Translate this page into:
Architectural aspects of cell-blocks as small biopsies

*Corresponding author: Liron Pantanowitz, Department of Pathology, University of Michigan, Michigan, United States. lironp@med.umich.edu
-
Received: ,
Accepted: ,
How to cite this article: Satturwar S, Pantanowitz L. Architectural aspects of cell-blocks as small biopsies. CytoJournal 2021;18:5.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/Cytojournal_4_2021
Abstract
Cell-block preparations have become an essential part of integrated cytology diagnosis. They are essentially microbiopsies that are formalin fixed and embedded in paraffin. This has become more prevalent with greater sample procurement due to the advent of newer biopsy techniques and needles. Cell-blocks allow retrieval of small tissue fragments from cytology specimens that sometimes cannot be processed by alternate cytologic techniques. They represent concentrated, cell-enriched preparations that provide cytologists with the opportunity to evaluate cellular architecture, as well as to perform ancillary testing. A cell-block compatible sample may thus obviate the need for a more invasive procedure such as a tissue biopsy. Microscopic examination of cell-blocks is quick, avoids obscuring material, permits cells to be evaluated in one focal plane, and allows the histologic architecture such as glandular differentiation, papillary formations, and sometimes invasion to be easily identified. This new era of “cytohistology” accordingly requires practicing cytologists to become more familiar with histopathology. This review article discusses the benefit of various architectural patterns identifiable in cell-blocks employed as an adjunct to Pap tests, exfoliative fluid specimens, and fine-needle aspirations.
Keywords
Biopsy
Cell-block
CellBlockistry
Small biopsy
FFPE
Surgical pathology
Histopathology
Cytohistology
Fine-needle aspiration

INTRODUCTION
Cell-blocks are essentially microbiopsies that are formalin fixed and embedded in paraffin. Cell-block preparation allows for the retrieval of small sample fragments in cytology specimens that often cannot be processed by other cytologic techniques (e.g., direct smear, and liquid-based preparations). In 1955, Richardson et al. utilized cell-blocks for fluid specimens and concluded that by allowing for histologic pattern recognition these preparations improved cancer diagnosis.[1] Subsequently, many laboratories began preparing cell-blocks for various specimen types.[2-5] Most opted for a more selective approach based on their anticipated need for ancillary testing.[6-8] Today, cytology laboratories routinely prepare cell-blocks for a variety of cytology specimen types such as body cavity fluids, washings, and fine needle aspirations (FNA) as a part of an integrated cytology diagnosis.[2-10]
Cell-block preparation methods have evolved considerably over the years. As a result, modern sophisticated methods have been able to generate cell-blocks with superior cellular yields, including the preservation of small tissue fragments.[11] This has become particularly important today with greater sample procurement from deep body sites made possible due to the advent of newer biopsy techniques (e.g., fiber optic endoscopy) [12,13] and next generation needles (e.g., SharkCore).[14-16] Hence, these endoscopy-guided procedures are now considered to be fine-needle biopsies. Moreover, rapid on-site evaluation of material has optimized specimen collection and triage. Additional dedicated FNA passes for cell-blocks have also become commonplace.
A well prepared cell-block creates the opportunity to examine the histological architecture of cytology material, as well as perform ancillary tests. As such, a cell-block compatible sample can sometimes obviate the need for a more invasive biopsy procedure. Microscopic examination of cell-blocks is quick, avoids obscuring material, permits cells to be evaluated in one focal plane, and allows for histologic architecture such as glandular differentiation, papillary formations, and sometimes invasion to be easily identified. This new era of “cytohistology” accordingly requires practicing cytologists to become more familiar with histopathology. This review article discusses the benefits of various architectural patterns identifiable in cell-blocks employed as an adjunct to Pap tests, exfoliative fluid specimens, and FNAs.
PAP TEST CELL-BLOCKS
Several publications have confirmed the diagnostic utility of cell-blocks as being complementary to conventional or liquid based cervico-vaginal cytology specimens.[16-20a] The use of cell-blocks in this context has increased Pap test screening sensitivity by identifying more malignancies and reducing the false positive rate of cervical cytology specimens. The value of making a cell-block from a Pap test sample is to uncover cellular architecture, or perform immunohistochemistry, to help reach a definitive diagnosis in challenging cases.[20b] As such, Pap test cell-blocks have accordingly been used to stratify the diagnosis of hyperchromatic crowded groups (HCGs),[20c,20d] distinguish glandular from squamous lesions involving glands, separate immature squamous metaplasia from high grade squamous intra-epithelial lesions (HSIL), discern repair from squamous cell carcinoma (SCC), and discriminate tubal metaplasia from atypical glandular cells (AGCs).
HCGs represent one of the most common cytologic patterns observed in Pap test litigation cases in false negative cases.[21] The differential diagnosis of a HCG is wide [Figure 1] and includes benign lesions (e.g., atrophy, tubal metaplasia, and immature squamous metaplasia) and neoplastic lesions (e.g., HSIL, endocervical adenocarcinoma in situ, endocervical or endometrial adenocarcinoma, and SCC) that present as syncytial aggregates of hyperchromatic cell clusters. The inability to see central areas of these HCGs and easily focus on all cells may lead to misdiagnosis in some cases. The identification of various cytologic abnormalities and the presence or absence of surface maturation can help distinguish certain squamous and glandular lesions of the cervix. For example, in low grade squamous intraepithelial lesions (LSIL), or cervical intraepithelial neoplasia (CIN) I, of the cervix the surface epithelium shows maturation, koilocytic atypia is limited to the upper layers, and the basaloid cells are present in the lower third of the epithelium. On the other hand, with HSIL abnormal cells occupy the lower two-thirds (CIN II) or entire thickness (CIN III) of the epithelium. Cell-blocks may contain fragments of intact cervical tissue that allows architecture to be analyzed such as full thickness nuclear abnormalities. In addition, ancillary tests like Ki-67 and p16 immunohistochemistry can aid in the diagnosis for equivocal cases.[22,23] Cell-blocks can also help avoid the overdiagnosis of tubal metaplasia as AGC by identifying endocervical glands with long terminal cilia [Figure 2]. Many studies have addressed the utility of cell-blocks in the work-up of AGCs and their role in distinguishing glandular from squamous lesions, confirming that cell-block preparations improve the diagnostic rate and can avoid unnecessary biopsies.[24-27] Cell-blocks are also useful for other glandular lesions. Manini et al. reported a specificity of 96% for diagnosing endometrial lesions using cell-blocks and Yang et al. similarly reported improved diagnostic efficacy for endometrial lesions.[28,29]
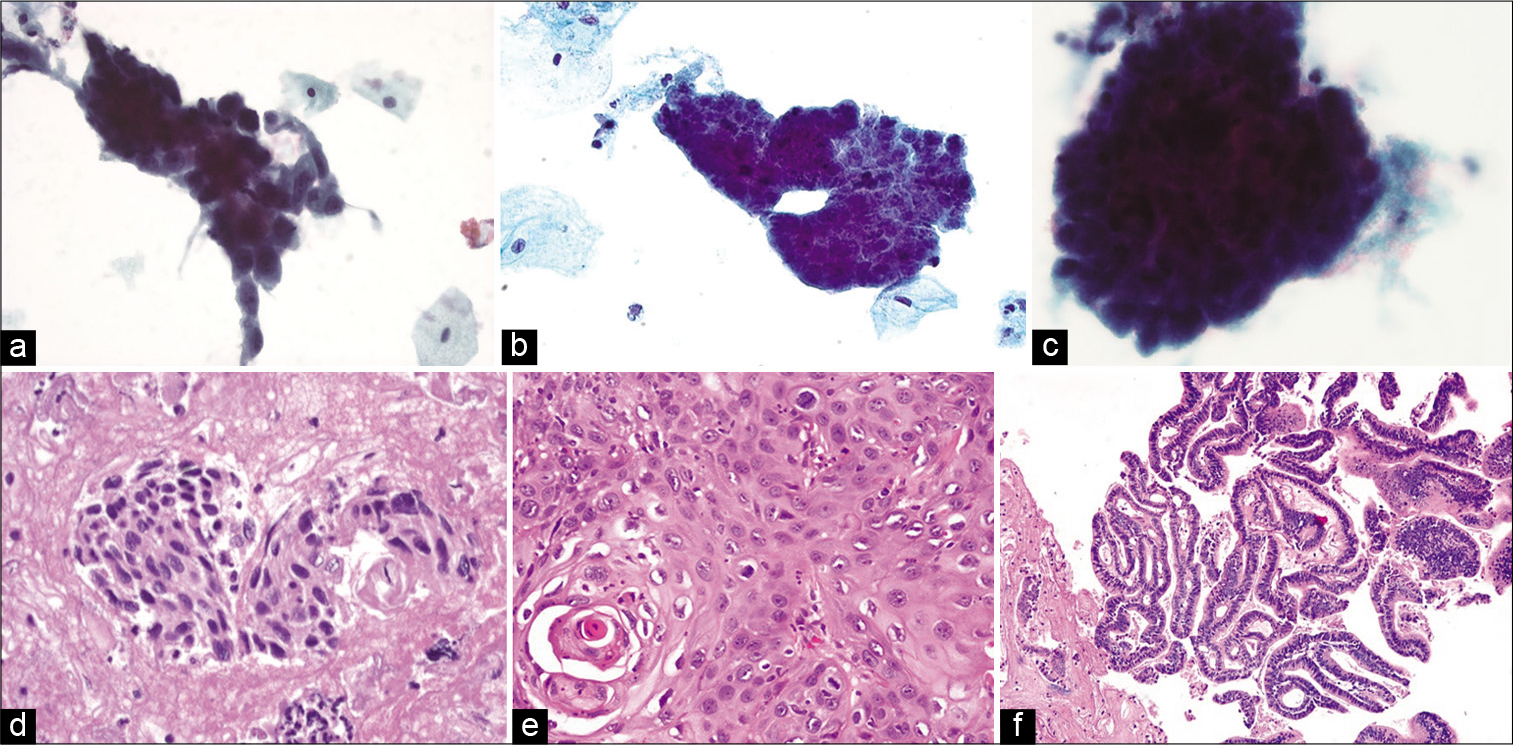
- Hyperchromatic crowded groups showing (top panel a-c) their appearance in liquid-based preparations (ThinPrep, Papanicolaou stain, ×400) with (bottom panel) their corresponding histopathologic appearance. (d) HSIL showing dense eosinophilic cytoplasm of squamous cells in the cell-block (hematoxylin and eosin, ×400). (e) Squamous cell carcinoma with a concurrent microbiopsy showing squamous differentiation (hematoxylin and eosin, ×400). (f) Endocervical adenocarcinoma where the cell-block highlights glandular lumens on cross-section (hematoxylin and eosin, ×200).
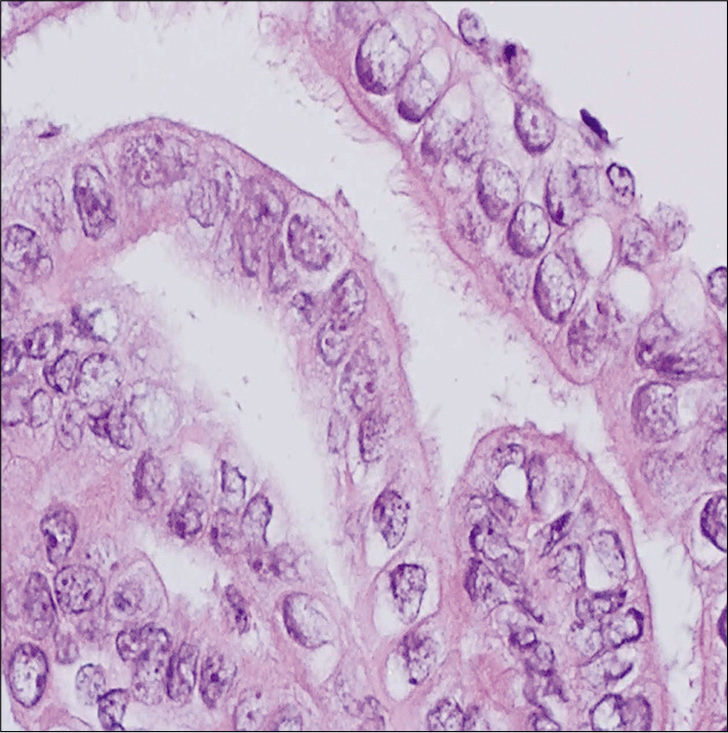
- Cell-block from a Pap test showing strips of endocervical glands with readily identified tubal metaplasia. Despite the nuclear enlargement, note the prominent terminal bars with cilia on the surface (hematoxylin and eosin, ×400).
NON-GYNECOLOGICAL SPECIMEN CELL-BLOCKS
Effusions
Cell-block preparations have been utilized for effusion specimens over many years and they are invaluable in the work-up of neoplastic effusions.[30-34] Effusion cytology along with cell-block preparation is superior to tissue biopsy of a body cavity as it is less invasive, allows wider sampling and conveys high diagnostic accuracy.[35,36] In most cases, the cytologic diagnosis of a malignant effusion is straightforward. While the optimum number of effusion specimens suggested for reaching a positive diagnosis is three, in the majority (up to 90% of cases) metastatic carcinoma can often be detected on the first specimen.[37-40] Malignancies can present as large clusters (e.g., metastatic adenocarcinoma) or single cells (e.g., gastric signet ring carcinoma, melanoma, and sarcoma). Rarely, effusions may manifest with a mesotheliomatous pattern of metastatic adenocarcinomas where the specimen contains only single malignant cells.[41-51]
Cytomorphologic features that are helpful in the identification of metastatic carcinoma include cell size (e.g., very large cells), cell shape (e.g., bizarre cells), presence of a second cell population other than mesothelial cells [Figure 3], numerous large three dimensional clusters, cell configuration (e.g., tumor cannibalism [Figure 4], spherical clusters, and lumina), cytoplasmic findings (e.g., intracellular mucin, and melanin), nuclear features (e.g., increased nuclear-cytoplasmic ratio, hyperchromasia, bizarre shapes, and atypical mitoses), and a bloody or mucinous background. In metastatic breast carcinoma, the finding of cannonball shaped cell clusters is a helpful clue [Figure 5]. The glandular differentiation of large cannonball clusters is most apparent on cell-blocks where a central lumen can be readily observed on cross section. Features that may help distinguish metastatic adenocarcinoma from mesothelioma include smooth, community borders in clusters of metastatic adenocarcinoma as opposed to knobby, flower-like outlined clusters of mesothelioma. While it may be easy to recognize papillae when present in effusions, these architectural structures are easy to appreciate in cell-blocks. Such papillae may be observed with metastatic carcinoma such as lung, breast or ovarian serous carcinoma [Figure 6], as well as with papillary mesothelial hyperplasia and malignant mesothelioma [Figure 7].
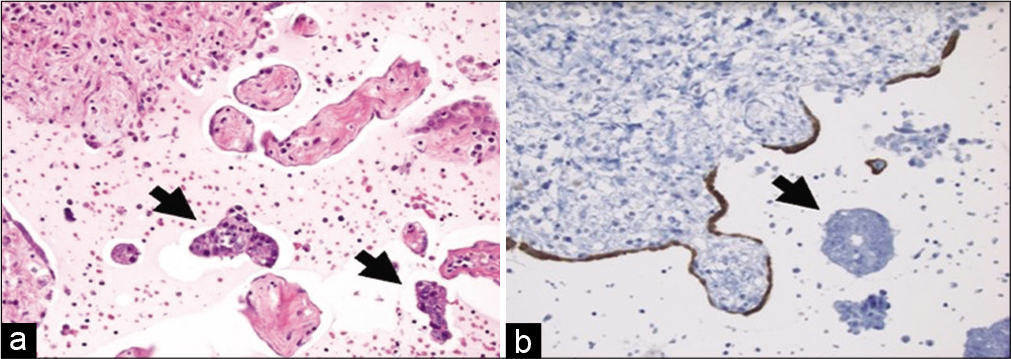
- Cell-block from a peritoneal wash with metastatic colorectal adenocarcinoma. (a) Note the clusters of malignant cells (arrows) that are distinct from the many fragments of mesothelial-lined peritoneum (hematoxylin and eosin, ×200). (b) A calretinin stain highlights an attenuated benign mesothelial lining (immunohistochemistry, ×200).
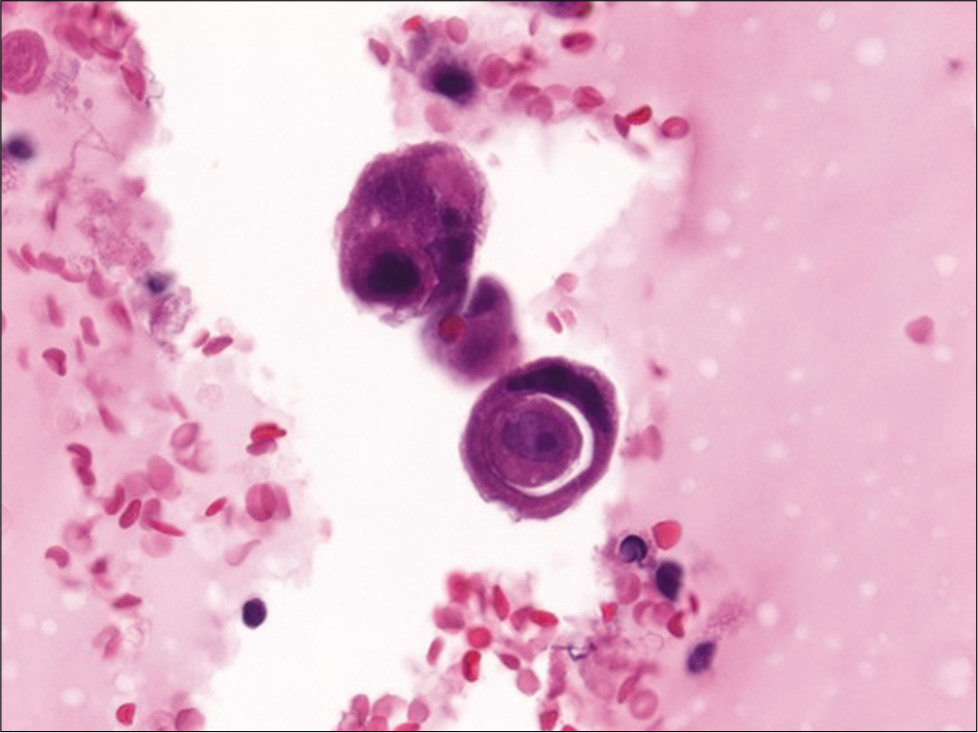
- Cell-block section of a pleural effusion with metastatic breast carcinoma showing a cell-in-cell appearance due to tumor cannibalism (hematoxylin and eosin, ×600).
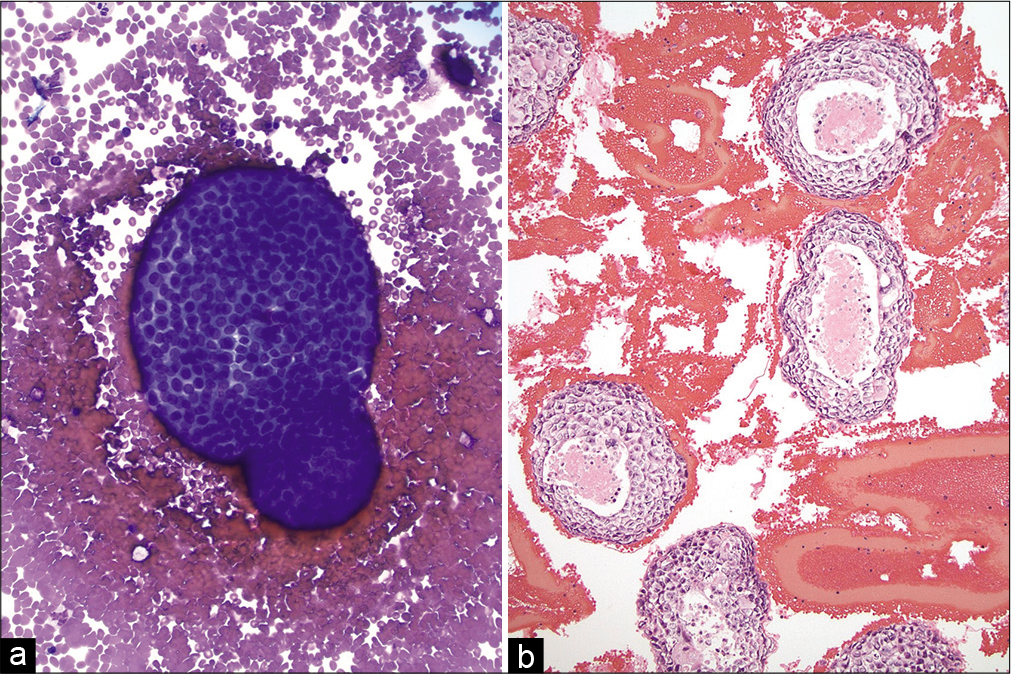
- (a and b) Pleural effusion with metastatic breast ductal carcinoma. (Left) A large cannonball cluster of metastatic carcinoma cells is shown with a smooth, community border (Cytospin, Diff Quick stain, ×200). (Right) Cell-block showing many large clusters of metastatic breast carcinoma with central comedo necrosis (hematoxylin and eosin, ×200).
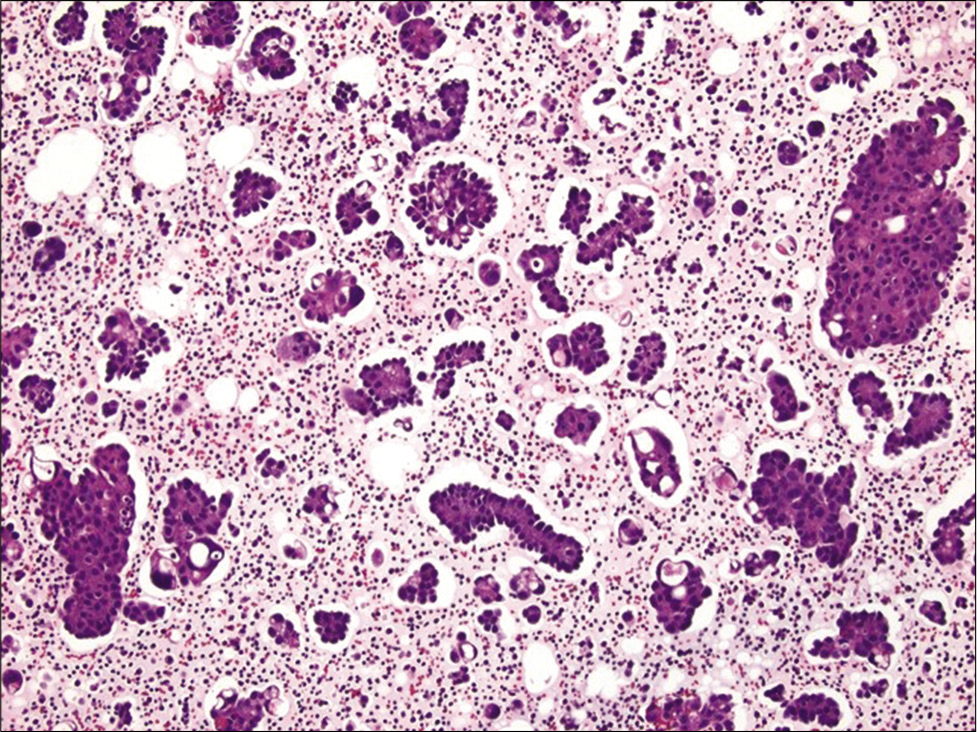
- Cell-block section of ascitic fluid showing numerous papillary clusters in a case of metastatic ovarian serous carcinoma (Hematoxylin and eosin, ×200). Also note the presence of pericellular lacunae in this case.
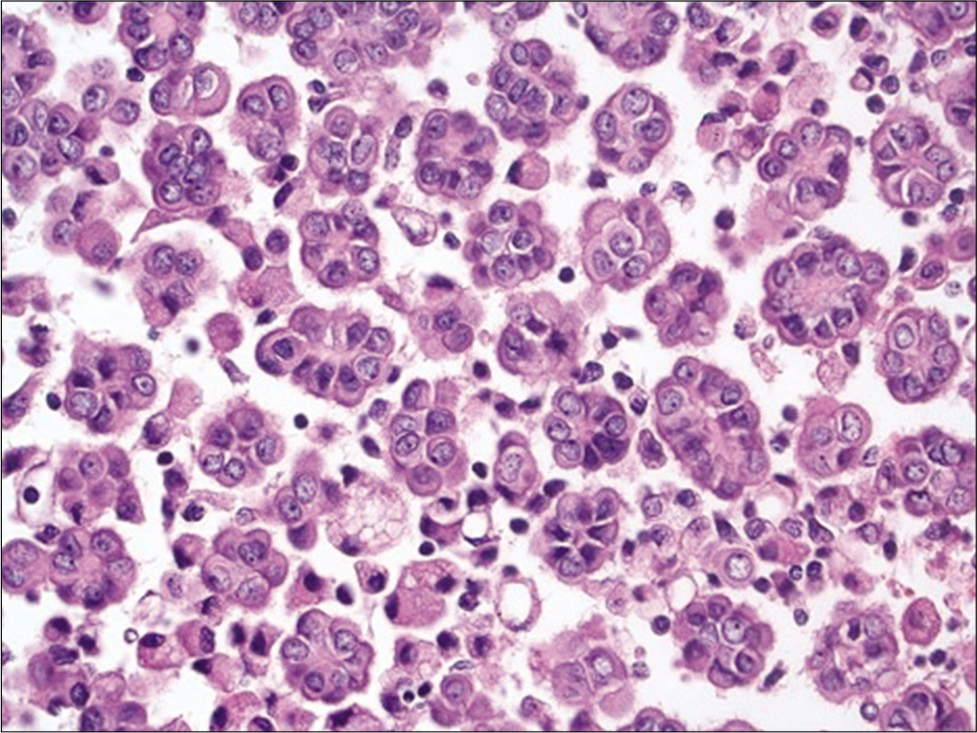
- Cell-block section of a well differentiated papillary mesothelioma showing numerous small clusters of mesothelial cells with knobby borders (Hematoxylin and eosin, ×200).
The presence of pericellular lacunae in cell-blocks is suggestive of metastatic adenocarcinoma [Figure 8]. As these lacunae are easily visible at low magnification, their recognition may help in screening cell-blocks. However, the concept of pericellular lacunae and their association with malignancy remains debated. Very few studies have addressed this issue. Price et al. studied over 200 cell-blocks of effusion specimens and reported that pericellular lacunae were found in malignant (although 75% of cases) and benign effusions.[52] From an architectural aspect, the majority of malignant cases in their study showed lacunae surrounding greater than two-thirds of the cells, whereas in the benign cases, when present, they were seen in less than one-third of the cells. In another study, McNeely divided effusions into three categories as no lacunae, <50% and >50% of cell clusters with pericellular lacunae. None of the benign effusions had >50% lacunae, whereas most malignant effusions had lacunae either in the <50% category (in 67% of cases) and >50% category (in 27% of cases). The author concluded that pericellular lacuna by itself is therefore not a reliable diagnostic criterion.[53] Similarly, Thomson and Hayes reported lacunae in both benign and malignant effusions.[54]
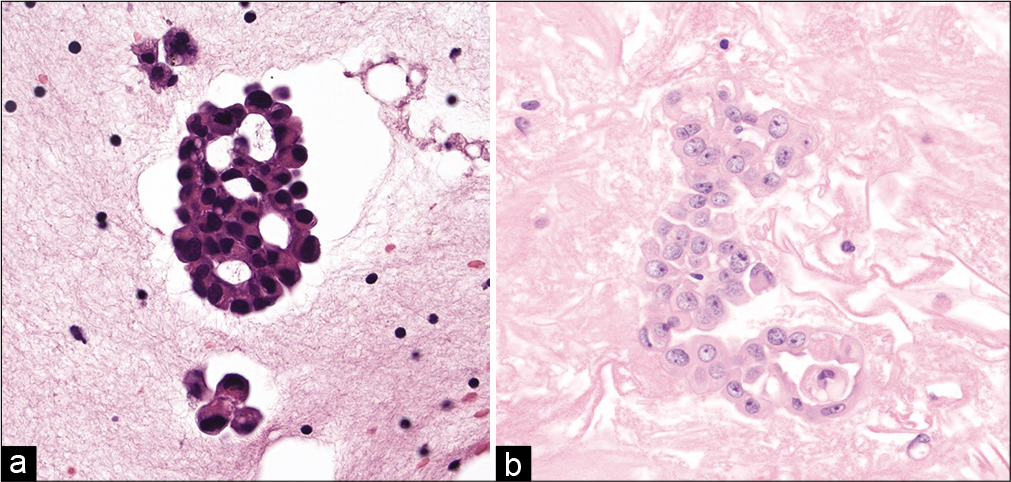
- (Left) Cell-block section showing a pericellular lacuna encircling more than two-thirds of the cell cluster in this case of metastatic adenocarcinoma involving the pleural cavity. Also note the presence of acini with nuclei polarized away from the center (hematoxylin and eosin, ×200). (Right) Cell-block section from a case of malignant mesothelioma. Note that in this case the large cluster of mesothelial cells has a solid to focal pseudo-acinar architecture and no pericellular lacuna (hematoxylin and eosin, ×200).
Urine
Urine cytology is the gold standard when screening for bladder cancer, especially for the detection of high-grade urothelial carcinoma (HGUC). The sensitivity of urine cytology for detecting HGUC ranges from 50 to 85% and is much lower (10–43%) for detecting low-grade urothelial neoplasia (LGUN).[55,56] After the adoption of the Paris system for reporting urine cytology, these rates are improving. McCroskey et al. demonstrated poor to fair correlation among different cytopathologists for diagnosing LGUN with a low a sensitivity (21–53%), but high specificity (81–95%).[57] According to the Paris system, the presence of fibrovascular cores is necessary to make a diagnosis of LGUN.[58] Several papillary urothelial neoplasms are in fact characterized by the presence of fibrovascular cores [Figure 9] because this category includes benign urothelial papilloma, low-grade papillary neoplasm of low malignant potential, low-grade papillary urothelial carcinoma, and high-grade papillary urothelial carcinoma.
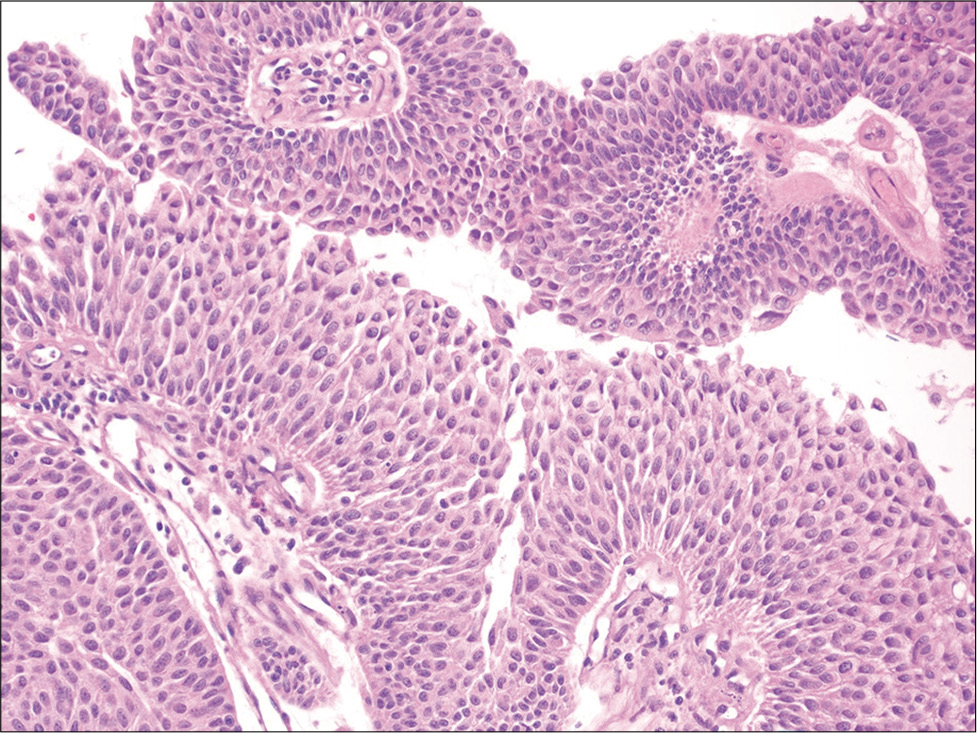
- Urine cell-block showing a three-dimensional cluster of cells with central fibrovascular cores. The cells show mild cytologic atypia and maintained polarity (hematoxylin and eosin, ×200). Follow-up biopsy revealed a low-grade papillary urothelial carcinoma.
Unfortunately, finding a papillary cluster of urothelial cells with a fibrovascular core in a urine specimen is uncommon. Cell-blocks are beneficial because they increase the likelihood of detecting a papillary architecture with visible fibrovascular cores. Furthermore, specimen types such as washings versus voided urine may increase the possibility of incorporating small tissue fragments in samples that make it easier to diagnose LGUN.[59] Although cell-blocks are an important part of integrated cytology diagnosis, very few studies have analyzed their utility for urine specimens.[5,59-65] Limiting factors for the routine adoption of cell-blocks when processing urine is that many specimens are paucicellular. Brisuda et al. found that female gender, positive urine cytology, and leukocytouria were associated with improved urine cell-block adequacy.[62] Nevertheless, Mansy et al. reported that cell-blocks for urine samples enhances the overall diagnostic rate.[63] Dantey et al. reported that routinely making urine cell-blocks only improves the diagnostic rate for the atypical urothelial cells (AUC) category.[64] Similarly, Chan et al. reported improved diagnostic rates for AUC, as well as the HGUC category with the adoption of the Paris system and cell-blocks.[65]
FNA
FNA, along with cell-blocks, are routinely used for the diagnosis of lesions within superficial (e.g., thyroid, salivary glands, and lymph nodes) and deep organs. The advent of newer biopsy techniques such as fiber optic endoscopy combined with using newer generation needles such as SharkCore has revolutionized the procurement of specimens from deep sites. Together with rapid onsite evaluation to better evaluate specimen adequacy and triage samples, which often includes dedicating extra material for cell-block processing, many cell-blocks today represent cell-enriched preparations that improve cytomorphology and provide material for ancillary studies. While cell-blocks can help expose unique cellular architecture in FNAs from many anatomic regions (e.g., distinct vasculature in myxoid soft-tissue neoplasms, and follicular growth pattern in lymphoma), the details of such tell-tale cytomorphologic findings will be illustrated in only few organs.
Thyroid gland
Cell-blocks are not required for most thyroid FNAs. In addition, cell-blocks made from bloody thyroid aspirates are often paucicellular. However, a cell-block can be very helpful in certain situations [Figure 10], especially when immunohistochemistry is needed to confirm a diagnosis (e.g., to differentiate medullary thyroid carcinoma from other thyroid and parathyroid neoplasms). Few studies have reported on the utility of cell-blocks for thyroid FNAs.[66-68] Li et al. Recommend the use of adjunct cell-blocks for radiologic thyroid category four and above lesions.[69] In general, these studies indicate that cell-blocks may lead to a reduction of the unsatisfactory rate and can improve the sensitivity and specificity of thyroid FNAs. However, Sanchez and Selvaggi reported that cell-blocks were contributory in only 31% of the 82 cases in their study.[70] Horton et al., who studied cell-blocks in 965 paired ThinPrep thyroid samples, did demonstrate an improved rate of non-diagnostic and atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) category cases.[71]
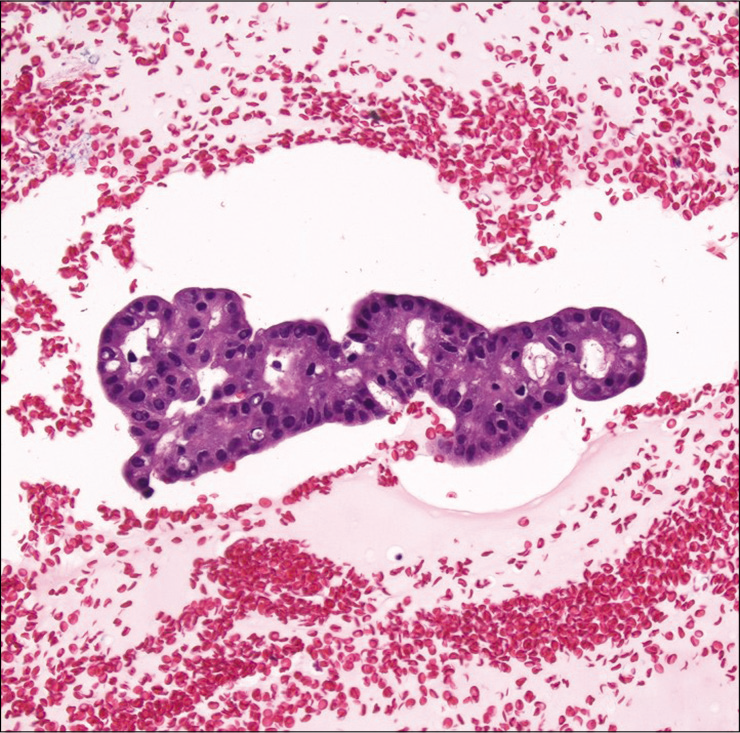
- Cell-block showing thyroid follicular cells with cribriform architecture in a case of cribriform/morular variant of papillary thyroid carcinoma. Note the presence of elongated nuclei with focal nuclear groves and intranuclear pseudoinclusions (Hematoxylin and eosin, ×200).
Salivary glands
Hong et al. reported an improved diagnostic rate for parotid FNAs when cell-blocks were utilized.[72] However, cell-blocks are mostly used with salivary gland FNAs to perform ancillary tests such as immunohistochemistry and fluorescent in situ hybridization.[73] Nevertheless, architectural patterns of salivary gland tumors that can be better appreciated on cell-block preparations include the double layer of epithelial cells resting on dense lymphoid stroma in Warthin tumor, cribriform glands of adenoid cystic carcinoma with central basement membrane-like material, biphasic tumors (e.g., epithelial myoepithelial carcinoma with luminal ductal cells and abluminal myoepithelial cells surrounded by extracellular stroma), variable tumor growth patterns (e.g., papillary, trabecular, and tubular), and possibly the infiltrating nature of some carcinomas.
Lung
Many patients with lung cancer primarily diagnosed by FNA require subclassification of their tumors for appropriate treatment triage. Hence, apart from distinguishing small cell versus non-small cell lung carcinoma, further subtyping of non-small carcinoma into squamous cell carcinoma or adenocarcinoma is required.[74,75] Thus, the routine use of cell-blocks for this purpose is warranted for lung FNAs. Loukeris et al. studied the value of cell-blocks in 62 cases of lung carcinomas. Their results showed that cell-blocks provided additional morphological clues for the classification of lung carcinomas.[76] For example, the presence of intercellular bridges may be better appreciated in cell-block sections of squamous cell carcinomas [Figure 11]. For basaloid squamous cell carcinoma a helpful finding that can be identified on cell-blocks is peripheral palisading in clusters of basaloid cells. Adenocarcinomas may exhibit helpful architectural patterns on cell-blocks such as strips of glandular cells, papillary groups, and acinar clusters of tumor cells with a cribriform pattern and the presence of glandular lumina. Ancillary tests are often needed for poorly differentiated non-small cell carcinomas, and although immunohistochemistry is helpful in challenging cases a mucicarmine stain can sometimes help to diagnose adenocarcinoma [Figure 12]. Demirci et al. demonstrated that cell-blocks are also important when distinguishing small cell and large cell neuroendocrine carcinoma (NECa).[77] Helpful morphologic features of large cell NECa on cell-block include the readily apparent abundant pink cytoplasm and conspicuous nucleoli of tumor cells, as opposed to small cell NECa which shows clusters of basaloid cells with minimal to absent cytoplasm.
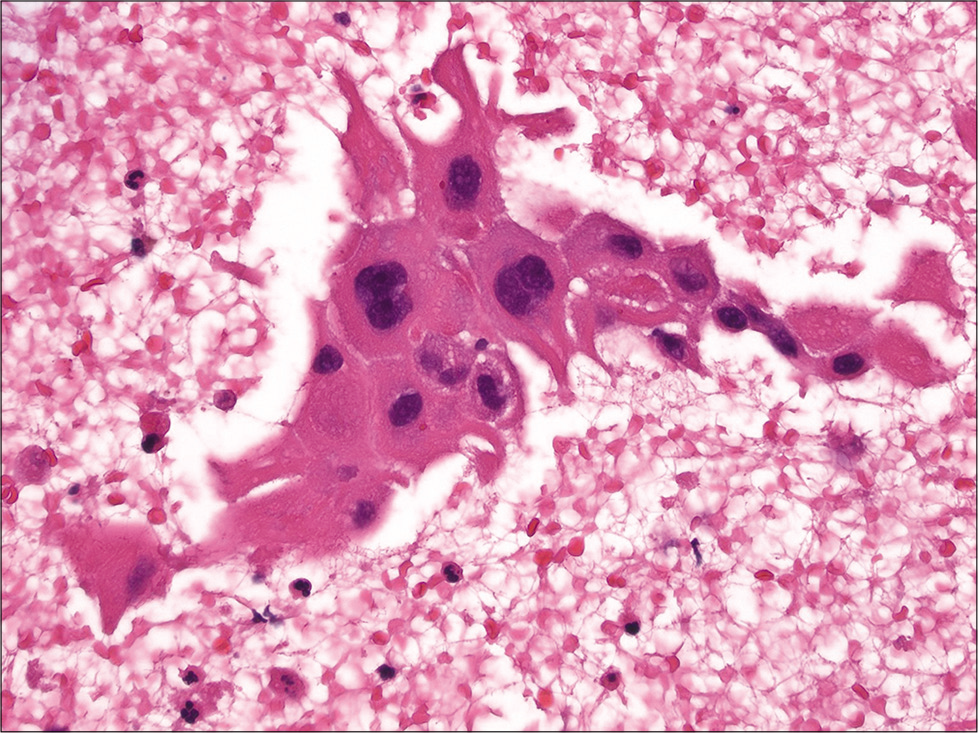
- Cell-block section of squamous cell carcinoma of the lung showing squamous cells with large central hyperchromatic nuclei and prominent intercellular bridges (hematoxylin and eosin, ×400).

- Cell-block section showing intracytoplasmic mucin in a case of lung adenocarcinoma (Mucicarmine stain, ×400).
Liver
In normal liver, hepatocytes are arranged in plates, where the apical surface faces sinusoids and the basal surface forms canaliculi. A bile canaliculus is not a true duct, but rather a dilated intercellular space between hepatocytes that lacks an epithelial lining. Recognition of this architecture in cell-blocks from liver aspirates can help distinguish benign liver from neoplasia. In normal liver, these plates are typically only two hepatocytes thick. However, in well-differentiated hepatocellular carcinoma (HCC), these plates can be 2–3 hepatocytes thick and in moderately differentiated HCC they are four or more hepatocytes thick. A reticulin stain can be used to easily highlight such abnormal liver architecture [Figure 13]. Other architectural features of HCC that may be helpful in cell-block sections include the absence of bile duct epithelial cells, the presence of many large solid clusters of hepatocytes with increased nuclear-cytoplasmic ratio, peripheral endothelial wrapping, and pseudo-acinar formation.[78,79]
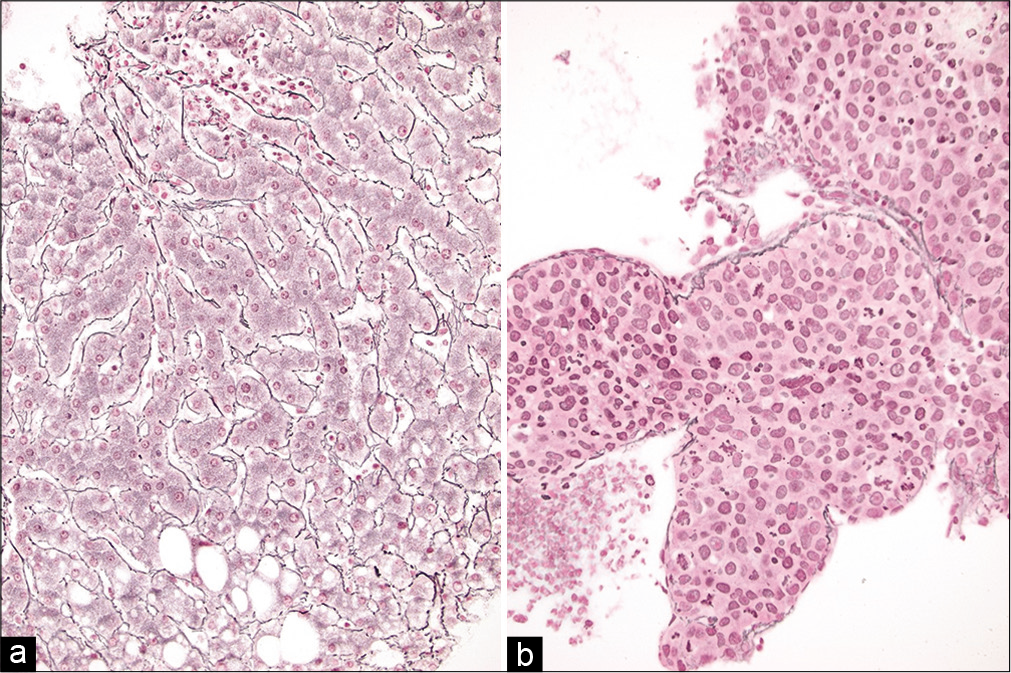
- (a and b) Reticulin stains on cell-blocks from liver FNAs showing (left) normal liver comprised of 1–2 layer thick hepatic plates and (right) a hepatocellular carcinoma with loss of normal architecture and very broad hepatic plates (Reticulin stains, ×200).
Pancreas
FNA remains the gold standard for diagnosing pancreatic lesions. Bang et al. along with multiple other studies demonstrated that new generation endoscopic biopsy needles (e.g., SharkCore) are very efficient for adequate collection of samples from solid pancreatic lesions.[12-16,80,81] These specimens often contain small fragments or microbiopsies showing intact tissue architecture [Figure 14]. Indeed, pancreas cell-blocks can be very helpful when they reveal features of malignancy such as complex glandular architecture and invasion of stroma with desmoplasia. Such features are particularly helpful for diagnosing well-differentiated pancreatic adenocarcinoma, particularly in cases with equivocal or suboptimal cytology smears. Furthermore, cell-blocks employed in conjunction with ancillary tests (e.g., immunohistochemistry for SMAD4 or neuroendocrine markers) can often help reach a definitive diagnosis.[82,83]

- (a-c) Pancreas FNA using SharkCore in which direct smears were suboptimal to reach a definitive diagnosis of malignancy. The cell-block in this case helped reach a diagnosis of adenocarcinoma because it was easy to appreciate (a) malignant glands, (b) tumor necrosis, and (c) invasion with desmoplastic stroma (hematoxylin and eosin, ×200).
DIGITAL CYTOLOGY
Innovative technologies, such as whole slide imaging (WSI), are beginning to revolutionize the way in which surgical pathology is practiced. However, for the practice of cytology WSI is largely limited to education and research applications. Unique technical challenges encountered when scanning glass slides with cytology preparations (such as smears of variable thickness, unevenly distributed cells, overcrowded clusters, and obscuring factors such as blood or mucus) remain key barriers to the adoption of WSI in routine cytology practice.[84,85] Utilization of Z-stacking when scanning addresses some of the aforementioned issues, but this process is often time consuming and creates large digital files. Compared to cytology smears or liquid-based preparations, cell-blocks are akin to tissue sections in that not only do they contain cells in the same (one) focal plane but they may also reveal cellular architecture. Therefore, it is not surprising that Tawfik et al. demonstrated that WSI of cell-blocks instead of liquid-based slides are a feasible method of screening Pap tests.[86] For the same benefits, Satturwar et al. demonstrated the feasibility of using WSI of cell-blocks for international teleconsultation for a variety of challenging non-gynecologic cytology cases.[87]
SUMMARY
Cell-blocks are small, concentrated, cell-enriched preparations derived from cytology samples that not only provide complimentary cytomorphology to smears and liquid-based preparations but often also reveal diagnostically helpful cellular architecture. Cell-blocks also permit recovery of valuable small tissue fragments which are increasingly being procured today with new and improved biopsy methods. A cell-block section stained with hematoxylin and eosin can portray a variety of cellular configurations and patterns including glandular differentiation, acinar arrangement, papillary formations, stromal invasion, and in effusions produce informative peri-cellular lacunae. Cell-blocks have thus become an essential part of integrated cytology diagnosis and have been shown to serve as a valuable adjunct to Pap tests, exfoliative fluid specimens and FNAs. Consequently, in this new era of “cytohistology” it is imperative that cytologists are accustomed with normal histology and histopathology when examining cell-blocks.
Key Features
Cell-blocks represent microbiopsies that have become an essential part of integrated cytology diagnosis
Cell-blocks allow small tissue fragments, that are procured using newer procedures and needles, to be processed and evaluated by the cytology laboratory
Recognition of cell-block architectural patterns has accordingly become important in this emerging era of cytohistology
Cell-blocks are suitable for whole slide imaging because they avoid focus problems plagued by other cytology preparations.
Acknowledgment
Editor thanks Janavi Kolpekwar, Kathy Rost, and Dr. Moumita Saha Roy Choudhury for their copy editing support.
LIST OF ABBREVIATIONS (IN ALPHABETIC ORDER)
AGCs – Atypical glandular cells
AUC – Atypical urothelial cells
AUS – Atypia of undetermined significance
CIN – Cervical intraepithelial neoplasia
FLUS – Follicular lesion of undetermined significance
FNA – Fine needle aspiration
HCC – Hepatocellular carcinoma
HCGs – Hyperchromatic crowded groups
HSIL – High grade squamous intra-epithelial lesions
HGUC – High-grade urothelial carcinoma
LSIL – Low grade squamous intraepithelial lesions
LGUN – Low-grade urothelial neoplasia
NECa – Neuroendocrine carcinoma
SCC – Squamous cell carcinoma
WSI – Whole slide imaging
References
- An evaluation of the concomitant use of cytological and histological techniques in the recognition of cancer in exfoliated material from various sources. Cancer. 1955;8:948-50.
- [CrossRef] [Google Scholar]
- Fine needle aspiration cytology of focal liver lesions, Results obtained with examination of both cytologic and histologic preparations. Acta Cytol. 1986;30:397-402.
- [Google Scholar]
- Fine-needle aspiration: Comparison of smear, cytospin, and cell-block preparations in diagnostic and cost effectiveness. Diagn Cytopathol. 1998;19:70-4.
- [CrossRef] [Google Scholar]
- Cell-block cytology, Improved preparation and its efficacy in diagnostic cytology. Am J Clin Pathol. 2000;114:599-606.
- [CrossRef] [PubMed] [Google Scholar]
- Can CD34 discriminate between benign and malignant hepatocytic lesions in fine-needle aspirates and thin core biopsies? Cancer. 2000;90:273-8.
- [CrossRef] [Google Scholar]
- Diagnostic value of electron microscopy on paraffin-embedded cytologic material. Diagn Cytopathol. 1993;9:282-90.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-block preparation as an adjunctive diagnostic technique in ThinPrep monolayer preparations: A case report. Diagn Cytopathol. 2001;24:142-4.
- [CrossRef] [Google Scholar]
- Cell-block preparation on residual ThinPrep sample. Diagn Cytopathol. 1999;21:427-31.
- [CrossRef] [Google Scholar]
- Cellblockistry: Chemistry and art of cell-block making. A detailed review of various historical aspects with recent advances. Cytojournal. 2019;16:20.
- [CrossRef] [PubMed] [Google Scholar]
- A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363-75.
- [CrossRef] [PubMed] [Google Scholar]
- A comprehensive review of endoscopic ultrasound core biopsy needles. Expert Rev Med Devices. 2018;15:127-35.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic yield of the SharkCore EUS-guided fine-needle biopsy. J Am Soc Cytopathol. 2019;8:212-9.
- [CrossRef] [PubMed] [Google Scholar]
- A Multicenter comparative trial of a novel EUS-guided core biopsy needle (SharkCore™) with the 22-gauge needle in patients with solid pancreatic mass lesions. Endosc Ultrasound. 2018;7:34-40.
- [CrossRef] [PubMed] [Google Scholar]
- Endoscopic ultrasound-guided sampling of solid pancreatic masses: 22-gauge aspiration versus 25-gauge biopsy needles. BMC Gastroenterol. 2015;15:122.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-block preparation as a diagnostic technique complementary to fluid-based monolayer cervicovaginal specimens. Acta Cytol. 1999;43:69-73.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-block as an adjunct to conventional Papanicolaou smear for diagnosis of cervical cancer in resource-limited settings. Cytopathology. 2007;18:309-15.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of high-grade lesions on cell-blocks from residual fluids of Pap smears diagnosed as low-grade abnormalities: A preliminary pilot study. Acta Cytol. 2012;56:247-50.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value, feasibility, and validity of preparing cell-blocks from fluid-based gynecologic cytology specimens. Cancer. 2002;96:204-9.
- [CrossRef] [PubMed] [Google Scholar]
- p16 INK4a immunocytochemistry on cell blocks as an adjunct to cervical cytology: Potential reflex testing on specially prepared cell blocks from residual liquid-based cytology specimens. Cytojournal. 2011;8:1. Available from: http://www.alturl.com/xobdb [Last accessed on 2021 Feb 22]
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation and significance of hyperchromatic crowded groups (HCG) in liquid-based paps. Cytojournal. 2007;4:2. Available from: http://www.alturl.com/r53gt [Last accessed on 2021 Feb 22]
- [Google Scholar]
- Hyperchromatic-crowded groups (HCG) in pap smears. Cytojournal. 2020;17:17.
- [CrossRef] [PubMed] [Google Scholar]
- Observations from Pap litigation consultations. Pathol Case Rev. 2011;16:78-82.
- [CrossRef] [Google Scholar]
- Diagnostic accuracy of conventional cell-blocks along with p16INK4 and Ki67 biomarkers as triage tests in resource-poor organized cervical cancer screening programs. Asian Pac J Cancer Prev. 2019;20:917-23.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of p16INK4a and Ki-67 staining on cell-blocks prepared from residual thin-layer cervicovaginal material. Cancer. 2004;102:142-9.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of pap cell-block preparations with liqui-PREP™ cell pellets to clarify the cytological diagnosis of atypical squamous cells of undetermined significance and atypical glandular cells. Diagn Cytopathol. 2017;45:520-5.
- [CrossRef] [PubMed] [Google Scholar]
- The cellient automated cell-block system is useful in the differential diagnosis of atypical glandular cells in Papanicolaou tests. Cancer Cytopathol. 2014;122:8-14.
- [CrossRef] [PubMed] [Google Scholar]
- Automated cell-block system for atypical glandular cells of cervical cytology: Is it feasible? Cancer Cytopathol. 2014;122:5-7.
- [CrossRef] [PubMed] [Google Scholar]
- Increased diagnostic accuracy of atypical glandular cells in cervical liquid-based cytology using cell-blocks. Cytopathology. 2011;22:253-60.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic value of micro-histology in endometrial brushing. Pathologica. 2010;102:46-50.
- [Google Scholar]
- Compact cell-blocks, Use for body fluids, fine needle aspirations and endometrial brush biopsies. Acta Cytol. 1998;42:703-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology of serous effusions, An investigation into the usefulness of cell-blocks versus smears. Am J Clin Pathol. 1978;70:855-60.
- [CrossRef] [PubMed] [Google Scholar]
- The cell-block for body cavity fluids: Do the results justify the cost? Mod Pathol. 1990;3:667-70.
- [Google Scholar]
- Diagnostic value of clot examination for malignant cells in serous effusions. Cytopathology. 2009;20:231-4.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of the thromboplastin-plasma cell-block technique for fine-needle aspiration and serous effusions. Diagn Cytopathol. 2009;37:86-90.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of cell-block to detect malignancy in fluid cytology: Adjunct or necessity? J Cancer Res Ther. 2017;13:425-9.
- [Google Scholar]
- Diagnostic efficacy of pleural biopsy as compared with that of pleural fluid examination. Mod Pathol. 1991;4:320-4.
- [Google Scholar]
- Diagnostic utility of pleural fluid cell-block versus pleural biopsy collected by flex-rigid pleuroscopy for malignant pleural disease: A single center retrospective analysis. PLoS One. 2016;11:e0167186.
- [CrossRef] [PubMed] [Google Scholar]
- Efficacy of pleural needle biopsy and pleural fluid cytopathology in the diagnosis of malignant neoplasm involving the pleura. Chest. 1975;67:536-9.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic evaluation of serous effusions, Processing techniques and optimal number of smears for routine preparation. Am J Clin Pathol. 1993;99:182-6.
- [CrossRef] [PubMed] [Google Scholar]
- Cytologic detection of malignancy in pleural effusion: Review of 5, 255 samples from 3, 811 patients. Diagn Cytopathol. 1987;3:8-12.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of efficacy of cell-block versus conventional smear study in exudative fluids. Niger Postgrad Med J. 2017;24:245-9.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of cell-block obtained by thoracentesis in the diagnosis of malignant pleural effusion. J Cytol. 2019;36:205-8.
- [CrossRef] [PubMed] [Google Scholar]
- Examination of cytological smears and cell-blocks of pleural fluid: Complementary diagnostic value for malignant effusions. Rev Clin Esp. 2017;217:144-8.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of conventional cytology and cell-block method in the diagnosis of pleural effusion. J Thorac Dis. 2017;9:3161-7.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of diagnostic value of cytological smear method versus cell-block method in body fluid cytology: Study of 150 cases. Ethiop J Health Sci. 2014;24:125-31.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic utility of the cell-block method versus the conventional smear study in pleural fluid cytology. J Cytol. 2012;29:11-5.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: Analysis of 414 cases. Mayo Clin Proc. 1985;60:158-64.
- [CrossRef] [Google Scholar]
- Peritoneal washing in borderline epithelial ovarian tumors in women under 25: The use of cell-block preparations. Diagn Cytopathol. 1998;18:212-4.
- [CrossRef] [Google Scholar]
- Is cell-block technique useful to predict histological type in patients with ovarian mass and/or body cavity fluids? Nagoya J Med Sci. 2020;82:225-35.
- [Google Scholar]
- Significance of pericellular lacunae in cell-blocks of effusions. Acta Cytol. 1992;36:333-7.
- [Google Scholar]
- Pericellular lacunae in effusions. Diagn Cytopathol. 1993;9:503-7.
- [CrossRef] [PubMed] [Google Scholar]
- Pericellular lacunae in the diagnosis of metastatic carcinoma in effusions: Is this a useful sign? Diagn Cytopathol. 1996;15:193-6.
- [CrossRef] [Google Scholar]
- Comparison of fluorescence in situ hybridization, nmp22 bladderchek, and urinary liquid-based cytology in the detection of bladder urothelial carcinoma. Diagn Cytopathol. 2013;41:852-7.
- [CrossRef] [PubMed] [Google Scholar]
- Is the performance of urinary cytology as high as reported historically? A contemporary analysis in the detection and surveillance of bladder cancer. Urol Oncol. 2014;32:27.e1-6.
- [CrossRef] [PubMed] [Google Scholar]
- Accuracy and interobserver variability of the cytologic diagnosis of low-grade urothelial carcinoma in instrumented urinary tract cytology specimens. Am J Clin Pathol. 2015;144:902-8.
- [CrossRef] [PubMed] [Google Scholar]
- Low-grade urothelial neoplasia In: Rosenthal DL, Wojcik EM, Kurtycz DF, eds. The Paris System for Reporting Urinary Cytology (1st ed). Switzerland: Springer; 2016. p. :75-86.
- [CrossRef] [PubMed] [Google Scholar]
- The cytomorphological features of low-grade urothelial neoplasms vary by specimen type. Cancer Cytopathol. 2016;124:552-64.
- [CrossRef] [PubMed] [Google Scholar]
- Benign-appearing urothelial tissue fragments in noninstrumented voided urine specimens are associated with low rates of urothelial neoplasia. Cancer Cytopathol. 2015;123:180-5.
- [CrossRef] [Google Scholar]
- Atypical urothelial tissue fragments in noninstrumented voided urine specimens are associated with low but significantly higher rates of urothelial neoplasia than benign-appearing urothelial tissue fragments. Cancer Cytopathol. 2015;123:186-92.
- [CrossRef] [Google Scholar]
- Clinical and cytopathological factors affecting the cellularity of urinary cell-blocks and the implication for diagnosis and follow-up of urinary bladder urothelial carcinoma. Cytopathology. 2018;29:537-44.
- [CrossRef] [PubMed] [Google Scholar]
- Value of the innovated technique agarose cell-block in improving the sensitivity of urine cytology in cases of bladder carcinoma. Ultrastruct Pathol. 2006;30:379-85.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-block preparation in urine cytology: Examination of utility and workflow in an academic practice. J Am Soc Cytopathol. 2019;8:61-8.
- [CrossRef] [PubMed] [Google Scholar]
- Improved diagnostic precision of urine cytology by implementation of the Paris system and the use of cell-blocks. Cancer Cytopathol. 2018;126:809-16.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-block alone as an ideal preparatory method for hemorrhagic thyroid nodule aspirates procured without onsite cytologists. Acta Cytol. 2008;52:139-44.
- [CrossRef] [PubMed] [Google Scholar]
- Increasing diagnostic effectiveness of thyroid nodule evaluation by implementation of cell-block preparation in routine USFNA analysis. Arch Endocrinol Metab. 2016;60:367-73.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic utility of cell-block in fine needle aspiration cytology of thyroid gland. Diagn Cytopathol. 2019;47:1245-50.
- [CrossRef] [PubMed] [Google Scholar]
- The KWAK TI-RADS and 2015 ATA guidelines for medullary thyroid carcinoma: Combined with cell-block-assisted ultrasound-guided thyroid fine-needle aspiration. Clin Endocrinol (Oxf). 2020;92:450-60.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of cell-blocks in the diagnosis of thyroid aspirates. Diagn Cytopathol. 2006;34:89-92.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of cellient cell-blocks in low-cellularity thyroid fine needle aspiration biopsies. Diagn Cytopathol. 2016;44:737-41.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical efficacy of the fine needle aspiration of the cell-block in the diagnosis of parotid gland masses. Hua Xi Kou Qiang Yi Xue Za Zhi. 2016;34:483-7.
- [Google Scholar]
- Usefulness of translocation-associated immunohistochemical stains in the fine-needle aspiration diagnosis of salivary gland neoplasms. Cancer Cytopathol. 2016;124:397-405.
- [CrossRef] [PubMed] [Google Scholar]
- Pathological diagnosis and classification of lung cancer in small biopsies and cytology: Strategic management of tissue for molecular testing. Semin Respir Crit Care Med. 2011;32:22-31.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of basaloid squamous cell carcinoma and small cell carcinoma-a comparison study. Diagn Cytopathol. 2013;41:81-4.
- [CrossRef] [PubMed] [Google Scholar]
- Cytological cell-blocks: Predictors of squamous cell carcinoma and adenocarcinoma subtypes. Diagn Cytopathol. 2012;40:380-7.
- [CrossRef] [PubMed] [Google Scholar]
- Contribution of cell-blocks obtained through endobronchial ultrasound-guided transbronchial needle aspiration for the determination of lung cancer subtypes. Clin Respir J. 2018;12:1623-7.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of aspiration cytology of the liver space occupying lesions by simultaneous examination of smears and cell-blocks. Diagn Cytopathol. 2009;37:557-63.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of FNAC in conjunction with cell-block for diagnosing space-occupying lesion (SOL) of liver with emphasis on differentiating hepatocellular carcinoma from metastatic SOL: Analysis of 61 cases. Oman Med J. 2016;31:135-41.
- [CrossRef] [PubMed] [Google Scholar]
- Randomized trial comparing the Franseen and Fork-tip needles for EUS-guided fine-needle biopsy sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2018;87:1432-8.
- [CrossRef] [PubMed] [Google Scholar]
- Cell-block technique and cytological smears for the differential diagnosis of pancreatic neoplasms after endosonography-guided fine-needle aspiration. Acta Gastroenterol Latinoam. 2008;38:246-51.
- [Google Scholar]
- Usefulness of cell-block cytology for preoperative grading and typing of intraductal papillary mucinous neoplasms. Pancreatology. 2013;13:369-78.
- [CrossRef] [PubMed] [Google Scholar]
- Immunohistochemical staining for S100P, SMAD4, and IMP3 on cell-block preparations is sensitive and highly specific for pancreatic ductal adenocarcinoma. J Am Soc Cytopathol. 2018;7:318-23.
- [CrossRef] [PubMed] [Google Scholar]
- Digital imaging in cytopathology. Patholog Res Int. 2011;2011:264683.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of glass slides and various digital-slide modalities for cytopathology screening and interpretation. Cancer Cytopathol. 2017;125:701-9.
- [CrossRef] [PubMed] [Google Scholar]
- Whole-slide imaging of pap cellblock preparations is a potentially valid screening method. Acta Cytol. 2015;59:187-200.
- [CrossRef] [PubMed] [Google Scholar]
- The utility of cell-blocks for international cytopathology teleconsultation by whole slide imaging. Cytopathology. 2020;31:419-25.
- [CrossRef] [PubMed] [Google Scholar]








