Translate this page into:
A diagnostically difficult case of a cellular pleural fluid: Morphology, immunohistochemistry, and fluorescence in situ hybridization study
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
An 80-year-old woman presented with complaints of nausea, dyspnea, and fatigue for the past 4 weeks. As per patient, she has a history of melanoma. She did not report any fever or chills. Radiologic evaluation showed right-sided pleural effusion. Thoracentesis was performed. Cytopathologic findings of the pleural fluid are shown in Figure 1. What is your interpretation?
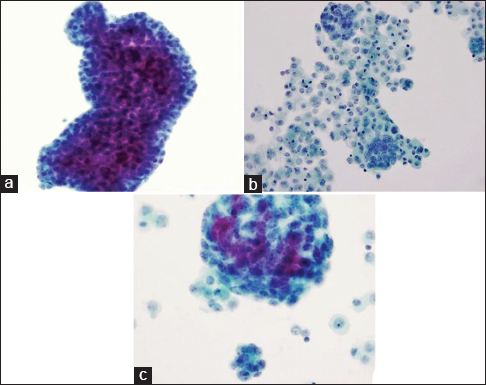
- (a-c) Cytomorphology of pleural effusion (Pap, ×200)
QUESTION
Which of the following entities SHOULD NOT be included in the differential diagnosis?
-
Metastatic adenocarcinoma
-
Atypical cell groups favor reactive mesothelial cells
-
Malignant mesothelioma
-
Metastatic melanoma
-
Lymphoma.
ANSWER
e. Lymphoma.
The differential diagnosis between reactive mesothelial proliferation, malignant mesothelioma (MM), and metastatic adenocarcinoma can be challenging. This pleural fluid specimen is relatively cellular, with one predominant population of cells. These cells have abundant dense perinuclear cytoplasm, centrally located nuclei, prominent nucleoli, and relatively normal nuclear-cytoplasmic (N/C) ratio, which suggest that these cells are mesothelial cells. Many large clusters are present, with scalloped, flower-like outlines. In both smear sample and cell block, atypical cells with binucleated or multinucleated cells are commonly seen. Therefore, MM is on the top of the differential diagnoses.
Melanoma is less likely the cause, in this case. MM cells seen in pleural effusion specimen usually have abundant cytoplasm with prominent nucleoli that can mimic mesothelial cells. However, the melanoma cells usually do not form cell clusters, and they often contain pigment and intranuclear pseudoinclusions. However, as the patient has a reported history of melanoma, it should not be immediately excluded.
Lymphoma is not in the differential diagnosis. Unlike lymphoid neoplasms, the cells in this patient's sample are cohesive with abundant cytoplasm and epithelioid morphology.
Follow-up of present case
Computerized tomography scan of the chest with intravenous contrast after the patient underwent thoracentesis revealed a 7 mm smooth nodule abutting the anterior pleural space of the right middle lobe. Furthermore, a 3 mm nodule is in the anterior right upper lobe. Subsequently, the patient underwent right pleural biopsy through video-assisted thoracoscopic (VAT) surgery. The thoracic cavity was inspected, and multiple plaques were noted over the pleura, as well as some studding over the lung and diaphragm.
ADDITIONAL QUIZ QUESTIONS
Q1. Which of the following immunohistochemistry (IHC) panels is most appropriate as first line markers to differentiate MM from adenocarcinoma?
-
Calretinin, CK7, CD56, and CD45
-
Cytokeratin 5/6 (CK5/6), CAM 5.2, CK7, and thyroid transcription factor-1 (TTF-1)
-
Calretinin, WT-1, Ber-Ep4, and MOC-31
-
Calretinin, CK7, TTF-1, and MOC-31
-
Calretinin, CK 5/6, WT-1, and TTF-1.
Q2. Which of the following features does not favor MM over reactive mesothelial proliferation?
-
Numerous large cell clusters with scalloped contour
-
Cell in cell engulfment
-
Epithelial membrane antigen (EMA) and glucose transporter-1 (GLUT-1) negativity
-
Loss of BRCA1-associated protein 1 (BAP1)
-
Giant atypical mesothelial cells.
Q3. Which of the following genetic markers is not associated with MM?
-
BRAF V660E
-
p16/CDKN2A
-
BAP1
-
NF2.
ANSWERS TO ADDITIONAL QUESTIONS
Q1 (c); Q2 (c); Q3 (a).
Q1 (c) - Calretinin, WT-1, Ber-Ep4, and MOC-31: The correct answer is C. In many cases, morphology alone is not sufficient to make a definitive diagnosis. IHC stains are very useful in differentiating metastatic adenocarcinoma from MM and establishing the primary origin of a metastatic adenocarcinoma.
IHC panels should include at least two markers for metastatic adenocarcinoma and two for MM.[1] Calretinin and WT-1 are considered as the first front-line mesothelial markers. Other mesothelial markers include D2-40 (podoplanin antibody, mesothelin, CK5/6, HBME-1, and thrombomodulin).[234] The diagnostic markers for adenocarcinoma include CEA, Ber-Ep4, BG-8, B72.3, and MOC-31.[35]
Q2 (c) - EMA and GLUT-1 negativity: The correct answer is C. The diagnosis of MM sometimes is challenging as reactive mesothelium can resemble neoplastic mesothelium. Generally speaking the presence of numerous large cell clusters (>50 cells) with scalloped border is characteristic of MM.[3] Cell-to-cell engulfment, cytomegaly, macronucleoli, and marked atypia are additional features which favor MM.
IHC markers might be useful in distinguishing between MM and benign mesothelial proliferation. EMA, p53, insulin-like growth factor messenger RNA-binding protein 3 (IMP3), and GLUT-1 appear to be preferentially expressed in neoplastic mesothelium.[678] EMA seems to be the best marker in this purpose when E29 clone is used in studies.[89] Immunolabeling for desmin appears to be in favor of reactive mesothelial cells.[6] However, IHC results should be interpreted with caution since the specificity and positive predictive values may not be high enough for the definitive diagnosis of MM.
Recently, mutations of BAP1 gene were reported in hereditary and sporadic MM.[10] BAP1 protein is frequently lost in MM and is commonly associated with homozygous BAP1 deletion. Loss of BAP1 IHC staining points to a diagnosis of MM.[11]
Q3 (a) - BRAF V660E: The correct answer is A. As BRAF v660E mutations are associated with melanoma, colorectal cancers, and other malignancies but not MM.
Many genetic changes have been detected in MM. The most common genetic alterations include inactivation of the tumor suppressor gene NF2, homozygous deletion of the 9p21 locus, and loss of BAP1.[12] The 9p21 locus encompasses p16INK4A (also called CDKN2A), p14ARF, MTAP, and p15INK4.[13]
Follow-up of present case (if any)
Evaluation of the cytology specimens, which contained numerous clusters of mild to moderately atypical epithelioid cells in cohesive groups of variable sizes, was suspicious for a malignant process. Biopsy from the pleura demonstrated chronic fibrinous pleuritis with an atypical mesothelial proliferation in a solid and glandular/cribriform pattern with superficial invasive growth pattern. Tumor cells showed loss of BAP-1 staining [Figure 2]. IHC studies performed on the cell block, and the biopsy specimen showed that mesothelial marker (calretinin) was strongly positive in the atypical cell groups. Other markers (CK 7, BerEp4, napsin A, TTF-1, PAX-8, and estrogen receptor [ER]) were negative. BerEp4 and calretinin IHCs are showed in Figure 3. CDKN2A (9p21) fluorescence in situ hybridization (FISH) study [Figure 4] performed on the cell block sample demonstrated the deletion in 91 of 92 cells examined (98.9%). Morphological features, FISH, and IHC stain results combined support the diagnosis of MM.
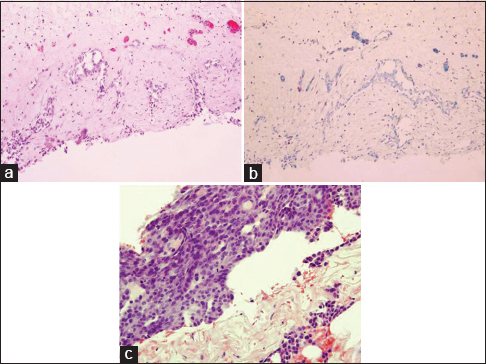
- (a) Pleural biopsy (H and E, ×100), (b) malignant cells lost BRCA1-associated protein 1 immunoreactivity (×100), (c) pleural biopsy (H and E, ×200)
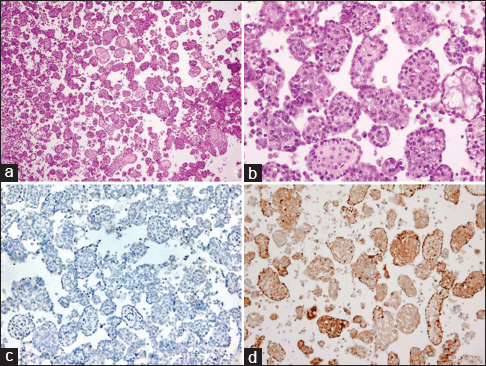
- Cell block from pleural fluid (a) H and E, ×40 and (b) H and E, ×200 (b) immunohistochemical stain (c) BerEp4 ×100, (d) Calretinin ×100
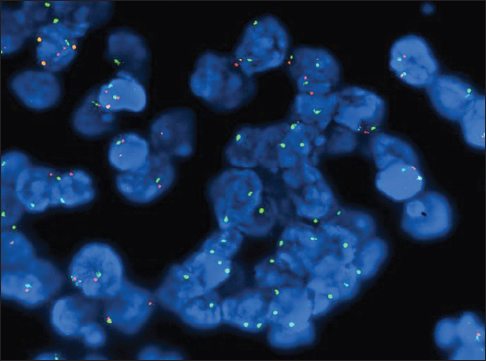
- Fluorescence in situ hybridization study for 9p21 on cell block
BRIEF REVIEW OF THE TOPIC
Role of cytology
MM is a rare primary serosal malignancy with an incidence 1–6/100,000 and accounts for <2% of malignant pleural effusion.[1415] In malignant pleural effusion, metastatic tumors are far more common than primary MM.[14] Most cases of MM are seen in male patients between the ages of 50 and 70 years and related to asbestos exposure.[16] MM patients often initially present with pleural effusion. The classic clinical scenario also includes chest pain, weight loss, shortness of breath, and persistent pleural effusion. Thoracocentesis is both therapeutic/palliative as well as a useful diagnostic procedure. The cytological examination of the pleural fluid is one of the primary diagnostic tools in these patients. The specificity of MM diagnosis is high when cytopathologic features are combined with ancillary tests.[317] Some groups propose that cytology alone is a reliable diagnostic tool when interpreted by “experienced cytopathologists” and combined with IHC characterization.[18] In addition, the presence of malignant mesothelial proliferation in pleural fluid may be sufficient for diagnosis in some patients when correlated with the clinical features and radiology studies, and when biopsy is contraindicated.[19] However, it should be noted that some guidelines indicate that the definitive diagnosis requires the demonstration of invasion by neoplastic mesothelium into stroma or subpleural fat either by histological examination or by imaging studies.[1219]
One major pitfall in attempting cytology-based effusion diagnosis of MM is relatively low sensitivity, ranging from 32% to 76%.[2317] To achieve a correct cytologic diagnosis, it is recommended that a minimum of 100 mL of effusion fluid is submitted for cytology.[16] Application of IHC and molecular techniques greatly improves diagnostic accuracy. In addition, only the epithelioid and mixed types of MM exfoliate malignant cells, while sarcomatoid and desmoplastic types are not usually detected in pleural fluid.
Focused differential diagnosis
In pleural fluid, the major differential diagnoses include MM, metastatic adenocarcinoma, and reactive mesothelial proliferation.
Malignant mesothelioma versus metastatic adenocarcinoma
In pleural fluid, numerous large clusters with prominent atypical cells and/or conspicuous atypical cells suggest malignant effusions. Metastatic adenocarcinoma is more common than primary MM.
-
The key feature for metastatic adenocarcinoma is detecting a foreign “second” population of cells in the pleural effusion, which are morphologically malignant
-
Cell clusters formed by mesothelial cells often show scalloped borders, while clusters formed by adenocarcinoma cells are more likely to have smooth or cannonball-like contours. Adenocarcinoma cells can also form acinar or glandular structures, with the central lumen containing secretion
-
Mesothelial cells contain relatively normal nuclear to cytoplasmic ratio; on the contrary, adenocarcinoma cells usually show increased N/C ratio and some degree of pleomorphism
-
Intracytoplasmic vacuoles of mesothelial cells appear empty or contain hyaluronic acid, while adenocarcinoma cells contain mucin that can be stained with mucicarmine
-
IHC stains are very helpful
-
Mesothelial markers: Calretinin, WT-1, D2-40, CK5/6, HBME-1, and thrombomodulin[234]
-
Markers for adenocarcinoma include CEA, Ber-Ep4, BG-8, B72.3, and MOC-31[35]
-
Depending on the differential diagnosis, additional markers can be added to the diagnostic panel[1220]
-
TTF-1 and Napsin A are particularly useful for lung primary carcinoma
-
CDX2 and CD20 can help distinguish gastrointestinal adenocarcinoma
-
PAX-8 and ER can suggest gynecologic primary site
-
Mammaglobin, GATA-3, and GCDFP-1 are compatible with metastatic breast carcinoma.
-
-
-
The immunocytochemical evaluation of effusion fluids can be facilitated by a strategy, “subtractive coordinate immunoreactivity pattern"
-
The cell blocks of the effusion fluids are serially sectioned, oriented identically, and labeled sequentially. Therefore, the same group of cells can be feasibly identified and evaluated for variable markers. This approach greatly assists the confirmation of the “second foreign population.”[21]
-
Malignant mesothelioma versus reactive mesothelial proliferation
-
The presence of large cell clusters (>50 cells per group) is probably the most useful clue for MM. On the contrary, benign mesothelial cell proliferation tends to disperse as isolated cells, forming monolayer cell aggregates or small clusters
-
Malignant mesothelial cells tend to be larger than reactive cells with macronucleoli and marked cytologic atypia. Reactive mesothelial cells can be very atypical with prominent nucleoli and morphologically indistinguishable
-
Ancillary tests could be helpful
-
IHC markers which favor MM when positive: EMA, p53, IMP3, and GLUT-1 and when negative: BAP1[678]
-
IHC markers which favor reactive mesothelial cells when positive: Desmin[6]
-
Detection of CDKN2A (9p21) deletion through FISH study. In the appropriate context, homozygous deletion of CDKN2A can support the definitive diagnosis of MM.
-
Molecular markers in malignant mesothelioma
One of the most common genetic mutations of MM is the homozygous deletion of the 9p21 locus, harboring p16INK4A (also called CDKN2A), p14ARF, MTAP, and p15INK4. The homozygous deletion of p16/CDKN2A should not be present in reactive mesothelial proliferation and is present in up to 80% of MM.[22232425] Therefore, detection of homozygous CDKN2A deletion can be a useful approach to detect MM. Studies have shown the sensitivity of p16 FISH lies between 58% and 79%, with almost 100% specificity, superior to IHC marker GLUT-1.[22225] It should be noted that the application of FISH is to confirm the malignancy, while IHC stain studies are necessary to identify the mesothelial origin. Homozygous deletion of the P16 gene is also a significant independent adverse prognostic factor.[26]
The BAP1 gene is a tumor suppressor gene located on chromosome 3p21, encoding a deubiquitinating enzyme, which regulates cell cycle, cellular differentiation, transcription, and DNA damage response.[27] Recently, mutations of BAP1 gene were reported in hereditary and sporadic MM, with 40%–60% in epithelioid MM and <20% in sarcomatoid MMs.[101628] More studies have shown that germline BAP1 mutations are associated with a novel cancer syndrome characterized by MM, uveal melanoma, cutaneous melanoma and melanocytic BAP1-mutated atypical intradermal tumors, and possibly by other cancers.[29]
BAP1 protein loss associated with homozygous BAP1 deletion is only seen in MMs.[30] BAP1 IHC (IHC) has been reported as a reliable marker to distinguish MM from reactive mesothelial proliferations. Studies have shown that loss of BAP1 IHC staining is highly specific (up to 100%) in distinguishing MM over benign mesothelial proliferation, with overall sensitivity of more than 50%.[11243132] Patients having MM with the presence of BAP1 mutation have a better prognosis.[33]
The finding of homozygous deletion of p16 by FISH or loss of BAP1 by IHC can be very useful diagnostic tools in differentiating benign mesothelial proliferation from MM. A drawback of p16 FISH and BAP1 IHC staining is that they have relatively low sensitivity and cannot be used to exclude the diagnosis. Co-testing with both the above-mentioned ancillary techniques improves the limited sensitivity of the individual tests.
The differentiation between MM and reactive mesothelial proliferation is diagnostically challenging due to their overlapping cytological features. FISH for p16/CDKN2A deletion and loss of BAP1 by IHC are useful tests for confirming the diagnosis of MM.
COMPETING INTERESTS’ STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All the authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author.
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is a quiz case without identifiers, our institution does not require approval from the Institutional Review Board.
LIST OF ABBREVIATIONS (In alphabetic order)
BAP1: BRCA1-associated protein 1
EMA - Epithelial membrane antigen
FISH - Fluorescence in situ hybridization
GLUT-1: Glucose transporter-1
IHC - immunohistochemistry
IMP3: Insulin-like growth factor messenger RNA-binding protein 3
MM - Malignant mesothelioma.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Guidelines for pathologic diagnosis of malignant mesothelioma: A consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2009;133:1317-31.
- [Google Scholar]
- Challenges and controversies in the diagnosis of mesothelioma: Part 1. Cytology-only diagnosis, biopsies, immunohistochemistry, discrimination between mesothelioma and reactive mesothelial hyperplasia, and biomarkers. J Clin Pathol. 2013;66:847-53.
- [Google Scholar]
- Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647-67.
- [Google Scholar]
- The diagnostic utility of D2-40, calretinin, CK5/6, desmin and MOC-31 in the differentiation of mesothelioma from adenocarcinoma in pleural effusion cytology. Acta Cytol. 2012;56:527-32.
- [Google Scholar]
- Cytologic malignancy versus benignancy: How useful are the “newer” markers in body fluid cytology? Arch Pathol Lab Med. 2008;132:23-8.
- [Google Scholar]
- The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology. 2003;43:231-8.
- [Google Scholar]
- Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using cytospin preparations. Diagn Cytopathol. 2011;39:395-401.
- [Google Scholar]
- Usefulness of EMA, GLUT-1, and XIAP for the cytologic diagnosis of malignant mesothelioma in body cavity fluids. Am J Clin Pathol. 2009;131:516-23.
- [Google Scholar]
- The value of epithelial membrane antigen expression in separating benign mesothelial proliferation from malignant mesothelioma: A comparative study. Diagn Cytopathol. 2005;32:156-9.
- [Google Scholar]
- Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022-5.
- [Google Scholar]
- BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 2015;28:1043-57.
- [Google Scholar]
- World Health Organization Committee for Tumors of the Pleura. The 2015 world health organization classification of tumors of the pleura: Advances since the 2004 classification. J Thorac Oncol. 2016;11:142-54.
- [Google Scholar]
- Introduction to the 2015 world health organization classification of tumors of the lung, pleura, thymus, and heart. J Thorac Oncol. 2015;10:1240-2.
- [Google Scholar]
- The malignant pleural effusion. A review of cytopathologic diagnoses of 584 specimens from 472 consecutive patients. Cancer. 1985;56:905-9.
- [Google Scholar]
- The established and future biomarkers of malignant pleural mesothelioma. Cancer Treat Rev. 2015;41:486-95.
- [Google Scholar]
- Clinical characteristics of patients with malignant pleural mesothelioma harboring somatic BAP1 mutations. J Thorac Oncol. 2013;8:1430-3.
- [Google Scholar]
- The role of cytologic evaluation of pleural fluid in the diagnosis of malignant mesothelioma. Chest. 1997;111:106-9.
- [Google Scholar]
- Second Italian consensus conference on malignant pleural mesothelioma: State of the art and recommendations. Cancer Treat Rev. 2013;39:328-39.
- [Google Scholar]
- Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis. 2013;5:E254-307.
- [Google Scholar]
- Evidence-based guidelines to optimize the selection of antibody panels in cytopathology: Pleural effusions with malignant epithelioid cells. Diagn Cytopathol. 2010;38:9-14.
- [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to SCIP approach. In: Shidham VB, Atkinson BF, eds. Cytopathologic Diagnosis of Serous Fluids (1st ed). Philadelphia: Elsevier, W.B. Saunders Company; 2007. p. :55-78.
- [Google Scholar]
- The diagnostic utility of p16 FISH and GLUT-1 immunohistochemical analysis in mesothelial proliferations. Am J Clin Pathol. 2011;135:619-27.
- [Google Scholar]
- Diagnostic importance of 9p21 homozygous deletion in malignant mesotheliomas. Mod Pathol. 2008;21:742-7.
- [Google Scholar]
- BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol. 2015;39:977-82.
- [Google Scholar]
- Cytologic differential diagnosis of malignant mesothelioma and reactive mesothelial cells with FISH analysis of p16. Diagn Cytopathol. 2016;44:591-8.
- [Google Scholar]
- Global gene expression profiling of pleural mesotheliomas: Overexpression of aurora kinases and P16/CDKN2A deletion as prognostic factors and critical evaluation of microarray-based prognostic prediction. Cancer Res. 2006;66:2970-9.
- [Google Scholar]
- Frequent inactivation of the BAP1 gene in epithelioid-type malignant mesothelioma. Cancer Sci. 2012;103:868-74.
- [Google Scholar]
- BAP1 cancer syndrome: Malignant mesothelioma, uveal and cutaneous melanoma, and MBAITs. J Transl Med. 2012;10:179.
- [Google Scholar]
- BAP1 immunohistochemistry and p16 FISH in the diagnosis of sarcomatous and desmoplastic mesotheliomas. Am J Surg Pathol. 2016;40:714-8.
- [Google Scholar]
- New markers for separating benign from malignant mesothelial proliferations: Are we there yet? Arch Pathol Lab Med. 2016;140:318-21.
- [Google Scholar]
- BRCA1-associated protein 1 (BAP1) immunohistochemical expression as a diagnostic tool in malignant pleural mesothelioma classification: A large retrospective study. J Thorac Oncol. 2016;11:2006-17.
- [Google Scholar]
- Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology. 2015;47:302-7.
- [Google Scholar]







