Translate this page into:
Cytologic features of tubular adenoma of ampulla causing distal common bile duct stricture: A case report and review of the literature
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Adenomas of the ampulla of Vater are distinctly rare, representing <10% of periampullary neoplasms. Very few reports of the cytologic features of ampullary adenomas are present in literature, particularly in bile duct brushing samples. A case report and review of the literature is presented. The typical cytologic features of ampullary adenomas on cytologic preparations include tall, thin columnar cells with mildly hyperchromatic elongated nuclei and nuclear pseudostratification, in a relatively clean background. The key differential diagnostic entities include invasive adenocarcinoma, thermal artifact, and reactive atypia.
Keywords
Adenoma
ampulla
bile duct brushing
ThinPrep
INTRODUCTION
Adenomas of the ampulla of Vater are distinctly rare, representing <10% of periampullary neoplasms.[1] Endoscopic biopsies, endoscopic ultrasound, and biliary brush cytology are established methods in the preoperative evaluation of the periampullary lesions.[12345] However, very few reports of the cytologic features of ampullary adenomas are present in literature.[56789] We present a case of ampullary tubular adenoma extending into the distal common bile duct diagnosed on a bile duct brushing using ThinPrep cytology preparation. The pertinent cytological features and key differential diagnosis are discussed.
CASE REPORT
Clinical history
A 53-year-old male presented to our institution with a 70-pound weight loss for evaluation of an ampullary lesion. His clinical history included Type 2 diabetes, diabetic gastroparesis, and a family history of lung, colon, and prostate cancer. Magnetic resonance cholangiopancreatography at that time showed a dilated extrahepatic biliary system with a filling defect within the distal common bile duct (CBD). The pancreatic duct was normal. No intrahepatic abnormality, vessel involvement, or pancreatic mass was seen. The considerations included a stone or a mass lesion. He had undergone esophagogastroduodenoscopy and colonoscopy at an outside facility about 2 months previously, where an ampullary polyp and an ileocecal tubulovillous adenoma were identified. Further workup at that time was precluded due to complications from a fall. He was referred to our facility for endoscopic retrograde cholangiopancreatography (ERCP) and endoscopic ultrasound (EUS).
ERCP performed at the Cleveland Clinic showed a large polyp at the ampulla along with bulging of the ampulla and extension into periampullary area as a flat, sessile component of about 2 cm in size [Figure 1]. The polyp was friable and bled on touch. There were a distal CBD stricture and intrahepatic ductal dilation seen on retrograde cholangiogram. On EUS, the lesion appeared confined to the mucosa, without extension into deeper wall layers. The polyp extended into the distal CBD causing a stricture, whereas the proximal CBD was mildly dilated at 9 mm. There was no vessel invasion or abutment, and no suspicious lymph nodes were found. During the ERCP, biopsies of the ampullary polyp were obtained together with brushings of the distal CBD stricture, and a biliary plastic stent was placed.
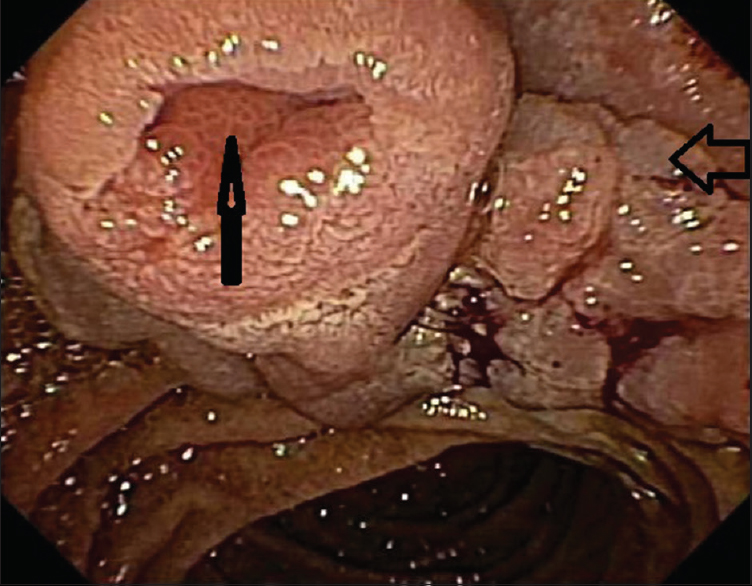
- Endoscopic image showing an ampullary adenoma involving the ampullary orifice (vertical arrow), entire ampulla and spreading laterally (horizontal arrow)
Subsequent computed tomography scan of the abdomen did not show any contiguous organ involvement, liver lesions, or enlarged lymph nodes and no ascites was noted. The patient was anicteric and liver function tests were normal. Serum tumor marker levels were within normal range (cancer antigen: 19.9–84 U/ml and carcinoembryonic antigen: 2.3 ng/ml).
Cytological findings
The CBD brushing sample was received in 30 ml of CytoLyt solution which was clear and colorless but containing multiple particles. A ThinPrep Papanicolaou-stained slide showed a cellular preparation containing cohesive clusters, strips, and single columnar cells dispersed in a clean background. Two distinct cell populations were present; the first showing bland flat honeycomb sheets of typical benign ductal epithelium [Figure 2a] and the second showing mild architectural atypia/disorganization and discernible nuclear pseudostratification/palisading, the latter seen best at the periphery of the larger two-dimensional groups [Figure 2b]. In this second population, the columnar cells were slender and taller than usual biliary epithelial cells, had basal, elongated, and slender nuclei occupying at least a third to half of the cell length, with smooth nuclear contours, and hyperchromatic evenly dispersed chromatin [Figure 2c]. Single dispersed cells were observed but did not display overt nuclear atypia [Figure 2d]. Mild inflammation was present, but necrosis not identified. A cytologic diagnosis of “Mildly atypical epithelial cells” was rendered with an accompanying comment that the cytological findings were similar to that seen in the surgical biopsy from the patient's ampullary polyp, which showed features of a tubular adenoma (TA). In this case, the use of “mildly atypical epithelial cells” was preferred over “low-grade dysplasia” or “adenomatous epithelium” at our institution as there are no defined criteria for “low-grade dysplasia” in cytology brushings, and the use was avoided to prevent misunderstanding and confusion with a clinically significant high-grade dysplasia by the clinical team.
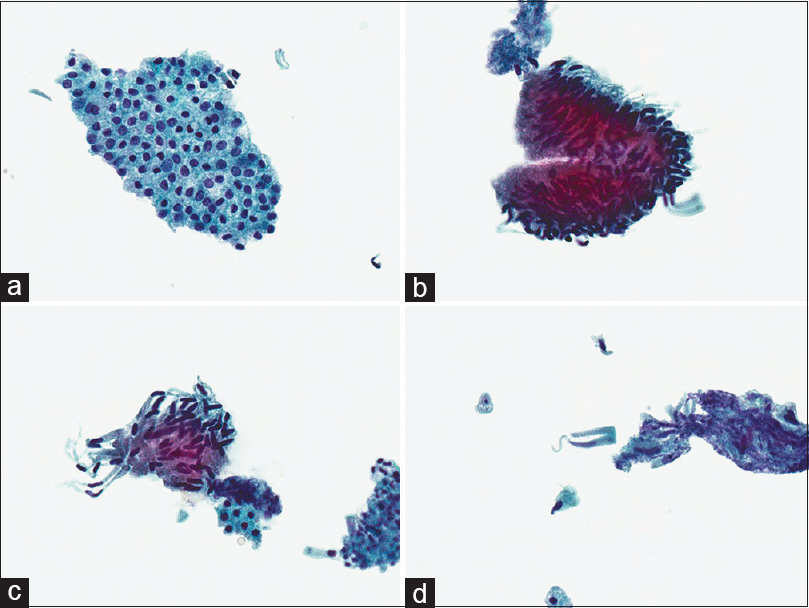
- Cytologic features of tubular adenoma on Papanicolaou-stained ThinPrep specimen. (a) Normal bile duct glandular epithelium (×400), (b) adenoma epithelium with nuclear palisading and hyperchromasia (×400), (c) adenoma epithelium with nuclear hyperchromasia, elongated thin nuclei and smooth nuclear contours (×400), (d) scattered single cells derived from the adenoma (×400)
Histological findings
Ampullary polyp biopsies were received in formalin as multiple pieces aggregating to 2.6 cm and hematoxylin- and eosin-stained sections showed features of a TA with low-grade dysplasia. No high-grade dysplasia or invasive carcinoma was identified.
Follow-up
The lesion was not amenable to local endoscopic resection based on its location, size, and involvement of distal 1 cm of the CBD. Based on the patient's significant weight loss, family history of cancer, and the large size of the lesion, a pancreatoduodenectomy (Whipple procedure) was performed to exclude an underlying malignancy.
Final histology confirmed an ampullary TA with low-grade dysplasia, 3 cm in largest dimension, extending into the distal CBD [Figure 3]. No high-grade dysplasia or mucosal invasion was seen. Thirty-four peripancreatic and periduodenal lymph nodes were examined and were negative for malignancy. The patient's postoperative course was uneventful, and he was discharged to home on day 8. At 1 month, he continues to feel well, with improved appetite and tolerating a regular diet.
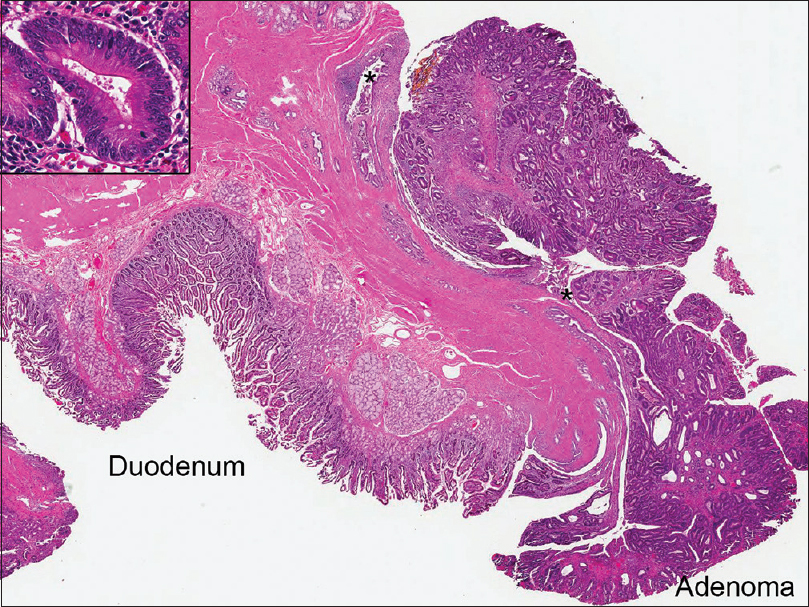
- Subsequent Whipple resection demonstrating the ampullary tubular adenoma involving the distal common bile duct (*) (×10). The insert shows the high power morphology including nuclear pseudostratification, hyperchromasia, and increased mitotic activity (×400)
DISCUSSION
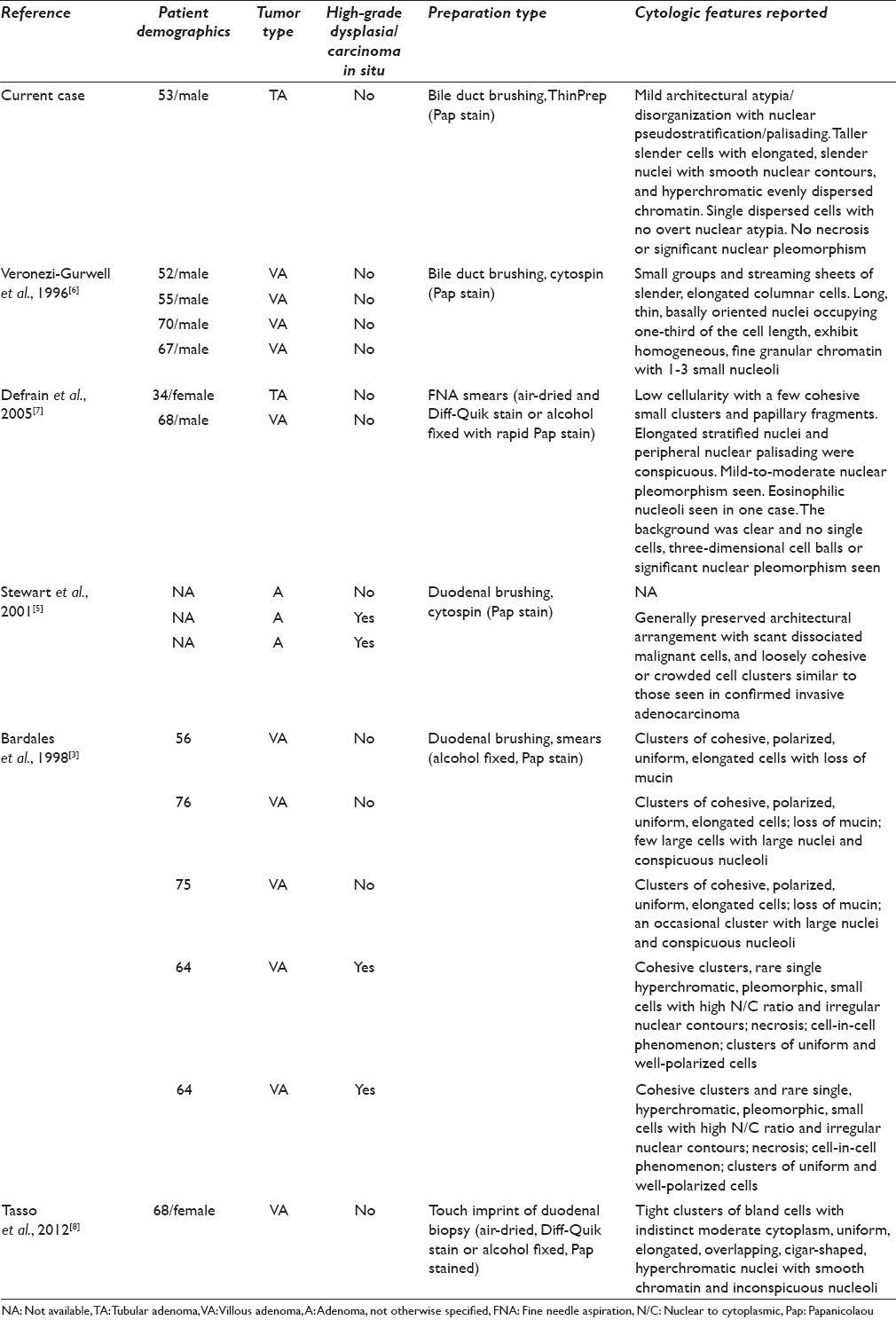
Adenomas of the ampulla of Vater are very rare neoplasms, representing 0.1%–0.2%, of the gastrointestinal tract tumors[12] and 10% of periampullary neoplasms.[1] Biliary brush cytology is an important established tool in evaluation of pancreatobiliary ductal lesions.[12345] A limited number of studies have examined the cytologic features of brush cytology for duodenal periampullary adenomas, most of which examine duodenal brushings,[359] fine needle aspiration,[7] or touch imprints of endoscopic biopsies [Table 1].[8] Only one prior publication, by Veronezi-Gurwell et al.,[6] reports the cytologic features of ampullary adenomas on bile duct brushings. The current report is the first to describe the cytologic features of an ampullary adenoma on a ThinPrep preparation identified on bile duct brushing cytology.
The cytologic features present in this case are similar to those previously reported in the literature: tall, thin columnar cells with mild disorganization, mild nuclear overlap, discernible nuclear pseudostratification, elongated nuclei with mild hyperchromasia and fine granular chromatin with small nucleoli, and a relatively clean background.[56789] In addition, we observed singly dispersed tall columnar cells which had identical nuclear features as the cells in clusters. This finding is more commonly seen in liquid-based preparations due to mechanical disruption of the groups and should not be misinterpreted as a sign of malignancy.[5] As previously reported, the ThinPrep preparation provides well-preserved lesional cells in a clean background unaccompanied by blood or air-drying artifacts which may overwhelm direct smears and hinder adequate interpretation.[9]
The differential diagnosis includes both benign/reactive conditions and malignant lesions. It is common to see significant reactive atypia in bile duct brushings. In both reactive changes and in adenomas, it is common to observe mild cellular disorganization, smooth nuclear contours, and nucleoli. However, adenomas show significantly more elongated and hyperchromatic nuclei with pseudostratification, rather than the typical round to slightly oval nuclei, vesicular chromatin, and prominent nucleoli of reactive change.[710] Furthermore, reactive changes are typically associated with more significant acute inflammation than seen in ampullary adenoma samples.
A more difficult problem is distinguishing thermal artifact from adenomatous change in bile duct cytology. Thermal artifact can cause significant nuclear elongation, hyperchromasia, and the appearance of pseudostratification.[11] Features which favor thermal artifact include elongated, stromal-like bizarre cells arranged singly or in loose clusters and stacks resembling sheaves of wheat, with streaming and cytoplasmic tails as well as elongated “taffy-pull” smudged nuclei.
The distinction of adenoma from malignancy is of particular concern. Recognition of an ampullary adenoma is important as these neoplasms harbor significant malignant potential, with invasive carcinoma in over 25% of patients;[2] therefore, resection is warranted.[1212] Ampullary adenomas can have high-grade dysplasia which is cytologically indistinguishable from invasive adenocarcinoma on brushing samples.[357] Thankfully, cytologic distinction of high-grade dysplasia from invasive adenocarcinoma is typically not necessary, as both require resection and histopathologic evaluation for further treatment. Cytologic features favoring high-grade neoplasia on bile duct brushings include marked nuclear pleomorphism (including >4-fold variation in nuclear size, marked nuclear enlargement, and nuclear membrane irregularities), high nuclear-to-cytoplasmic ratio, prominent macronucleoli, and singly dispersed abnormal cells.[3579] The cytologist should be vigilant to not overlook a subset population of malignant cells, even in the background of an ampullary adenoma.
SUMMARY
We have described in detail the characteristic cytologic findings of TA of the ampulla involving the distal CBD as observed in a ThinPrep liquid-based preparation. The presence of single dispersed cells in the background does not prompt the diagnosis of malignancy as long as they show the typical elongated, hyperchromatic nuclei with minimal nuclear pleomorphism typical of adenomas.
COMPETING INTERESTS’ STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author.
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. SM performed the literature review, participated in study data acquisition, coordinated the study completion, and drafted the manuscript. MS participated in study data acquisition and critically reviewed the manuscript draft. DC conceived of the study, participated in the design, critically reviewed the manuscript draft and is corresponding author. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved. As this is case report without identifiers, our institution does not require approval from the Institutional Review Board (or its equivalent).
ETHICS STATEMENT BY ALL AUTHORS
As this manuscript is a descriptive case report with without identifiers, institutional review board approval was not required.
LIST OF ABBREVIATIONS (In alphabetic order)
A - Adenoma, not otherwise specified
CBD - Common bile duct
EGD - Esophagogastroduodenoscopy
ERCP - Endoscopic retrograde cholangiopancreatography
EUS - Endoscopic ultrasound
FNA - Fine needle aspiration
MRCP - Magnetic resonance cholangiopancreatography
N/C - Nuclear to cytoplasmic
NA- Not available
TA - Tubular adenoma
VA - Villous adenoma.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Ampullary adenoma: Clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649-69.
- [Google Scholar]
- Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am. 2013;23:95-109.
- [Google Scholar]
- Diagnostic value of brush cytology in the diagnosis of duodenal, biliary, and ampullary neoplasms. Am J Clin Pathol. 1998;109:540-8.
- [Google Scholar]
- The role of endoscopy in ampullary and duodenal adenomas. Gastrointest Endosc. 2015;82:773-81.
- [Google Scholar]
- Brush cytology in the assessment of pancreatico-biliary strictures: A review of 406 cases. J Clin Pathol. 2001;54:449-55.
- [Google Scholar]
- Cytologic features of villous adenoma of the ampullary region. Diagn Cytopathol. 1996;14:145-9.
- [Google Scholar]
- Cytologic features and diagnostic pitfalls of primary ampullary tumors by endoscopic ultrasound-guided fine-needle aspiration biopsy. Cancer. 2005;105:289-97.
- [Google Scholar]
- Endoscopic ultrasound guided fine-needle aspiration diagnosis of duodenal high grade neuroendocrine carcinoma underlying a villous adenoma: Report of a case. Diagn Cytopathol. 2012;40:62-8.
- [Google Scholar]
- Pancreatic and bile duct brushing cytology in 1000 cases: Review of findings and comparison of preparation methods. Cancer. 2006;108:231-8.
- [Google Scholar]
- Accuracy and morphologic aspects of pancreatic and biliary duct brushings. Acta Cytol. 1995;39:11-8.
- [Google Scholar]
- Cytomorphology of laser/thermal artifact in ductal cells in a post-laser surgery bile duct brushing specimen. Acta Cytol. 2005;49:226-8.
- [Google Scholar]
- A case of ampullary adenoma that developed to cancer 7 years after initial diagnosis. Am J Case Rep. 2015;16:586-9.
- [Google Scholar]








