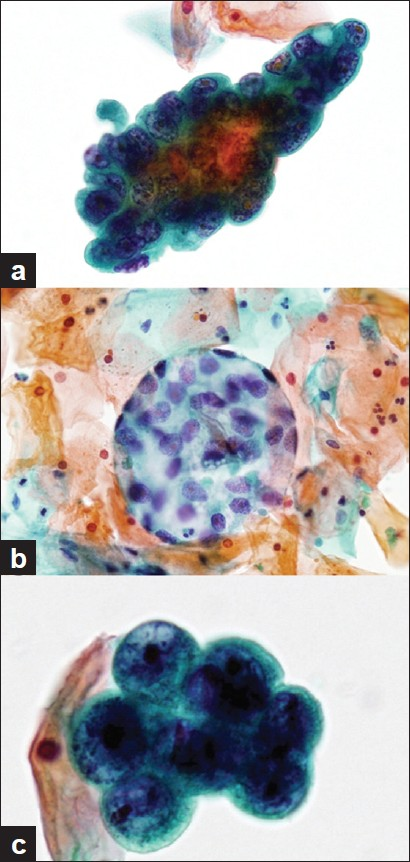
- Cytological findings of the urine sediments. Papanicolaou staining, a) A papillary cluster of tumor cells. The tumor cells with hyperchromatism are piled up irregularly (1000×), b) A spherical cluster of clear cells (400×), c) Nuclei of the tumor cells showing fine to granular chromatin and prominent nucleoli (1000×)
Histopathologic features
The biopsy and TUR samples showed that the tumor comprised papillo-tubular lesions [Figure 4a]. The epithelial cells covering the tumor were cuboidal and single layered, and some of these cells showed a “hobnail pattern.” Most of the cells had eosinophilic cytoplasm, except for a few that had clear cytoplasm. The cells with a clear cytoplasm were positive for periodic acid-Schiff reaction. The epithelial cells showed relatively mild cytological atypia and did not invade the stroma. Mitotic figures were observed at a frequency of 10/10 high-power fields. Necrotic debris was frequently observed in the lumen of the tubular structures. In immunohistochemistry, the tumor cells were positive for cytokeratin (CK) 7 [SP52, ready-to-use (RTU) prediluted, Ventana Medical Systems, Tucson, AZ, USA], epithelial membrane antigen (EMA) (E29, RTU prediluted, Nichirei Bioscience, Tokyo, Japan), and carbohydrate antigen (CA) 125 (M11, 1:25, Dako, Glostrup, Denmark), and were also focally positive for CD15 (80H5, RTU prediluted, Immunotech, Marseille, France) [Figure 4b]. The tumor cells were negative for CK 5/6 (D5/16 B4, 1:50, Dako), CK 20 (SP33, RTU prediluted, Ventana Medical Systems), carcinoembryonic antigen (12-140-10, RTU prediluted, Leica Microsystems, Milton Keynes, UK), thrombomodulin (1009, 1:50, Dako), uroplakin III (AU1, RTU prediluted, Nichirei Bioscience), prostate-specific antigen (polyclonal, Dako), calretinin (5A5, RTU prediluted, Leica Microsystems), estrogen receptor (6F11, 1:50, Leica Microsystems), and progesterone receptor (312, 1:50, Leica Microsystems). Histological findings suggested diagnosis of CCA, although definite diagnosis of the malignancy could not be elicited because of absence of stromal invasion in both biopsy and TUR samples. In the surgically resected specimens, clear atypical cells with papillo-tubular structure invaded almost all layers of the urethra and vaginal muscular layer. The final definite diagnosis was CCA.
The Ki-67 labeling index of these tumor cells was approximately 20% [Figure 4c] and approximately 5% of the tumor cells showed strong p53 positivity in the nucleus.
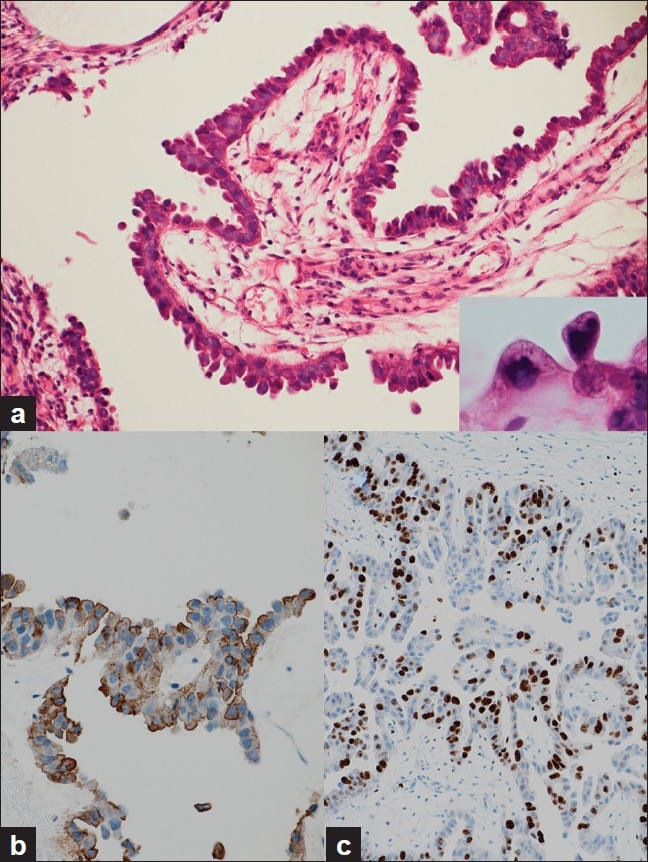
- Histological and immunohistochemical findings, a) A biopsied specimen showing a papillary lesion covered with cuboidal cells. A hobnail pattern configuration was observed without any stromal invasion. A few clear cells were also observed (inset) (H and E 200×), b) The tumor cells were positive for CD15 (H and E 200×), c) The Ki-67 labeling index was approximately 20% (H and E 100×)
DISCUSSION
Carcinomas rarely arise from the urethral diverticulum. Hamilton and Leach were the first to describe the carcinomas arising from the urethral diverticulum.[1] Approximately 100 cases of urethral diverticulum-associated carcinomas have been reported so far.[2] Although squamous cell carcinomas represent the most common histology of urethral carcinomas, adenocarcinomas most commonly develop from the urethral diverticulum. According to the data presented by Clayton et al. and Evans et al., 46-56% of the urethral diverticulum-associated carcinomas are adenocarcinomas and only 15-18% are squamous cell carcinoma.[34] CCAs are especially associated with the urethral diverticulum, and 56% of the urethral CCAs are associated with the urethral diverticulum.[6]
CCA has also been called mesonephric adenocarcinoma, but the hypothesis that CCAs originate from the mesonephric tissue has now been abandoned. The histogenesis of the urethral diverticulum-associated CCAs remains unclear. Based on the fact that more than half of the bladder CCAs contain focal components of urothelial carcinoma or remnants of benign urothelium within the tumor,[911] most CCAs are supposed to be derived from the peculiar glandular differentiation of urothelial neoplasms.[11] The present case showed a transition between CCA and benign urothelium of the diverticulum, which was consistent with urothelial origin of the tumor.
Washino et al. described that urine cytology is relatively sensitive for the detection of atypical cells with a positive rate of 85%[8] however, definitive identification of histological subtype based on the cytological findings is often difficult. In most of the reported cases, diagnosis based on urine cytology was urothelial carcinoma. In the present case, urine cytology could not determine the histological subtype of the tumor; however, in the retrospective review of the specimens, the spherical clusters of atypical cells with clear, abundant cytoplasm were suggestive of CCA.
Histological diagnosis of CCA is usually not difficult if stromal invasion is observed. However, if the tumor is minimally invasive and present in limited specimens, it is very difficult to distinguish between CCA and nephrogenic adenoma. Herawi et al. described seven cases with minimally invasive CCAs histologically similar to nephrogenic adenoma.[10] They reported that nuclear atypia, increased mitotic figures, intraluminal necrosis, focal clear cells, focal solid areas, and elevated Ki-67 and p53 indices were the key features for distinguishing between nephrogenic adenoma-like CCA and nephrogenic adenoma. Other differential diagnoses of CCA are urothelial carcinoma, adenocarcinoma not otherwise specified (adenocarcinoma NOS), and metastatic adenocarcinoma. A systematic workup of the primary tumor site and close histological examination for the identification of the hobnail pattern are essential for differentiating CCA from other tumors. Immunohistochemistry for CD15 is also useful for the differentiation of CCA from other carcinomas.
In this report, we presented a rare case of CCA arising from the urethral diverticulum. In the presence of a urethral diverticulum, clear cell tumor cells clustering like mirror balls in urine cytology are suggestive of CCA and, because these tumors have a propensity to arise in urethral diverticula, the presence of malignant clear cells in urine sample should raise the possibility of origin from urethral diverticulum. When CCA gives a nephrogenic adenoma-like appearance in limited histologic specimens, cytologic findings in urine sediment and positivity for p53 and Ki-67 immunohistochemical stains might be helpful in making an accurate diagnosis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. All authors are responsible for the conception of this study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case without patient identifiers, approval from the Institutional Review Board (IRB) is not required at our institution.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGEMENTS
The authors thank Mr. H. Ishimaru (Kansai Rosai Hospital) for his technical assistance.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/11/95528.
REFERENCES
- Adenocarcinoma arising in a diverticulum of the female urethra. AMA Arch Pathol. 1951;51:90-8.
- [Google Scholar]
- Laparoscopic radical cystourethrectomy in a patient with adenocarcinoma of the female urethral diverticulum. Korean J Urol. 2010;51:145-8.
- [Google Scholar]
- Adenocarcinoma of a female urethral diverticulum: case report and review of the literature. J Urol. 1981;126:124-6.
- [Google Scholar]
- Clear cell adenocarcinoma arising from a urethral diverticulum. J Urol. 1995;153:1914-5.
- [Google Scholar]
- Clear cell adenocarcinoma of the urethra: a clinicopathologic analysis of 19 cases. Mod Pathol. 1996;9:513-20.
- [Google Scholar]
- Clear cell adenocarcinoma in a female urethral diverticulum. Scand J Urol Nephrol. 2000;34:136-8.
- [Google Scholar]
- A case of adenocarcinoma in female urethral diverticulum. Hinyokika Kiyo. 2007;53:593-6.
- [Google Scholar]
- Histogenesis of clear cell adenocarcinoma in the urinary tract: evidence of urothelial origin. Clin Cancer Res. 2008;14:1947-55.
- [Google Scholar]
- Clear cell adenocarcinoma of the bladder and urethra: cases diffusely mimicking nephrogenic adenoma. Hum Pathol. 2010;41:594-601.
- [Google Scholar]
- Clear cell carcinoma of the urinary bladder: a report and comparison of four tumors of mullerian origin and nine of probable urothelial origin with discussion of histogenesis and diagnostic problems. Am J Surg Pathol. 2002;26:190-7.
- [Google Scholar]








