Translate this page into:
Amyloidoma secondary to insulin injection: Cytologic diagnosis and pitfalls
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Amyloidomas are rare tumors composed of deposits of amyloid protein not associated with systemic amyloidosis. They can present as an initial manifestation of a systemic disease process or can be a completely localized phenomenon. We present a case of amyloidoma associated with insulin injection site found incidentally in an 80-year-old male with multiple co-morbidities who presented with diverticulitis associated bleeding. A subcutaneous abdominal mass was found on physical examination. Imaging revealed a 5 cm × 1.6 cm homogenous subcutaneous lesion. A fine-needle aspiration (FNA) and core biopsy were performed under ultrasound guidance to reveal amorphous material proven to be amyloidosis at insulin injection sites (AIns) type amyloid. The patient had no treatment for this lesion and has had his care triaged to his more serious health problems. This is the first case of AIns type amyloidoma associated with insulin injection site reported in cytology literature. We highlight the cytologic findings and diagnostic pitfalls. As the incidence of diabetes is increasing, cytopathologists may encounter this lesion more often on FNA.
Keywords
Amyloid
amyloidoma
amyloidosis at insulin injection sites
diabetes
fine-needle aspiration
insulin injection site
INTRODUCTION
Amyloidosis is a heterogeneous group of diseases caused by extracellular deposition of amorphous amyloid material that can result in tissue damage. Amyloidosis is associated with many different underlying disorders such as plasma cell dyscrasia, chronic renal failure, and chronic inflammatory conditions such as tuberculosis and malignancies.[12] Systemic forms are more common and can affect many organs. An amyloidoma, or localized deposition of amyloid, is a rare form of amyloidosis. Amyloidomas have been reported in many sites including upper respiratory tract, lung, skin, gastrointestinal (GI) and urinary tracts, thymus, thyroid gland, brain, soft tissues, and bone.[345678910111213] Local amyloid depositions have also been reported in at insulin injection sites of diabetics.[1415161718192021] In fact, in diabetics, injection associated localized amyloid deposition is more common than systemic amyloidosis; an alternative name for this is iatrogenic endocrine amyloidosis. The insulin peptide (either porcine or human recombinant) is a precursor to amyloidosis at insulin injection sites (AIns) (insulin) type amyloid. This is in contrast to systemic amyloid which is more typically composed of amyloid light (AL) or amyloid A (AA) amyloid proteins.
We present a case of an amyloidoma in an 80-year-old man with multiple medical problems including long-standing type 2 insulin dependent diabetes. To our knowledge, all reported cases of amyloidomas associated with insulin injection sites have been diagnosed on surgical resections or punch biopsies; this is the first report of an insulin injection-related amyloidoma encountered on a fine-needle aspiration (FNA) specimen. Amyloidoma was not suspected at the time of on-site evaluation or as the final diagnosis. In this report, we emphasize the cytologic characteristics of this lesion and point out the potential diagnostic pitfalls.
CASE REPORT
An 80-year-old male with a significant medical history including myasthenia gravis diagnosed in 2006 controlled with azathioprine, coronary artery disease status postcoronary artery bypass graft in 1982, and long standing insulin dependent diabetes, first presented with a GI bleed due to diverticulitis. A mobile subcutaneous mass in the abdominal wall was noted on physical exam. CT scan showed a 5 cm × 1.6 cm homogenous mass [Figure 1]. An ultrasound guided FNA and core biopsy were done.
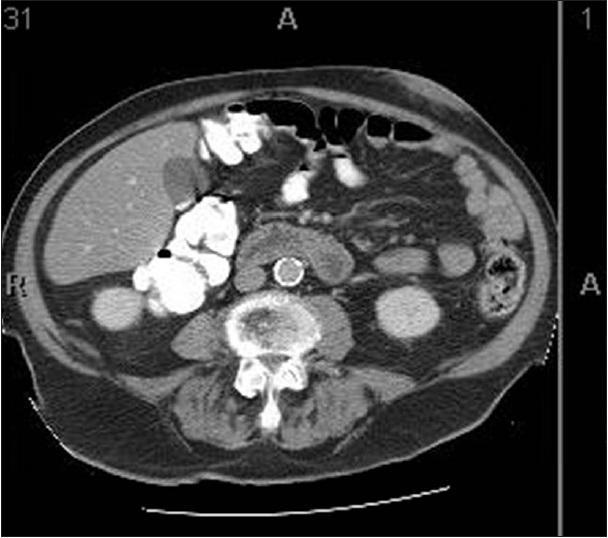
- Computed tomography image of abdominal wall lesion. Sections were taken at 5 mm intervals. Image 31 of series #2 shows greatest dimensions of lesion as well as its homogenous nature
Cytologic findings
Immediate adequacy assessment during the procedure was deemed to be inadequate for all three passes. Needle rinses were collected for a cell block. Smears contained abundant material and showed small and large aggregates of thick orangeophilic material with a flaky texture on Papanicolaou stain and fluffy magenta colored acellular material on Diff Quik stain [Figures 2 and 3]. Smaller aggregates mimicked anucleated squamous cells whereas larger collections of this material were interpreted as proteinaceous fluid and keratin debris. Scattered spindled cells were also present. Cell block contained scant pink acellular proteinaceous material. The FNA was signed out as “anucleated squamous cells, and acellular keratin debris, consistent with epidermal inclusion cyst or dermoid cyst.”
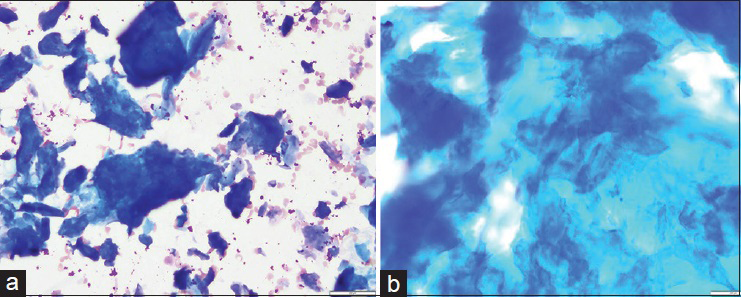
- Fine-needle aspiration smears of amyloid, Diff Quik stain. (a) Smear shows abundant deep blue/purple colored amorphous material (×20). (b) On high power, this material resembles has tissue paper appearance, resembling keratin debris (×40)
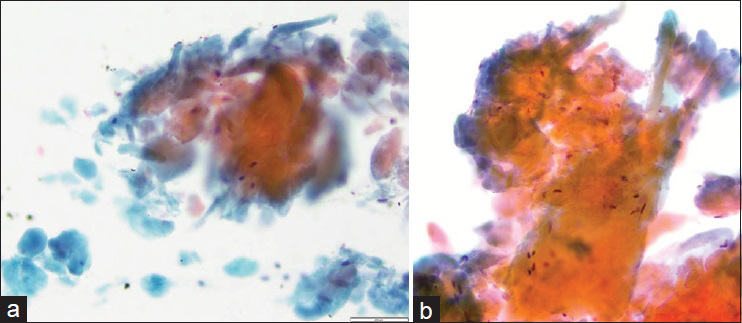
- Fine-needle aspiration smears of amyloid, Papanicolaou stain. (a) Orangophilic waxy material is abundant on this smear. Smaller globules in the lower left corner mimic anucleated squamous cells (×20). (b) High power demonstrates dense waxy appearance of amyloid; a few spindled cells are seen intermixed with amyloid (×40)
Core biopsy findings
Core biopsy showed fragments of a cellular pink waxy material and hyalinized tissue [Figure 4a]. Cytokeratin stains were performed on the surgical specimen and were negative. Congo red stain was positive; green birefringence was seen under polarized light [Figure 4b]. Lambda and kappa immunohistochemical stains did not show restriction.
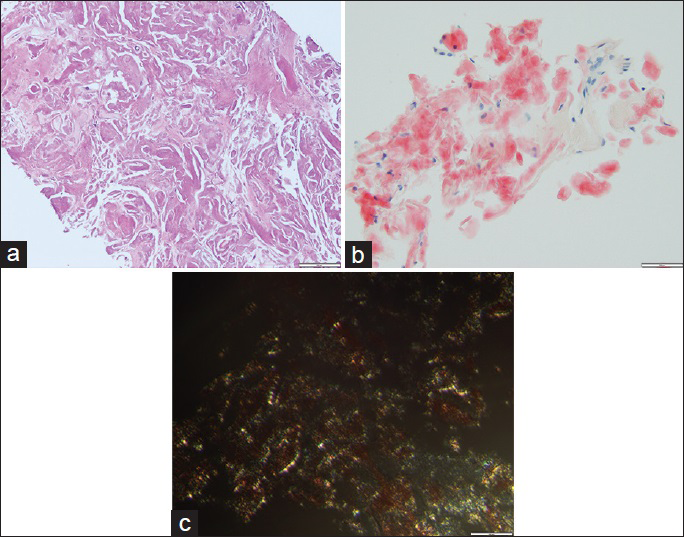
- Core biopsy. (a) This core biopsy slide shows amorphous eosinophilic dense material (H and E, ×20). (b) The material is homogeneous and red with Congo stain (Congo, ×20). (c) The core biopsy shows apple green/yellow birefringence (Congo, under polarized light, ×40)
Amyloid subtyping was done by mass spectrometry (MS) on core biopsy. Liquid chromatography tandem MS (LC MS/MS) was performed on peptides extracted from Congo red-positive/micro dissected areas of paraffin-embedded tissue. LC MS/MS detected a peptide profile consistent with AIns (insulin)-type amyloid deposition. AIns amyloidosis was diagnosed on this sample.
Follow-up
The lesion was not resected. The patient has since required several hospitalizations for recurrent GI bleeding but has not had any complaints related to his amyloidoma.
DISCUSSION
Systemic amyloidosis as part of an underlying disease such as epithelial and hematologic malignancies and chronic inflammatory or autoimmune conditions is relatively common compared to localized amyloid depositions (amyloidomas). Amyloidomas have been reported in many anatomic sites ranging from head and neck to bone and soft tissues.[34567891011121322232425] Most amyloidomas are not clinically suspected and are associated with AL or AA amyloid proteins. The AIns subtype has only been described in patients with diabetes and then only in amyloidomas associated with insulin injection site.[19] These lesions are not known to recur or progress. However, they have been associated with poor absorption of insulin.
Fine-needle aspiration diagnosis of amyloidoma has been described in various sites.[3491322232425] Amyloid appears as homogenous aggregates of waxy cyanophilic to eosinophilic material on Papanicolaou-stained smears and irregular clumps of metachromatic to purple/blue material on Diff Quik stained smears. A foreign body giant cell reaction and/or lymphoplasmacytic inflammation may be seen. When amyloid forms a tumoral mass, cytologic diagnosis can be elusive in the absence of clinical suspicion. Amyloid can mimic necrotic material/debris, fibrous tissue, scar/sclerotic collagen, or cartilage, anucleated squamous cells suggesting an epidermal inclusion cyst, acellular proteinaceous material suggesting any other type of cyst such as a ganglion cyst based on aspiration site, fibrin in cell blocks and dense colloid.[342627] A diagnosis of a nondiagnostic aspirate can be rendered due to the presence of debris without cells.[26] In each of these instances, an accurate recognition of amyloid is possible once the thought is entertained in the differential. For example, there are subtle differences between amyloid and necrotic debris; necrotic debris appears more dirty and granular with ghost of cells compared to the more waxy, dense and homogenous nature of amyloid. In the case presented herein, there was more than the adequate material on smears and cell block for diagnosis. The cytologic features were misinterpreted as nucleated and anucleated squamous cells in the background of amorphous proteinaceous debris, therefore, a diagnosis of an epidermal inclusion cyst was rendered. We were not aware at the time that the patient had diabetes and was injecting insulin at this site. Diagnosis of amyloidoma was established on the core biopsy performed at the same time through Congo red staining and mass spectrometry.
It is important that as cytopathologists, we are aware of this entity and consider it in the differential diagnosis of a subcutaneous mass in a patient who is a known insulin dependent diabetic. To the best of our knowledge, this is the first cytologic description of AIns type amyloidoma occurring at insulin injection site in the cytology literature. Imaging findings are often suggestive of neoplasm in these cases. An accurate diagnosis on FNA will eliminate a surgical intervention.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without patient identifiers, our institution does not require approval from the Institutional Review Board (IRB) (or its equivalent).
LIST OF ABBREVIATIONS
FNA - Fine-needle Aspiration
AIns - Amyloidosis at Insulin Injection Sites
GI - Gastrointestinal
AL - Amyloid light
AA - Amyloid A
MS - Mass spectrometry
IRB - Institutional Review Board
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through the automatic online system.
REFERENCES
- Systemic amyloidosis in England: An epidemiological study. Br J Haematol. 2013;161:525-32.
- [Google Scholar]
- Amyloidomas of soft parts: Diagnosis by fine-needle aspiration. Diagn Cytopathol. 2012;40(Suppl 2):E126-30.
- [Google Scholar]
- Fine-needle aspiration diagnosis of an intraosseous amyloidoma. Diagn Cytopathol. 2012;40(Suppl 2):E114-7.
- [Google Scholar]
- Primary localized amyloidosis of the bladder with a monoclonal plasma cell infiltrate. Pathology. 1988;20:67-9.
- [Google Scholar]
- Amyloidosis presenting in the respiratory tract. Pathol Annu. 1989;24(Pt 1):253-73.
- [Google Scholar]
- Tumoral presentation of amyloidosis (amyloidomas) in soft tissues. A report of 14 cases. Am J Clin Pathol. 1993;100:135-44.
- [Google Scholar]
- Amyloid tumor. A clinicopathologic study of four cases. Am J Surg Pathol. 1978;2:141-5.
- [Google Scholar]
- Nodular goiter with amyloid deposition in an elderly patient: Fine-needle cytology diagnosis and review of the literature. BMC Surg. 2013;13(Suppl 2):S43.
- [Google Scholar]
- Cutaneous amyloidosis and possible association with systemic amyloidosis. Int J Dermatol. 2002;41:127-32.
- [Google Scholar]
- Localized thymic amyloidosis presenting with myasthenia gravis: Case report. J Korean Med Sci. 2014;29:145-8.
- [Google Scholar]
- A first report of endoscopic ultrasound for the diagnosis of pancreatic amyloid deposition in immunoglobulin light chain (AL) amyloidosis (primary amyloidosis) JOP. 2013;14:283-5.
- [Google Scholar]
- Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-9.
- [Google Scholar]
- Human recombinant insulin and amyloidosis: An unexpected association. Neth J Med. 2010;68:138-40.
- [Google Scholar]
- Localized insulin-derived amyloidosis in patients with diabetes mellitus: A case report. Hum Pathol. 2009;40:1655-60.
- [Google Scholar]
- Examination of insulin injection sites: An unexpected finding of localized amyloidosis. Diabet Med. 2002;19:881-2.
- [Google Scholar]
- Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-61.
- [Google Scholar]
- Localized amyloidosis at the site of repeated insulin injection in a diabetic patient. Intern Med. 2010;49:397-401.
- [Google Scholar]
- Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:e200.
- [Google Scholar]
- Localized insulin-dependent amyloidosis with scar-tissue formation. J Am Acad Dermatol. 2014;71:e160-2.
- [Google Scholar]
- Nodular pulmonary amyloidosis with an unusual protein composition diagnosed by fine-needle aspiration biopsy: A case report. Diagn Cytopathol. 2009;37:286-9.
- [Google Scholar]
- Primary amyloidosis involving mesenteric lymph nodes: Diagnosis by fine-needle aspiration cytology. Acta Cytol. 2011;55:296-301.
- [Google Scholar]
- Fine-needle aspiration cytology of amyloid associated with nonneoplastic and malignant lesions. Diagn Cytopathol. 1998;18:270-5.
- [Google Scholar]
- Amyloid tumor: A clinical and cytomorphologic study. Diagn Cytopathol. 2003;28:325-8.
- [Google Scholar]
- Soft tissue amyloidoma of the extremities: A case report and review of the literature. Arch Pathol Lab Med. 2004;128:1270-3.
- [Google Scholar]








