Translate this page into:
Cell-blocks and hematolymphoid lesions

*Corresponding author: Ali Gabali, Professor, Director of Hematopathology and Hematopathology Fellowship, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center, Detroit, Michigan, United States. agabali@med.wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Alrajjal A, Choudhury M, Yang J, Gabali A. Cell-blocks and hematolymphoid lesions. CytoJournal 2021;18:7.
Abstract
Cell-blocks are an important component for evaluation for hematolymphoid lesions. They are especially critical for immunocharacterization of the lymphoid population especially when flow cytometry is not available or cannot be performed. In addition, cell-blocks allow various molecular pathology tests including gene rearrangement studies and FISH, proteomics analysis, and microbiology/histochemical special stains. Fine-needle aspiration (FNA) for mass lesions, lymphadenopathy, and effusion fluids are common cytopathology specimens which are frequently cell-blocked. The differential diagnosis of enlarged lymph nodes (LNs) and mass lesions is broad and includes reactive processes, granulomatous lesions and malignancies including solid tumor metastases and various types of hematological malignancies, of which lymphoma would be most common. Depending on the patient population, most lymphomas may be diagnosed with immunocharacterization on cell-block or/and flow cytometry in concert with excellent cytomorphology in Diff-Quik stained FNA aspirate smears. However, a proportion of lymphoma cases (up to 12-30%) may still require an excisional LN biopsy to evaluate architectural parameters. Similarly, various effusion fluids suspicious for lymphoma can be immunocharacterized by immunostaining of cell-block sections (or/and by flow cytometry). Availability of quantitatively and qualitatively optimum cell-blocks of specimens to be evaluated for hematolymphoid processes is critical for immunohistochemistry, polymerase chain reaction, in situ hybridization (FISH), and gene expression profiling studies.
Keywords
CellBlockistry
Cell-blocks
Cell-blocks
Cellblocks
Cell-blocking
Cell-blocked
NextGen CelBloking
FFPE
Molecular
PCR
FISH
CISH
Hematolymphoid lesions

INTRODUCTION
Cell-block of any cytopathology specimen undergoing evaluation for hematolymphoid lesions allows for immunocharacterization (especially when flow cytometry is not possible or available). In addition, cell-blocks are critical for various molecular pathology tests including gene rearrangement studies, Fluorescent situ hybridization (FISH) / Chromogenic situ hybridization CISH), proteomics, and microbiology/histochemical stains. The most common cytopathology specimens processed for such indications include fine-needle aspiration (FNA) aspirates and effusion fluids.
FNA of lymph node is an excellent tool to rule out malignancy, including lymphoma. But this should not be practiced without on-site adequacy evaluation and without proper application of ancillary studies as applicable. The most common causes of lymphadenopathy include reactive processes, granuloma, and malignancies including metastases and various types of lymphomas. Other less common hematologic malignancies that can present as lymphadenopathy include plasmacytomas and myeloid sarcomas.[1,2] Depending on the patient population, only a small subset of cases turns out to be lymphoma, most of which can be ruled out based on immunocharacterization on flow cytometry or/and cell-block in concert with excellent cytomorphology in Diff Quik (DQ) stained FNA aspirate smears [Figure 1].[3-5] Major benefit of FNA is that it is a minimally invasive office procedure (without potential scar formation and morbidity related to the excisional biopsy).
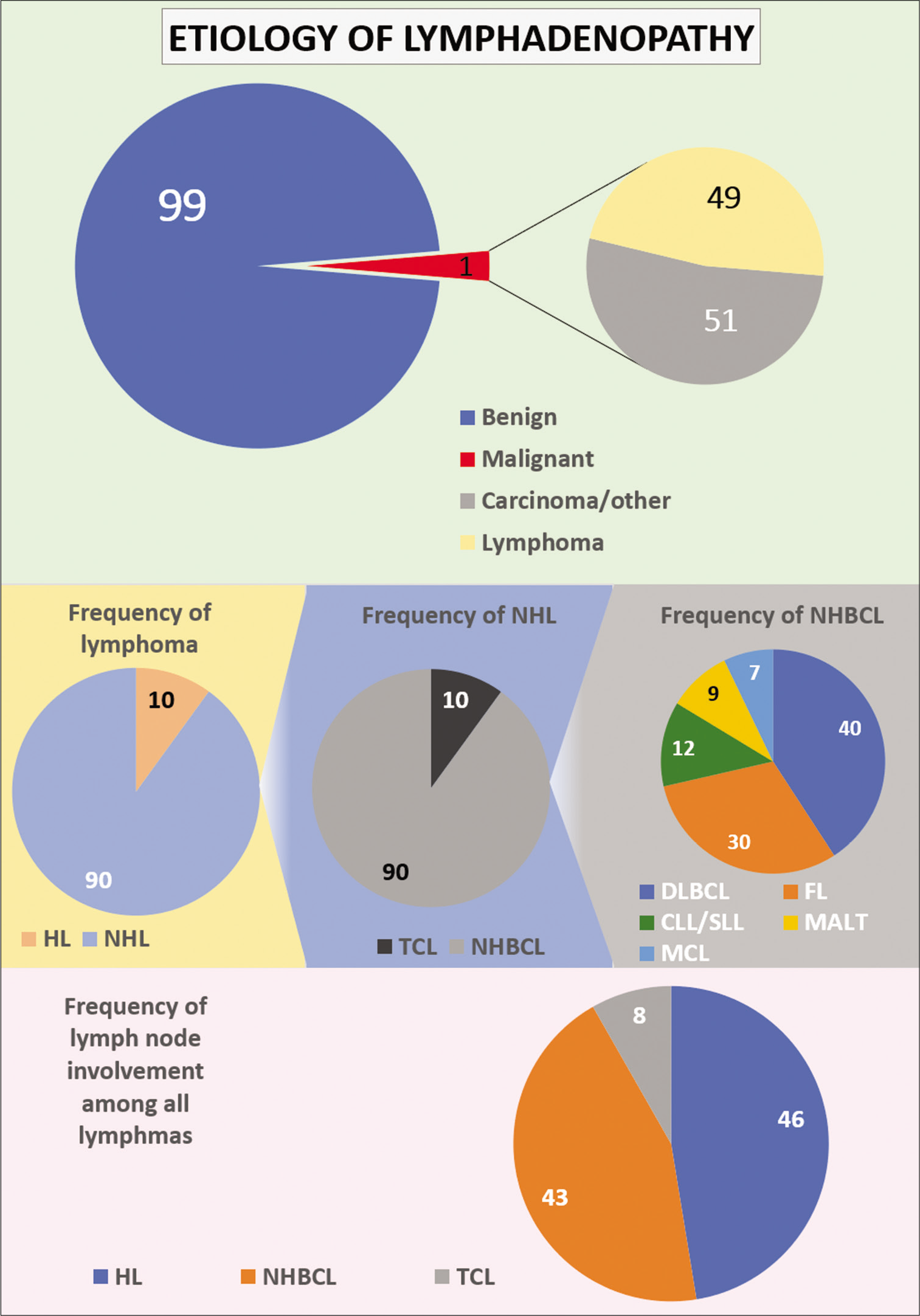
- The most common causes of lymphadenopathy, depending on the patient population. Only a small subset of cases turns out to be lymphoma (Hodgkin lymphoma (HL), non-HL, non-Hodgkin B-cell lymphoma, T-cell lymphomas, Follicular lymphoma, Mantle cell lymphoma).
Cytomorphological evaluation during Rapid On-Site Evaluation (ROSE) is critical for recommending appropriate ancillary studies such as immunocharacterization and molecular testing. B-cell clonality and T-cell receptor (TCR) gene rearrangements studies can be performed on optimal cell-block.
Availability of quantitatively and qualitatively optimum cell-blocks is critical for immunohistochemistry (IHC), polymerase chain reaction (PCR), in situ hybridization (FISH and CISH), and gene expression profiling studies. In fact, the significance of ancillary testing for the proper diagnosis and classification of malignancy is greatly emphasized in hematopathology.
When lymphoma is the primary differential diagnosis, excisional biopsy is usually preferred for the best diagnostic yield. However, when excisional biopsy is not obtainable, FNA should be the preferred diagnostic tool. In fact, the diagnostic yield of FNA, if done properly, with ancillary studies (flow cytometry/cell-block-IHC) is comparable to needle core biopsy (NCB) of lymph nodes (LNs) with a definite lymphoma diagnosis (88% and 90% for FNA and NCB, respectively).[6-11] Both NCB and FNA of LNs require an excisional biopsy in only a subset of lymphadenopathy cases based on the preliminary findings. This translates into only 0.15% cases with lymphadenopathy that is lymphoma and require an excisional biopsy (30% of 0.5% lymphoma cases) [Figure 1].[6,12,13a]
The full diagnostic value of FNA in lymphoproliferative disorders is achieved only through appropriate integration of clinical information, light microscopic cytology, and proper utilization of ancillary studies. With increasing refinements in various ancillary studies including molecular tests, the requirement for excisional biopsy for the diagnosis of lymphoma may be avoided in a significant number of cases. Based on one of the strong recommendations by Laboratory Workup of Lymphoma in Adults, FNA cytomorphology alone should not be used without ancillary testing for definitive diagnosis of lymphoma.[13b]
Similarly, various effusion fluids may have to be evaluated for hematolymphoid lesions. The hematolymphoid components in the effusion specimens should be immunocharacterized by immunostaining of cell-block sections or/and by flow cytometry of a fresh unfixed effusion sample.
Evaluation with cytopathology, including FNA, is increasingly performed to diagnose, stage, and monitor lymphoma progression.[13-16] A detailed practical guide on the role of cell-blocks in relation to hematolymphoid lesions is presented.
ADVANTAGES OF FNA WITH CELL-BLOCK/ FLOW CYTOMETRY FOR EVALUATION OF HEMATOLYMPHOID DISORDERS
FNA is a minimally-invasive procedure and better tolerated by patients. A superficial enlarged LN represents an excellent target. Compared to other sampling methods, LN-FNA offers a shorter turn-around time, is more economical, and provides readily available and content-rich sample for accurate flow cytometry and molecular biology results. In addition, ROSE offers procedural guidance and appropriate triage input for providing material for ancillary testing. The most distinguishing advantage of LN-FNA is the method’s ability to reach anatomically tight spots (technically difficult locations) when assisted by endoscopy and imaging modalities (e.g., endobronchial ultrasound guided FNA) for acquiring diagnostic material that otherwise would be difficult or impossible to be sampled with other modalities such as core needle biopsy or excisional biopsy.
LIMITATIONS OF FNA WITH CELL-BLOCK/ FLOW CYTOMETRY FOR EVALUATION OF HEMATOLYMPHOID PATHOLOGY
The main limitation of LN-FNA in the realm of lymphoid proliferations is the lack of architectural details required for diagnosing and monitoring some of the hematolymphoid processes.
In general, because of the potential of sampling artifact, which is also applicable in NCBs, the possibility of focal large cell transformation cannot be excluded with certainty.[13]
Another relative limitation is sampling artifact due to:
Lack of sampling of focal pathology partially involving the LN (metastatic carcinoma/granuloma/in situ follicular neoplasia)
Inability to assess the proliferation index (Ki-67 index) accurately due to lack of architectural insight.
Nodal fibrosis – dry aspiration such as in nodular sclerosis type Hodgkin lymphoma (HL).
Excessive necrosis or inflammation – without viable cells for examination (high grade B and T cell lymphomas).
INDICATIONS OF FNA WITH CELL-BLOCK/ FLOW CYTOMETRY
Unexplained lymphadenopathy, with a prevalence of around 1% in the general population, is an important indication for FNA. The risk of malignancy in these patients is variable (around 1–50%), depending on the clinical scenario, size, and location of LNs.[1,14a] Although the risk might be low, clinicians and patients are usually in fear of missing a serious diagnosis.[3] The request for a LN-FNA starts when there is a suspicion for malignancy in cases with unexplained lymphadenopathy.
The most common LN-FNA indication is to prove or exclude malignancy and potentially avoiding the hurdle of undergoing a surgical procedure such as excision of a mass or LN. In addition, LNs may also be sampled for the staging of lymphoma or metastasis. Finally, FNA can be used for identification of a LN, where physical examination and imaging studies are inadequate to confirm the nature of the lymphadenopathy, usually in the head and neck area (e.g., intra-salivary gland and intra-thyroid LN).
Indications for LN-FNA is some patients may be for reasons other than to establish diagnosis of malignancy. These include and are not limited to anxiety-relief in a nervous patient where clinical suspicion for malignancy is low and in situations where sample collection is indicated for research and/or clinical trial purposes. These indications are endorsed by an international panel of experts (The Sydney system for reporting LN-FNA 2020) [Table 1].[14b]
| Lymph node FNA issues | Clinical scenario | Course of action |
|---|---|---|
| Clinical data to review when interpreting lymph node FNA | Single or multiple lymph nodes without relevant history | Mandatory |
| Single or multiple lymph node with known pathology | Mandatory | |
| Clinical data to review when interpreting lymph node FNA | Age, symptoms, site, size, time of onset, imaging (US) | Mandatory |
| Remote and current medical history | Mandatory | |
| Basic serology (ESR, LDH, ToRCH complex, ANA, others) | Recommended | |
| Specific serology (known or suspected disease) | Recommended | |
| Indications for performing lymph node FNA | Differential diagnosis: lymph node versus not lymph node in atypical sites (intercostal, intra-mammary, epitrochlear, intra-parotid, retroperitoneal, and other sites) | Recommended |
| Exclude malignancy and avoid lymph node excision for benign/reactive processes | Suggested | |
| Diagnosis and staging of lymphoma or metastasis | Recommended | |
| Diagnosis and microbial culture material for infectious etiologies | Suggested | |
| Relieve anxiety for benign/reactive processes | Suggested | |
| Cell collection for diagnostic and predictive tests | Suggested | |
| Cell collection for clinical trials or other research tests | Suggested |
US: Ultrasound, ESR: Erythrocyte sedimentation rate, LDH: Lactate dehydrogenase, ToRCH: Toxoplasmosis, rubella, cytomegalovirus, herpes simplex, and HIV, ANA: Antinuclear antibodies
Abnormal LNs are typically defined based on size, consistency, and/or imaging findings. As per radiology criteria, lymphadenopathy is considered pathological if LN is larger than 1.0 cm along the short axis in most body sites.[1,15] A lower threshold is considered at specific sites such as popliteal, supraclavicular, and epitrochlear regions, where LNs larger than 0.5 cm are considered abnormal.[15] However, other radiologic features such as obliterated hilum, echogenicity, and round shape might trigger a lymphoma suspicion demanding further investigation. Although without any specific rationale, LN-FNA is less commonly performed in pediatric patients as compared to adult population.[1]
LYMPHOMA STATISTICS AND FREQUENCY BASED ON NODAL SITE OF INVOLVEMENT
Lymphadenopathy may be secondary to carcinoma or lymphoma based on the patient’s clinical history, demographics, and radiologic findings. Cytomorphologic findings during onsite adequacy evaluation with clinical differential diagnoses provide guidance for proper triaging and utilization of ancillary studies including IHC panels on cell-block/flow cytometry and molecular pathology testing.
Considering the frequency of lymphoma in the general population, only 10% of all lymphoma cases are classified as HL. The remaining 90% of lymphoma cases fall into the category of non-HL (NHL). The majority of NHL (90%) are of B cell origin categorized as non-Hodgkin B-cell lymphoma (NHBL) and only about 10% are T-cell lymphomas (TCL). Non-Hodgkin B-cell lymphoma is further subclassified into diffuse large B cell lymphoma (DLBCL) (37%), follicular lymphoma (FL) (29%), chronic lymphocytic leukemia/ lymphoma (CLL/SLL) (12%), Mucosa-associated lymphoid tissue (MALT) lymphoma (9%), and mantle cell lymphoma (MCL) (7%), among others [Figure 1].
Specific flow cytometry and cell-block IHC panels are developed for proper evaluation of LN-FNA specimens. Application of such panels is primarily based on the initial cytomorphological assessment of the aspirates during ROSE. This underscores the importance of adequate, properly processed, and triaged high quality aspirate specimens as Diff-Quik stained (with Pap strained) direct smears and FNA rinses for good quality cell-block and flow cytometry in isotonic medium such as RPMI medium or saline or Isotonic Medium S™ [Figure 2].[16]
![Suggested algorithm for processing of FNA of hematolympoid lesions based on the cytomorphological details of the lymphocytes during on-site adequacy evaluation of Diff Quik stained air-dried direct smears. The needle rinses including dedicated passes are submitted in isotonic mediums such as RPMI and Isotonic Medium S[16] for flow cytometry (with or without cell-blocking).](/content/105/2021/18/1/img/Cytojournal-18-7-g003.png)
- Suggested algorithm for processing of FNA of hematolympoid lesions based on the cytomorphological details of the lymphocytes during on-site adequacy evaluation of Diff Quik stained air-dried direct smears. The needle rinses including dedicated passes are submitted in isotonic mediums such as RPMI and Isotonic Medium S[16] for flow cytometry (with or without cell-blocking).
THE ROLE OF FLOW CYTOMETRY IN LN-FNA AND HEMATOLYMPHOID LESIONS
The value of flow cytometry in the realm of hematopathology is indispensable. It provides unique information that otherwise may be difficult to elucidate by other ancillary studies. First and foremost is the incredible ability of the technique to allow for testing on a small sample size. Second, flow cytometry offers evaluation of multiple phenotypic and physical markers on the same cell, such as coexpression of CD5 and CD23 in chronic lymphocytic leukemia/small lymphocytic lymphoma(CLL/ SLL). In addition, the forward and side scatter plot provides details on cell size and cytoplasmic complexity. In addition, immunoglobulin (Ig) ƙ and λ chain analysis with flow cytometry provides the simple and best approach to identify B cell clonality.[6] Furthermore, evaluation of the mean fluorescence intensity in some cases can have diagnostic implications, as is the case in CLL/SLL which is characterized by low-intensity CD20 and surface immunoglobulin. Additionally flow cytometry is vital for identifying minor populations of neoplastic cells in a reactive polyclonal background, especially useful where the LN is only partially involved by lymphoma. In addition, the ability of identifying T-cell clonality based on a wide range of antibodies against the variable region of the TCRβ gene has been described recently.[17]
However, in certain cases, flow cytometry is of a limited diagnostic value.[13] For example, in HL and T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL), flow cytometry analysis will not be useful in identifying the pathology; on the contrary, it may lead to a false negative interpretation. Such findings are largely due to the polymorphous reactive cellular background in these lesions with limited number of malignant cells. As such, we recommend prioritizing cell-block over flow cytometry in cases where the initial evaluation of the specimen (ROSE) showed a polymorphous background.
A dedicated pass should be sent for flow cytometry in LNFNA, especially if lymphoma is suspected. In general, if the initial findings of FNA-LN during ROSE are negative with reference to a specific pathology, additional sampling should be sent for flow cytometry and cell-block as indicated. If ROSE findings suggest metastatic carcinoma or infectious process (granuloma), cell-block should be prioritized over flow cytometry for ancillary IHC, molecular studies, and special stains for microorganisms as applicable.
FNA of LN suspicious of lymphoma can be categorized into three main cytomorphological groups based on ROSE:
Monomorphic small lymphocytes
Monomorphic medium/large lymphocytes
Polymorphic background with or without large, atypical cells.
Monomorphic small lymphocytes: (CD3, CD5, CD20, CD79a, CD10, CD23, SIg, K, and L)
Most cases with monomorphic small lymphocytes fall into the category of Non-Hodgkin B cell lymphoma (NHBCL) with FL and CLL/SLL being most common. Flow cytometry analysis is efficient in identification and sub-classification of NHBCL including MCL, CLL, FL, hairy cell leukemia, and lymphoplasmacytic lymphoma (LPL). Marginal zone lymphoma (MZL) is a diagnosis usually reached by excluding other NHBCL. As known, FL cannot be graded with flow cytometry, while grading of FL has important management implications. Nevertheless, flow cytometry will reliably identify a monoclonal population of germinal center B-cells (GCB), which allows for an excisional biopsy to be recommended as next step [Table 2].
| CD5 | CD10 | CD23 | CD43 | BCL2 | BCL6 | Cyclin D1 | Annexin-A1 | IgD | |
|---|---|---|---|---|---|---|---|---|---|
| CLL/SLL | + | – | + | + | + | – | – | – | + |
| FL | – | + | – | – | + | + | – | – | – |
| MCL | + | – | – | + | + | – | + | – | + |
| MZL | – | – | ± | ± | + | – | – | – | – |
| LPL | – | – | – | – | + | – | – | – | – |
| HCL | – | – | – | – | + | – | ± | + | – |
CLL/SLL: Chronic lymphocytic leukemia/small lymphocytic lymphoma, FL: Follicular lymphoma, MCL: Mantle cell lymphoma, MZL: Marginal zone lymphoma, LPL: Lymphoplasmacytic lymphoma, HCL: Hairy cell leukemia
Monomorphic medium/large lymphocytes: (CD3, CD5, CD20, CD79, CD138, CD34, CD10, kappa, lambda, MUM1, BCL2, and TdT)
The other relatively frequent scenario is preponderance of medium/large lymphocytes on ROSE. The findings range from large, atypical lymphocytes to large cells with blastoid, immunoblastic, centroblastic to anaplastic bizarre cytomorphology. DLBCL is the top differential in this category and the proper subclassification into several subtypes is of clinical value. (See further discussion in cell-block section below). Large monomorphic lymphocytes are also characteristic of High grade B-cell lymphoma, blastoid or pleomorphic MCL, lymphoblastic lymphoma (LBL), Burkitt lymphoma (BL), high grade FL (grade3), some cases of LPL with blastoid morphology, and occasionally plasmacytoma with blastic morphology. It is crucial to entertain the most likely diagnoses based on individual basis which would allow for proper triaging.
Polymorphic background ± RS cells: (CD2, CD3, CD4, CD5, CD7, CD8, CD20, CD79a, K, L, CD56, and CD57)
The most commonly encountered entity that falls into this cytomorphological category is Classic Hodgkin lymphoma (cHL). Although flow cytometry is not diagnostic in such cases, it may demonstrate value in ruling out clonal B cell processes in the background. Other conditions, less frequently encountered but are necessary to consider, include peripheral TCL (PTCL), Angioimmunoblastic TCL, THRLBCL, and anaplastic large TCL (ALCL) [Figure 2] along with the recommended ancillary study [Figure 2a and b for details].
THE ROLE OF CELL-BLOCK IN LN-FNA AND HEMATOLYMPHOID LESIONS
Quantitatively and qualitatively optimal Formalin Fixed Paraffin Embedded (FFPE) of cell-block allows for proper application of ancillary techniques, including IHC, molecular testing, special stains, and proteomics. This increases the diagnostic accuracy and allows for more definite diagnoses on different cytology specimens.[18] Cell-block sections can also be used in lymphadenopathy cases suspected to be of infectious etiology for performing various microbial stains such as for mycobacteria and fungal organisms with Ziehl-Neelsen stain for acid fast bacilli (AFB) and Gomori Methenamine Silver (GMS) stain, respectively.[19]
IHC on surgical pathology specimen of LN (NCB/excision biopsy) provides two valuable morphological inputs: Antigen expression profile and cell localization “architecture.” In contrast, IHC on cell-block of LN-FNA lacks this architectural benefit. Yet, immunophenotyping using IHC on cell-block can be particularly useful in certain hematolymphoid malignancies, especially in which the antigen expression pattern is unique irrespective of architecture.
Two main challenges while interpreting IHC on cell-blocks include:
Evaluation of the same cells present in serial sections in adjacent levels to evaluate coordinate immunoreactivity pattern for different immunomarkers
Intricacy of immunophenotypic patterns in benign and malignant hematolymphoid conditions with some discrepancies which may lead to misinterpretation.
For example, a monoclonal population of B-cells with light chain restriction and aberrant expression of CD5 on flow cytometry is correctly diagnosed as positive for malignancy, consistent with Non-Hodgkin B-cell Lymphoma. However, such results combined with immunoreactivity with CyclinD1 and CD20 on cell-block allows for a more specific diagnosis of MCL.[20] The critical task for the cytopathologist is to identify B-lymphocytes (e.g., CD20+) that are also immunoreactive for CyclinD1. The cytomorphology helps in distinguishing endothelial cells (large oval nuclei) that might be CyclinD1 positive from lymphocytes (small, round). Application of SCIP approach[21] and dual color immunostaining[22,23] method using CD20/CyclinD1 may be valuable for proper interpretation. It allows for objective evaluation of this coordinate immunoreactivity for detection of the lymphocyte population with aberrant expression. Both SCIP (Subtractive Coordinate Immunoreactivity Pattern) approach[21] and dual color immunostaining[22,23] methods are relatively simple techniques to be applied by any lab.
It is important to understand the differences in IHC applications on cell-blocks acquired from lymph node aspirates versus non-lymph node body sites (e.g. serous fluids). Due to the variable nature of antigen expression in hematolymphoid cells depending on their level of maturity and location (lymph node, germinal center versus parafollicular), the application of IHC on cell-block may not be straightforward and can be misleading. This is specially applicable to the disease entities with precursor nonlymphoma stage such as in-situ follicular lymphoma and in situ-mantle cell lymphoma.
For example, a monomorphic population of lymphocytes with cyclinD-1, CD5, and CD20 immunoreactivity is virtually always abnormal and raises the differential of MCL, or hairy cell leukemia. However, distinction between in situ mantle cell neoplasia (ISMCL) versus mantle cell lymphoma is not possible on LN-FNA cell-block due to the lack of architectural details. However, a similar finding in cell-blocks of non-lymph node cytology specimens such as effusion fluids would be diagnostic of mantle cell lymphoma involving effusion fluid (although it would be a rare presentation for MCL).
As another example follicular lymphoma cells express germinal center markers with coexpression of CD10, bcl-6, and bcl-2. However, such findings in LN-FNA cell-block would not allow distinction between in situ process versus lymphoma; again due to lack of architectural details. However, for non-lymph node sites, a population of lymphocytes immunoreactive for CD20 with coexpression of germinal center markers (CD10, bcl-6, and bcl-2) would be diagnostic of non-Hodgkin B-cell lymphoma with germinal center immunophenotype.
Another example of how cell-block-IHC can be very useful is in cases where Reed-Sternberg cells or Hodgkin cells are noted on light microscopy in suspected cases. Adequately cellular cell-block can be utilized with a proper immunopanel with SCIP approach to confirm the diagnosis of Hodgkin lymphoma. It should be pointed here that cells with RS like morphology can be seen in other malignant and reactive pathologies. In some cases, a recommendation for excisional biopsy, could be made as next step for subclassification as clinically indicated.
According to the three possible morphologic scenarios, a recommended algorithmic IHC panel is presented with the most commonly encountered hematolymphoid malignancies [Figure 3].
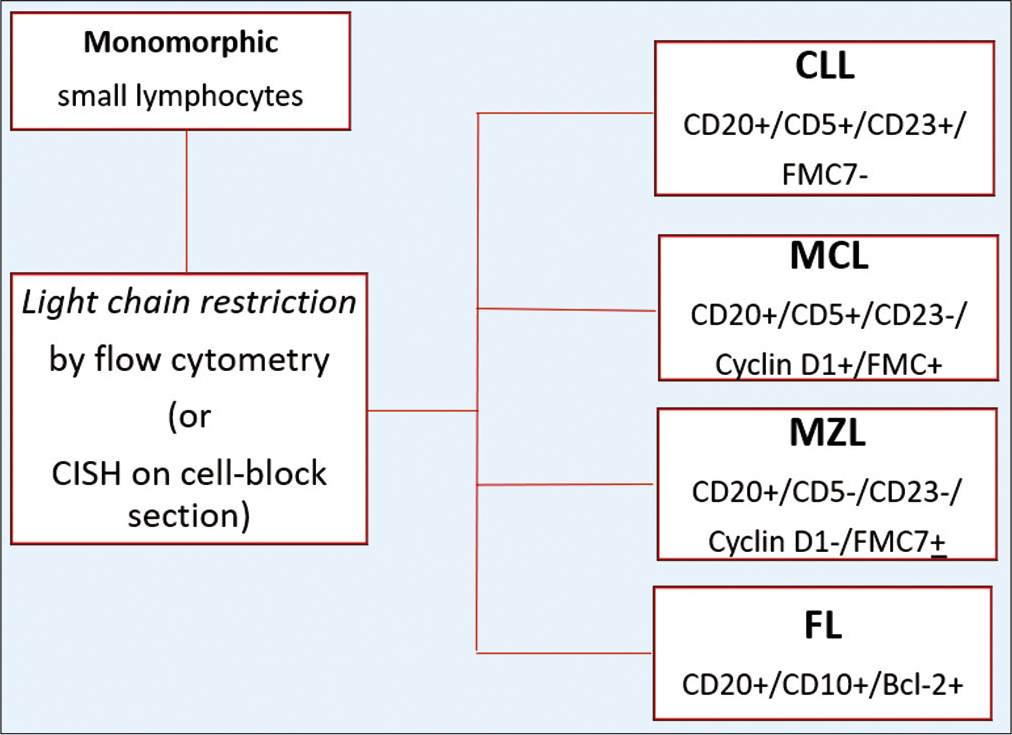
- The immunoprofiles of monomorphic lymphoid proliferations based on IHC on CB (CLL, Chronic lymphocytic leukemia; MCL, Mantle cell lymphoma; MZL, Marginal zone lymphoma; FL, follicular lymphoma).
Suggested IHC battery for the most common morphologic presentation based on the European Society for Medical Oncology:[12]
(Proposed only for subclassification purposes after confirmation of malignancy).
Small monomorphous lymphocytes
Cytomorphological features can be helpful for broad morphological differential [Figure 1] but subtyping of NHBCL is suboptimal if performed solely on cytomorphology.[13] Professional organizations recommend against making a diagnosis of lymphoma based solely on cytology smears including the most recent CAP guidelines (CAP 2020).[3]
A panel of antibodies against CD45, CD3, CD20, CD5, CD10, CD23, Ki-67, BCL-2, and CyclinD1 are useful in subtyping NHL.[4,18,24] Given a confirmed malignancy (recurrent lymphoma or documented evidence of monoclonality by flow cytometry), the differential diagnosis of a small monomorphous lymphocytic population could be narrowed down and accurately subclassified. For example: CD10-/CD5+/CD23+ points toward (CLL/SLL); while CD10-/CD5+/CyclinD1+ is consistent with (MCL), and CD10+/Bcl-2+/CD5- is consistent with a lymphoma with follicle center phenotype suggestive of FL [Table 2 for typical immunophenotype of the common small B cell lymphomas].
Large monomorphous lymphocytes
The most common entity that falls into this group is (DLBCL) in addition to others such as ALCL, BL, and T-cell and B-cell LBL (T/B-LBL).
DLBCL is a clinically and biologically heterogeneous group. Based on the cell of origin concept, DLBCL is further subclassified into activated B-cell-like (ABC) germinal center B-cell (GCB) subtypes, with the latter carrying better prognosis.[25] In addition, DLBCL is impossible to distinguish from the morphologically similar, but more aggressive high-grade B-cell Lymphoma (HGBCL) based on cytomorphology and flow cytometry only.
According to the revised 4th edition of the World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues, HGBCL includes two major entities; HGBCL – not otherwise specified (NOS), the majority of cases, and high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangement, that is, “double/triple hit” lymphoma (DHL), accounting for 6–9% of all aggressive B cell lymphomas.
Another provisional subgroup was proposed based on Bcl-2 and c-Myc protein expression and termed double expresser lymphoma (DEL) which encompasses a subset of DLBCL and HGBCL-NOS, with MYC (more than 40% positive cells) and BCL2 protein (more than 50% positive cells) over-expression, accounting for 25–30% of remaining aggressive B-cell lymphomas. Overall, DHL and DEL represent distinct but overlapping subsets of mature NHBCL with aggressive clinical behavior, poor response to standard (i.e., R-CHOP) chemotherapy and high relapse rates.[26,27] While DHL has a worse prognosis than that of DEL, both show poor progression-free survival compared to conventional DLBCL.[28,29] Due to the diverse and overlapping nature of DLBCL, further subclassification carries a clinical value.[25] Proper subtyping of DLBCL into GCB- and ABC- subtypes can be made following a well-established IHC algorithm.
Multiple algorithms have been proposed. One of the most popular and user-friendly is Hans algorithm [Figure 4].[25] On the other hand, Choi algorithm requires more IHC markers that are not readily available in most clinical settings, but it shows the highest correlation with gene expression profiling studies.[30] The suggested IHC panel for large cells includes CD45, CD3, CD20, CD5, CD10, CD23, Ki-67, BCL-2, BCL-6, MuM-1, CD30, EBV, ALK, and c-Myc. DLBCL- Germinal center (GC) subtype CD10+, CD20+, BCL6+, MUM-1- Versus DLBCL-ABC subtype CD10-, CD20+, BCL6-, MUM-1+.
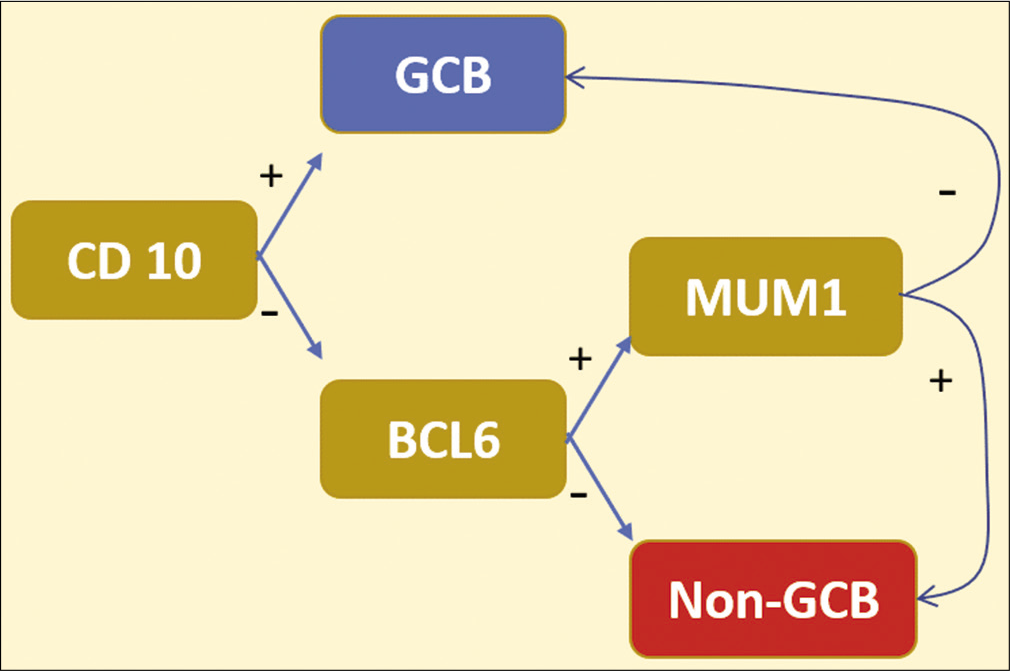
- Hans algorithm: CD10 with BCL-6 as germinal center markers. MUM1 is activated in post-germinal center B-cells (post-GCB). ~80% concordance with the gene expression profiling classification of DLBCL.
A valuable role of cell-block of LN-FNA is the ability to identify DEL and raise suspicion for DHL types of DLBCL. The expression of MYC along with BCL-2 is the hallmark of DEL. Further, cell-block of LN-FNA could be used for molecular studies such as FISH analysis detecting rearrangements in MYC and BCL2 or BCL6 that would point to a DHL diagnosis (see further discussion under molecular genetics below). A diagnosis of lymphoblastic lymphoma (LBL) is entertained when markers of immaturity are detected (TdT, and CD34). Aside from the distinctive cytomorphologic features of ALCL (i.e., hallmark cells), immunoreactivity with CD3, CD30, and ALK is diagnostic. Burkitt’s lymphoma (BL) also features a somewhat distinctive immunophenotype with CD10+, BCL2 negative, and high (near 100%) Ki-67 immunoreactivity (but this may also be seen in DHL also).
Polymorphous background with or without RS cells
cHL and THRLBCL along with non-neoplastic, autoimmune, and infectious causes are the most probable differential diagnoses entertained with this pattern. Flow cytometry does not provide a definitive diagnosis when applied to this category [Figure 5]. Therefore, such specimens are best investigated using morphology combined with IHC on cell-block. The recommended IHC panel to rule in/out cHL is (CD45, PAX-5, CD30, CD15, CD20, CD3, ALK, and EMA). Identification of RS cells on cytomorphologic grounds [Figures 5 and 6] is aided by immunoreactivity with the typical RS cells immunophenotype [Figure 6].
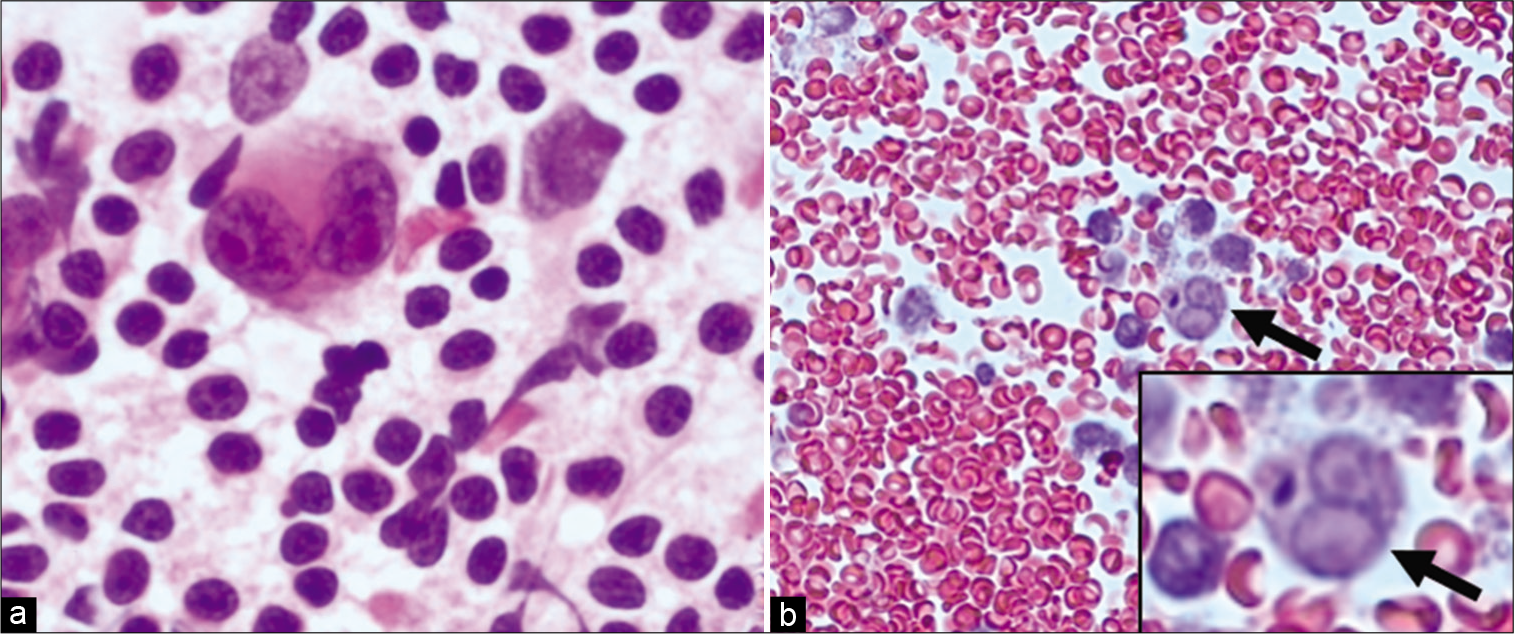
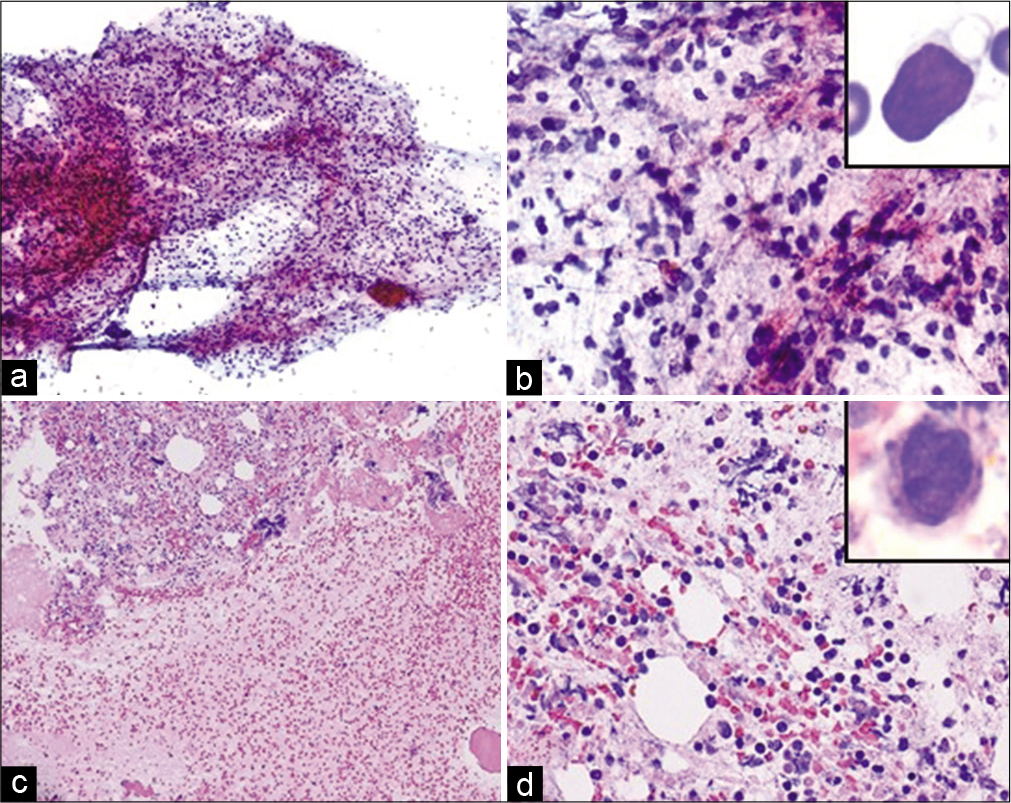
- Mediastinal lymph node EBU-FNA- Hodgkin lymphoma (a) PAP 100X: Aspirate smears with Reed-Sternberg (RS) cells in a polymorphous background; (b) Cell block H&E 40X: shows a few lymphocytes and a single RS cell (arrows).
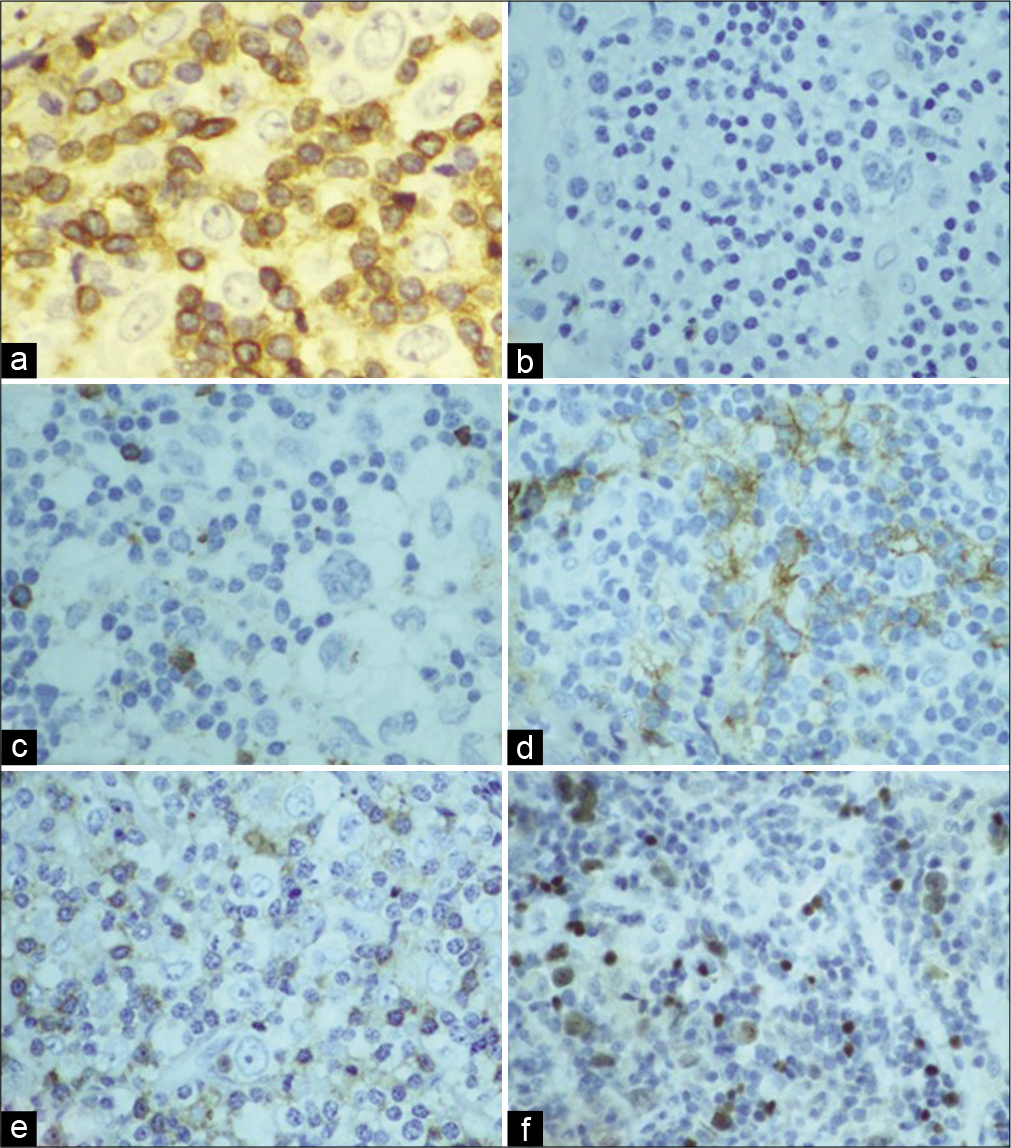
- Mediastinal lymph node EBU-FNA- Hodgkin lymphoma IHC (a) CD3 IHC 40X: negative in neoplastic large cells while positive in background T-cells; (b & c) CD15 and CD20 IHC 20X, respectively, negative in Hodgkin cells; (d) CD30 IHC 20X: is positive in neoplastic cells; (e) CD45 IHC 20: is positive in small background cells; (f) PAX5 IHC 20X: dim-nuclear positivity in Hodgkin and RS cells.
Overall, cell-block allows application of IHC studies that can establish diagnosis and subclassify a lymphoma. Furthermore, tumor immunomarkers (e.g., CD19, CD22, and CD30) are becoming an essential part of lymphoma workup with therapeutic implications as more immunochemotherapy-based treatment options are becoming widely available.[2]
Table 2 shows an example of the most common IHC studies applicable to the work up of small B cell lymphomas.
APPLICATION OF MOLECULAR GENETICS FOR EVALUATION OF HEMATOLYMPHOID LESIONS IN CYTOLOGY SPECIMENS INCLUDING LN-FNA
Hematolymphoid malignancies are progressively being associated with genetic changes. Identification of specific genetic alterations may be essential for both diagnostic and prognostic evaluation. Molecular testing encompasses FISH and PCR based assays that can be performed on FFPE of cell-blocks of cytology material.[31] The cytopathologists should be aware of what testing is feasible based on the quality of the cell-block and the differential diagnosis. While it is not an absolute requirement for a lymphoma diagnosis, it can provide useful information in terms of lineage designation and clonality in addition to prognostic and therapeutic input.
In general, the most frequent molecular testing for distinguishing lymphoma versus reactive lymphoid proliferation is evaluation of clonality through analysis of Ig gene as well as TCR gene rearrangements. This can be tested on FFPE of cell-block by PCR-based assay.
Clonality testing may likewise be helpful for detection of minimal residual disease. One of the most valuable application of molecular diagnostics on cell-block is in fact the capacity to identify DHL, which carries the worst prognosis and requires intensive treatment.[32,33] In some cases, flow cytometry is not available or can not be performed due to submission and processing of aspirate material in formalin. Demonstration of monoclonality in B-cell or T-cell is important for establishing a diagnosis of malignancy (although monoclonality is not always equivalent to neoplasia). PCR can be performed on FFPE of cell-block to detect mutation/clonality at the immunoglobulin heavy chain gene locus for B-cells and at the Gamma chain in TCR for T-cells [Tables 3 and 4].
| Lymphoma | Most common genetic alteration | Variants | Utility |
|---|---|---|---|
| DLBCL | BCL-6 translocation or mutation | BCL2(translocation), MYC(translocation) | MYC translocation infers aggressive disease |
| FL | t(14;18)(q32;q21), IGH-BCL2 | BCL6 translocation | The presence of a t(14;18), IGH-BCL2 facilitates the diagnosis |
| CLL | del(13q14) | del(11q22–23), +12, del(17p13), del(6q21) | del(13q14)-favorable prognosis, del(11q22–23), del(17p13)-unfavorable prognosis |
| MCL | t(11;14)(q13;q32) CCND1-IGH | Rare translocations involving cyclin D2 or D3 | The presence of a t (11;14), CCND1-IGH facilitate the diagnosis |
| MZL | t(11;18)(q12;q21), BIRC3-MALT1 | t(14;18)(q32;q21), IGH-MALT1 t(3;14)(p14.1;q32), FOXP1-IGH t(1;14)(p22;q32), IGH-BCL10 | t(11;18), BIRC3-MALT1 in gastric MALT lymphoma is associated with resistance to antibiotic therapy |
| BL | t(8;14)(q24;q32), MYC-IGH | t(2;8)(p12;q24), IGK-MYC t(8;22)(q24;q11), MYC-IGL | The presence of a t(8;14), MYC-IGH facilitates the diagnosis |
| Burkitt-like lymphoma with 11q aberration | -11q23.3-q25 Chromosome 11 proximal gain and telomeric losses |
Not applicable | The presence of aberration facilitates the diagnosis |
DLBCL: Diffuse large B cell lymphoma, FL: Follicular lymphoma, CLL/SLL: Chronic lymphocytic leukemia/small lymphocytic lymphoma, MCL: Mantle cell lymphoma, MZL: Marginal zone lymphoma, BL: Burkitt’s lymphoma
| Lymphoma | Most common genetic alteration | Variants | Utility |
|---|---|---|---|
| Anaplastic Large T-Cell Lymphoma (ALCL) | t(2;5) (p23;q35) NPM1-ALK | t(x; 5) and other numerous variants ALK translocation | ALK translocations: favorable |
| Peripheral T-cell lymphoma | Gains: 7q, 8q, 17q, 22q; Losses: 4q, 5q, 6q, 9q, inv14q (q11;q32) | Losses: 10q, 12q, 13q | Not established |
| T-cell prolymphocytic leukemia | inv14q (q11;q32) | t(8;8)(p11–12;q12), trisomy 8q, idic (8p11) | inv(14q)(q11;q32) facilitates the diagnosis |
| Hepatosplenic T-cell lymphoma | i(7)(q10) | i(7)(q10) facilitates the diagnosis | |
| Enteropathy-associated T-cell lymphoma | 9q31.3 complex amplifications | del16q12.1 | 9q amplification facilitates the diagnosis |
| Extranodal NK/T-cell lymphoma, nasal type | del (6)(q21q25), i (6)(p10) | Not established |
Molecular testing such as PCR or NGS (Next generation sequencing) can also be performed on cell-block or FNA samples to aid in determining a lymphoma subtype. For example, CD5-/ CD10- clonal small B cells raise the differential of MZL versus LPL.[12] Cell-block can be used here for identification of MYD88 mutation pointing toward a diagnosis of LPL. Another potential role for molecular testing is to identify targetable mutations.
Challenges
The immunophenotypic profiles are sufficiently distinctive to permit classification of low-grade B-cell lymphomas based on cytology and flow cytometry in most cases.[32] The diagnostic yield is further enhanced by the proper application of IHC on cell-block; however, challenges may still exist needing excisional biopsy for evaluation of architecture.
The grading of FL is based on evaluating the relative proportions of centrocytes and centroblasts, an exercise that has poor reproducibility on histologic sections and probably is less reproducible on cytology preparations.[12] As such, diagnosis of FL based on FNA always requires a surgical resection specimen for grading purposes as well as to rule out transformation. Although criteria for grading of FL by aspiration cytology have been proposed by Young et al.;[34]such criteria are not widely applied in clinical practice. Further research on this issue with application of appropriate immunomarkers on qualitatively and quantitatively optimal cell-block[35-37] with special approaches including application of SCIP[21] and dual/ multicolor immunostaining[22,23] is recommended.
Primary mediastinal large B-cell lymphoma (PMBL) versus HGBCL with features intermediate between DLBCL and PMBL: The extensive overlap in morphologic features of these two entities precludes a definitive distinction in FNA specimens and requires surgical excision biopsy.
CD45 and epithelial markers such as EMA (with or without cytokeratin) are common immunostains performed by pathologists as an initial step for identification of the lineage of malignancy (carcinoma vs. lymphoma). In selected cases of lymphoma (e.g., cHL, ALCL), CD45 may be nonimmunoreactive with EMA immunoreactivity (e.g., ALCL) leading to the false interpretation as epithelial lineage. Similarly, a subset of plasma cell neoplasms (PCNs) is immunoreactive for EMA. Some of the frequent unexpected immunostaining patterns are summarized in Table 5.
| IHC | Common discrepancies |
|---|---|
| CD45 | Lost expression in DLBCL (10%), T-LBL, and plasmacytoma/myeloma Aberrant expression in a subset of seminoma and mammary carcinoma |
| CD20 | B-cells lack CD20 s/p immunotherapy “Rituximab” nucleolar staining of large number of cells of many different tissue types. Not indicating B-cell origin |
| CD3 | Positive in NK-cells. ALCL is usually positive for CD3 but can be CD3 negative |
| CD10 | Positive in Germinal center B and T-lymphocytes, Mature granulocytes, and subet of MZL |
| CD23 | Positive in 20% of follicular lymphoma Normal in monocytes and dendritic cells |
| CD68 | Positive in a subset of DLBCL Histiocytes |
| EMA | Positive in a subset of large B cell lymphoma, plasma cell myoma, Nodular lymphocyte predominant Hodgkin lymphoma, ALCL |
STUDY CASES
Case 1
History
A 55-year-old male with medical history of prostate cancer, Gleason score 4+3. Recently, he presented to the emergency room with left neck enlarged LNs, fatigue, weight loss, and night sweats for 1 month. The LNs were painful with waxing and waning over the past few weeks. Computed Tomography (CT) scan of the neck with IV contrast showed multiple large LNs throughout the mediastinum with lymphadenopathy in the left supraclavicular area, left carotid triangle, left submandibular fossa, and right supraclavicular region. Ultrasound-guided FNA was performed with onsite adequacy on a 1.5 cm left supraclavicular LN with central necrosis. Needle rinses including dedicated pass were submitted for flow cytometry.
Cytomorphology
Diff-Quik stained direct smears of FNA aspirates were mildly cellular with a few medium to large sized lymphocytes with occasional prominent nucleoli and slightly open chromatin [Figure 7]. The cell-block was mildly cellular and showed similar findings. The aspirates were negative for carcinoma.
- Case 1, Left supraclavicular lymph node (1.5 cm)-FNA: (a) PAP stain 20X: Mild cellularity with singly scattered non-cohesive lymphocytes; (b) PAP stain 40X: Majority of small lymphocytes with occasional large cells with prominent nucleoli (inset); (c 10X: d 40X) Cell-block H&E: Mildly cellular with monomorphic lymphocytes with occasional atypical large cells with prominent nucleoli (inset).
Cytopathologic interpretation
Lymphocytes present, final characterization pending immunophenotyping.
Immunophenotyping by flow cytometry
Flow cytometry was performed with 86% viability and identified 81% lymphocytes, 70% of which are monoclonal kappa-restricted B-cell population positive for CD19, CD20 bright, CD10, CD38, and FMC7. They were negative for CD2, CD3, CD4, CD5, CD7, CD8, CD11C, CD23, CD25, and lambda light chain. The majority of lymphocytes are small in size based on the side scatter analysis.
Final interpretation
Non-Hodgkin B cell lymphoma (NHBCL) with GC phenotype.
Discussion
Although lymphoma typically presents as painless lymphadenopathy, it is important to note that local physical pressure due to enlarged LNs could explain the painful lymphadenopathy presented in this patient. A dedicated pass was sent for flow cytometry analysis to exclude lymphoma as no clear-cut pathology (i.e., metastatic carcinoma, suppurative lymphadenitis, and granuloma) that would explain the existing lymphadenopathy was found during on-site adequacy. As discussed earlier, the flow cytometry is more sensitive when compared to immunocharacterization by IHC. Although we recommend submitting two FNA passes to flow cytometry analysis; adequate viable cells can be obtained with a single pass to demonstrate the clonal lymphocytic population and the expression of GC marker (i.e., CD10). The major differential diagnoses of a clonal population of B-cells with GC phenotype include DLBCL, BL, and FL. While the observation of a monomorphic population of large lymphocytes on morphologic assessment would direct the interpretation towards DLBCL or gray zone lymphoma, the finding of a monomorphic medium sized lymphocytes with cytoplasmic vacuoles favor BL. Although the majority of the lymphocytes are small in size as demonstrated by FC side scatter and on morphologic assessment, partial involvement by a higher-grade process like DLBCL cannot be reliably excluded. In addition, a follow-up excisional biopsy is recommended in such cases to grade FL (low vs. high), and subclassify FL (diffuse vs. follicular) architecture.
Case 2
History
A 64-year-old African American female with gamma heavy chain plasma cell leukemia status post-chemotherapy presented with shortness of breath due to a large pericardial effusion with pericardial tamponade. Pericardiocentesis was performed and flow cytometry confirmed 18% atypical plasma cells.
Cytomorphology
Cytological analysis showed hypercellular specimen with many atypical plasma/plasmacytoid cells in a background of mixed inflammatory cells and blood. The atypical plasma/ plasmacytoid cells are large in size and show slightly irregular nuclear contours, variable chromatin, many with prominent eosinophilic nucleoli and pale cytoplasm. The cell-block is cellular and shows similar findings [Figure 8].
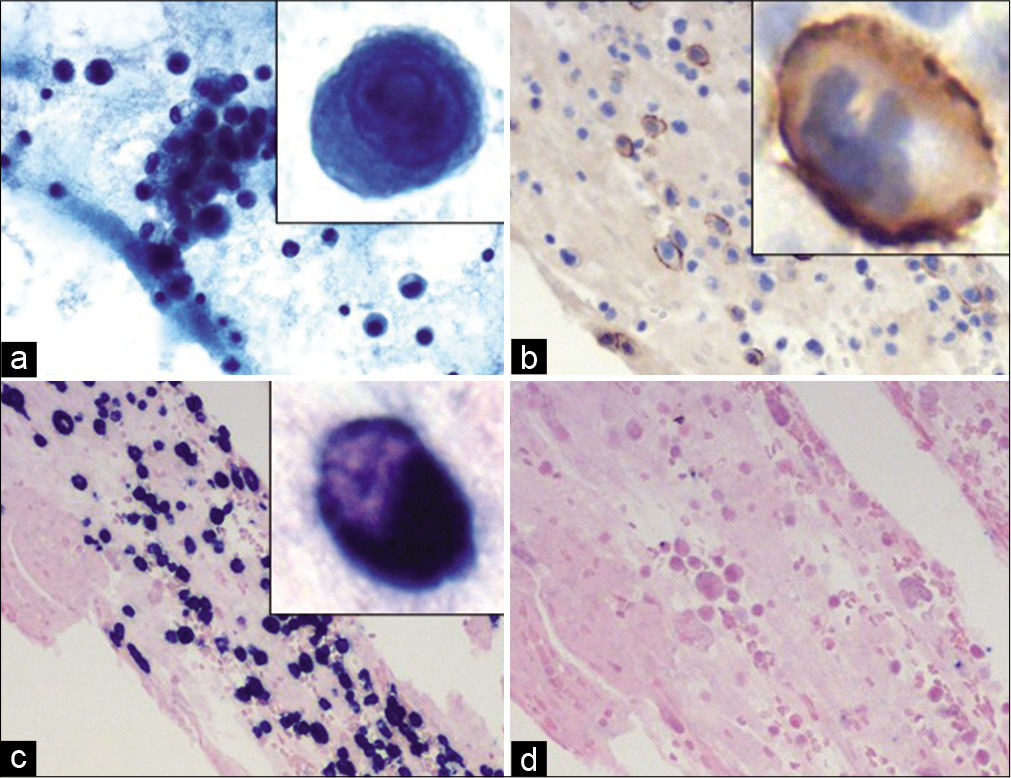
- Case 2, Pericardial fluid: (a) PAP 20X: Hypercellular specimen with many atypical plasma/plasmacytoid cells with prominent nucleoli (inset); (b) CD138 IHC on cell-block 20X: The plasma cells are immunoreactive, higher magnification (inset); (c) Lambda CISH on cell-block section 20X: Positive in virtually all plasma cells confirming clonality; (d) Kappa CISH on cell-block section 20X: Negative in most plasma cells.
Cytopathologic interpretation
Positive for malignancy, final characterization pending immunophenotyping
Immunophenotyping by flow cytometry
Immunophenotypic analysis of the pericardial fluid detected approximately 30% atypical plasma cells expressing bright CD38, CD56, dim CD138 and negative for cytoplasmic kappa and lambda, CD19, and CD45.
Final interpretation
Plasma cell neoplasm.
Discussion
Although body cavities are a common site for metastases, they are less commonly involved by a lymphomatous process (20% incidence).[38] Infiltration of monoclonal “neoplastic” plasma cells into pleural fluid is rare; around 5% of patients with plasma cell neoplasm (PCN) develop it during the course of their disease. Usually, the circulating neoplastic plasma cells are displaced mechanically into the pleural fluid as a physical phenomenon secondary to amyloid related heart failure instead of true invasion into pleural cavity by the neoplastic plasma cells.[38] It is rarer for PCN to present as a direct infiltration of plasma cells into the pleural cavity. Patients known to have PCN usually present with effusion as a sign of relapsed disease or disease progression. In cases with high clinical suspicion, flow cytometry analysis should be performed to confirm monoclonality and further characterize the cells. The differential diagnosis of monoclonal plasmacytoid cell in pleural specimen includes PCN, small NHBCL with plasmacytoid differentiation, plasmablastic lymphoma (PBL), and primary effusion lymphoma (PEL). Different types of NHBCL including CLL/SLL, MCL, FL, and MZL can show plasmacytoid differentiation on morphologic assessment; however, they can be excluded based on immunophenotype. Demonstration of CD20 negativity (B-lymphocyte marker) using FC or CB-IHC and expression of CD138 (plasma cell marker) confirms the plasmacytic lineage and essentially excludes lymphomas with tumor cells committed to lymphocytic lineage. Other tumors with neoplastic plasma cells (CD138+) include PEL, PBL, and HHV8 positive DLBCL, among others which may pose a diagnostic challenge. Clinical scenario is extremely helpful here as PEL is almost always seen in immunocompromised Human Immunodeficiency Virus (HIV) positive patients and would show pleomorphic lymphoplasmacytic infiltrate on morphologic examination. PBL commonly involves the nasopharynx and also associated with HIV infection. The previous history of PCN in this case along with the matching immunophenotype of absent k and l light chain expression on the plasma cells facilitated the interpretation as consistent with an aggressive, immature PCN.
Case 3
History
A 73-year-old male with acute myeloid leukemia, status post bone marrow transplant presented with worsening abdominal distention and ascites. The patient underwent ultrasound-guided paracentesis, and 2.5 L fluid was sent for cytomorphologic analysis.
Cytomorphology
Diff Quik (DQ) stained slides showed a hypercellular specimen with a monomorphic population of large cells featuring high nuclear to cytoplasmic ratio, open chromatin with one to three nucleoli, and scant pale cytoplasm [Figure 9].
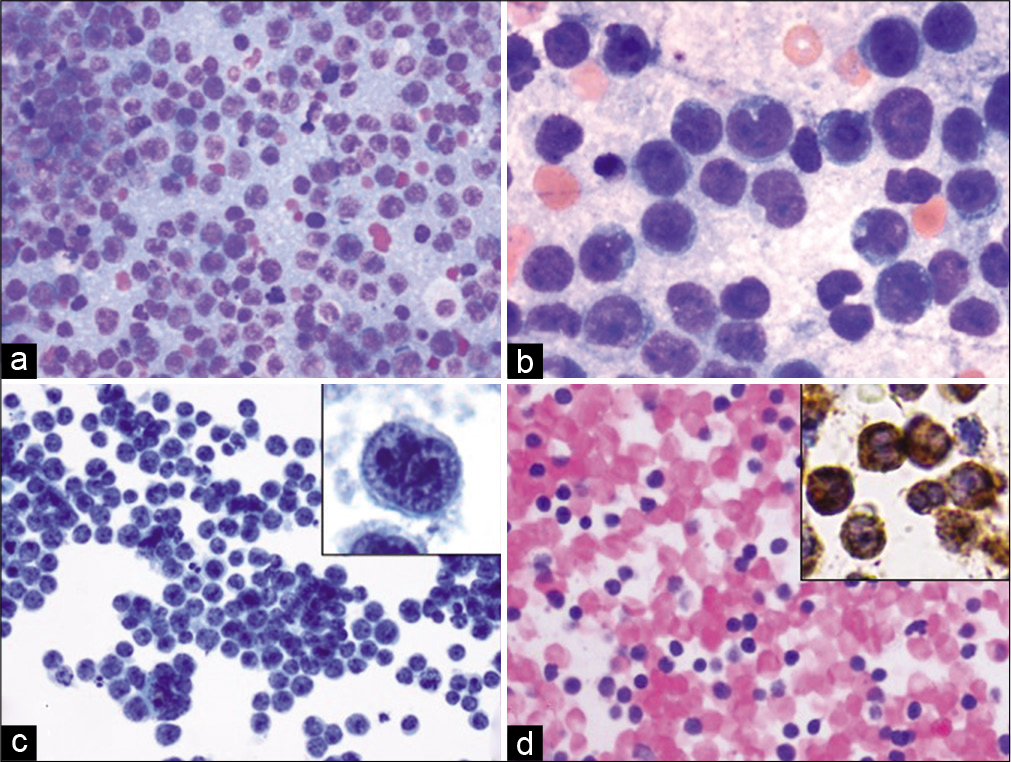
- Case 3, peritoneal fluid: (a) Diff-Quik 20X: Monomorphic large cells with blast morphology; (b) Diff- Quik 40X: Monomorphic large, cleaved cells; (c) PAP 20X: Large non-cohesive single cells with bilobed/cleaved nuclei (inset); (d) Cell-block 20X: Cellular specimen with myeloperoxidase special stain (inset) confirming myeloid lineage.
Cytopathologic interpretation
Positive for malignancy, final characterization pending immunophenotyping.
Immunophenotyping by cell-block IHC
Immunohistochemical stains performed on the cell-block and showed that the blasts are positive for Myeloperoxidase (MPO).
Final interpretation
Acute myeloid leukemia.
Discussion
The defining morphologic findings of “myeloblasts” are clearly demonstrated on an adequately prepared Diff Quik stained smear. The blast are large cells with high nuclear to cytoplasmic ratio, the nuclei are variable in shape (round, oval, delicate) with smooth nuclear membrane and open chromatin. One to three nucleoli can be observed (pale/ eosinophilic with Diff-Quik stain and dark with Pap stain), surrounded by scant pale cytoplasm. Myeloblasts with monocytic differentiation often demonstrate cytoplasmic vacuoles. Around 20% of leukemia cases (including MDS, AML, and ALL) develop malignant pericardial effusion, the majority of which are clinically insignificant.[39] FC or CB-IHC could be used for immunophenotypic analysis. The impetus to fully characterize the leukemia is often not clinically indicated in a previously diagnosed leukemia patient. The main purpose of immunocharacterization of “blast-like cells” is to confirm immaturity by demonstrating blast markers (CD34 and/or TdT on CB-IHC or CD34 and/or HLA-DR by FC). Further characterization of blasts into myeloid and lymphoid blasts (CD13, CD33, and CD117, MPO/TdT vs. CD3 or CD19, CD10, TdT, and CD99, respectively) might be indicated in cases where a cross-lineage progression phenomenon is suspected. A common example is the lymphoblastic leukemia presenting as a blastic phase of chronic myeloid leukemia. In this case, the cell-block was adequately cellular and TdT immunostaining showed nuclear reactivity. A follow-up flow cytometry was performed on a liver nodule that was suspicious for myeloid sarcoma and showed acute myeloid leukemia with monocytic differential. The atypical mononuclear/blastoid cells were positive for MPO (strong), CD43, CD68, CD56 (weak; subset), and p53 (few/focal) and were negative for CD20, CD3, CD5, CD79a, CD117, CD15, CD30, CD4, CD123, CD56, CD34, CD45, and CD99.
Case 4
History
A 37-year-old African American male presented with left-sided cervical lymphadenopathy along with B symptoms including fatigue, generalized weakness, 30 pounds weight loss, anorexia, and night sweats. CT scan of the neck showed enlargement of lingual tonsils and multiple enlarged cervical LNs bilaterally. PET-CT scan showed increased fluorodeoxyglucose avidity of the LNs both above and below the diaphragm. Ultrasound-guided FNA was performed on an enlarged neck lymph node with cell-block and flow cytometry for cytomorphologic analysis.
Cytomorphology
FNA and cell-block showed a hypercellular specimen with many atypical lymphocytes [Figure 10].
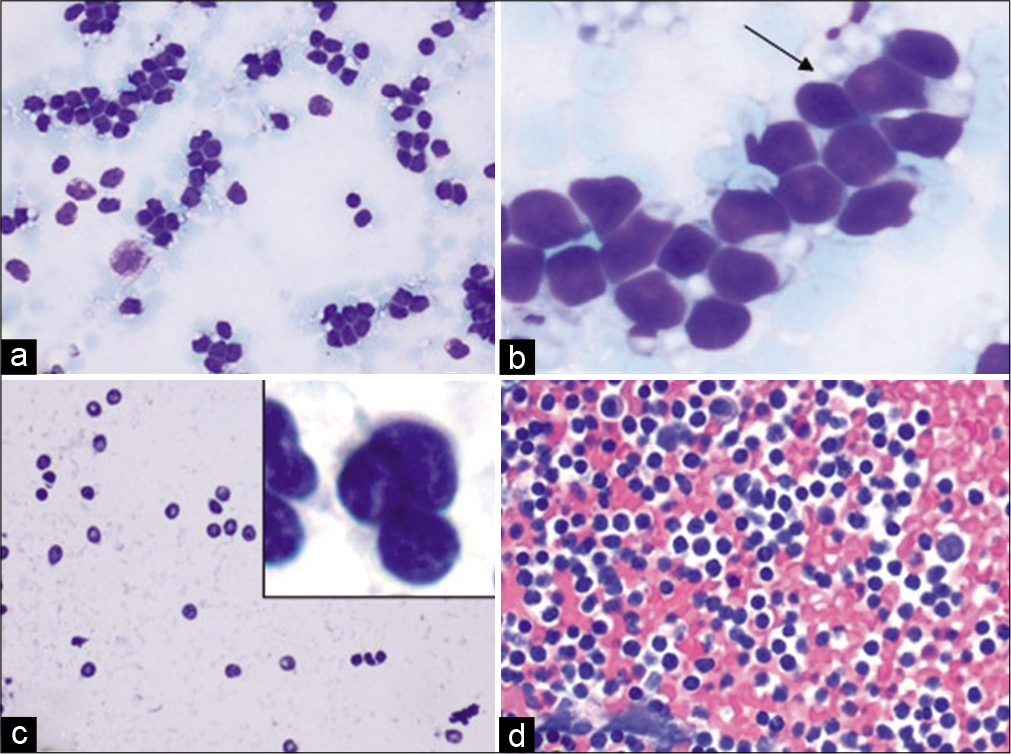
- Case 4, Neck lymph node FNA: (a) Diff-Quik 20X: Monomorphic lymphocyte; (b) Diff-Quik 40X: Lymphoglandular bodies (arrow); (c) PAP 20X: Irregular shapes and sizes of atypical lymphocytes with an inset showing atypical lymphocytes with irregular nuclei; (d) Cell-block H&E 20X: Hypercellular specimen; adequate for an expanded panel for immunostains (See Figure 11).
Cytopathologic interpretation
Atypical lymphocytes, final characterization pending immunophenotyping.
Immunophenotyping on CB-IHC and FC
Flow cytometry analysis detected around 98% lymphocytes. Around 70% of those T cells were abnormal; positive for CD2, CD3, CD7, CD38, and TCR A/B. They were negative for CD5, CD4, CD8 (dim in subset), CD10, CD19, CD25, CD57, and TCR G/D. This population was showing abnormal expression of the B cell marker CD20. The immunohistochemical analysis showed that the majority of lymphocytes are T-cells immunoreactive for CD2, CD3, with downregulation of CD5 and CD7 [Figure 11].

- Case 4, Neck lymph node FNA, IHC on cell-block sections: (a) CD2 IHC 20X: diffusely positive; (b) CD3 IHC 20X: diffusely positive; (c) CD5 IHC 20X: partial loss of expression; (d) CD7 IHC 20X: partial loss of expression; (e & f) showing loss of expression of CD4 and CD8, respectively.
Final interpretation
Mature T-cell lymphoma (TCL), NOS subtype.
Discussion
The morphologic features of lymphocytes are best demonstrated on an adequately prepared Diff-Quik stained cytology slide. In this case, non-cohesive cells with high N/C ratio, fine chromatin, and scant clear cytoplasm are noted. The differential diagnosis of predominantly small to medium sized lymphocytes includes a wide range of mature B and TCL among other malignant non-lymphoid small round cell tumors (small cell carcinoma, neuroendocrine tumors, and small cell tumor variants [e.g., melanoma]). Given the clinical scenario of diffuse lymphadenopathy associated with B-symptoms; the probability of a benign reactive or infectious lymphoid process is less likely. The discohesive nature of those cells and the lymphoglandular bodies in the background confirms the lymphocytic nature of those cells and essentially rules out other non-lymphoid malignancies. Morphologic identification of lymphocytes is essential allowing for proper specimen triaging as flow cytometry plays an essential role in identifying lymphoid malignancies in general and TCL in particular, as the majority of TCL would demonstrate aberrant CD markers expression profile. While normal T-cells express CD2, CD3, CD4/8, CD5, and CD7; neoplastic T-cells often are missing (downregulated) some CD markers (commonly CD5, and CD7). The majority of mature TCL are identified according to their specific morphologic and immunophenotypic expression profiles. T-cell proliferations that do not correspond to the current specifically defined entities by WHO are classified as PTCL, NOS. In our case, both flow cytometry analysis and CB-IHC demonstrated abnormal T-cell supported by the aberrant expression profile (downregulation of CD5 and CD7). The absence of BCL-6, CD10, and CXCL-13 precludes a diagnosis of T-cell lymphoma with T-follicular helper (TFH) phenotype. In addition, the absence of specific morphologic and immunophenotypic features that would otherwise allow categorization into a defined TCL entity; and the mature nature of lymphocytes along with the aberrant expression profile qualifies this case to be interpreted in a more broad and general diagnosis of mature TCL. The patient had a follow-up excisional biopsy of a neck LN which showed peripheral TCL, NOS.
Case 5
History
A 75 year-old white male with past medical history of left renal cancer (S/P nephrectomy), previous seizure episode, and heart failure presented with shortness of breath, loss, yellowing of the skin, and pale/white/ clay colored stools for around 2 weeks. Lab results were notable for elevated bilirubin and liver enzymes. CT scan of abdomen/pelvis with contrast showed a mass in the head of the pancreas encasing the superior mesenteric artery and celiac vein, extensive retroperitoneal and paraaortic lymphadenopathy, with Gall bladder dilation, and moderate amount of ascites. Endoscopic ultrasound guided FNA was performed on the pancreatic mass and sent for flow cytometry and cytological analysis.
Cytomorphology
The aspirates were moderately cellular with crushing artifact. The specimen showed uniform population of medium to large lymphocytes featuring large vesicular nuclei, occasional nucleoli, and scant amount of cytoplasm in background of tingible body macrophages. H&E stained sections of the cell-block showed sheets of medium to large lymphocytes with similar morphology, infiltrating unremarkable pancreatic acinar glands in background of necrosis [Figure 12].
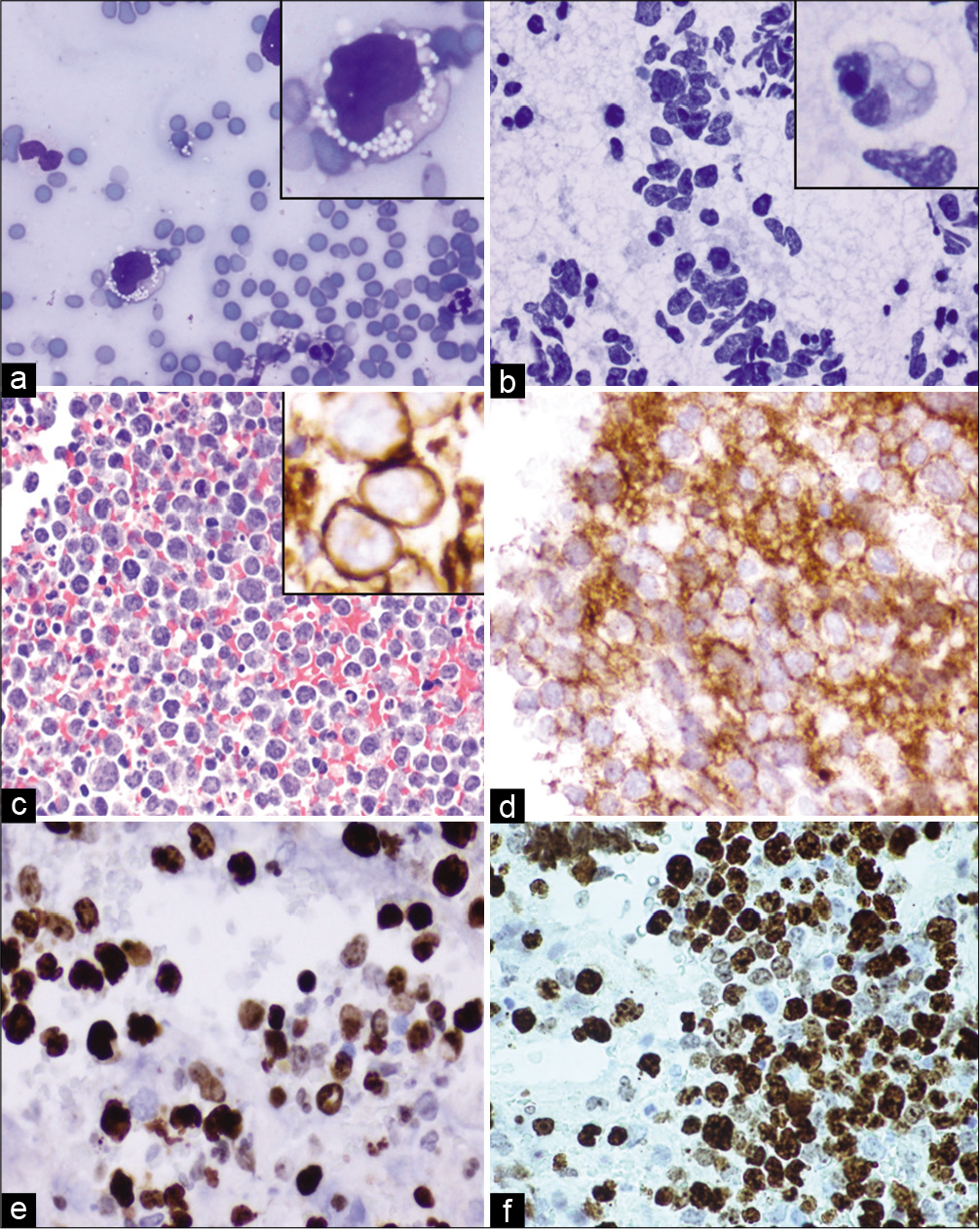
- Case 5, Pancreatic head mass EUS-FNA: (a) DiffQuik 20X: Large, atypical lymphocyte with irregular nucleus and cytoplasmic vacuoles (inset); (b) PAP 20X: Cellular specimen with uniform population of medium to large lymphocytes featuring large vesicular nuclei, occasional nucleoli and occasional vacuolated cytoplasm (inset); (c) Cell-block H&E 20X: Medium to large lymphocytes, immunoreactive for CD20 (inset of c), CD10 diffusely (d), BCL-6 (e) (d,e,f: 40X:). (f) KI-67 labeling index was high with expression >40% (40X).
Cytopathologic interpretation
Large B-cell lymphoma, final characterization pending immunophenotyping.
Immunophenotyping
The cell-block sections immunostained with SCIP approach showed that the malignant cells were immunoreactive with CD20, PAX-5, CD10, and bcl-6 with high proliferation index (Ki-67 80%). They were non-immunoreactive with CD3, CD5, bcl-2, CD30 (0%), MuM-1, C-MYC, CD34, and TdT. CD3 and CD5 highlight background T-cells also immunoreactive with BCL-2. The malignant lymphocytes composed around 80% of total viable nucleated cells in the cell-block.
Molecular testing
FISH analysis was performed on the cell-block and failed to detect rearrangement in IGH/BCL-2, BCL-6, or C-MYC. However, increased copy gains of IGH/BCL-2, BCL-6, and C-MYC were detected suggestive of polyploidy.
Final interpretation
Large B-Cell Lymphoma with Germinal Center immunophenotype and high Ki-67. (See comment).
Comment: The main differential here would be DLBCL and Follicular lymphoma, Grade III. Given the tendency of high grade follicular lymphoma (grade III) to be negative for the conventional IGH/BCL-2 rearrangement, follicular lymphoma cannot be excluded, leading to the final interpretation of Large B-Cell Lymphoma with Germinal Center immunophenotype and high Ki-67.
Discussion
Although B-cell lymphoma could, essentially, affect any given body site, presenting as a pancreatic mass is an uncommon scenario. The required skill to identify the variable morphologies of atypical lymphocytes for the pathologist/ cytotechnologist performing on-site adequacy is essential for proper triaging that subsequently yields a more accurate interpretation.
The observation of large non-cohesive atypical cells with high N/C ratio and the presence of lymphoglandular bodies are the basis of requesting a dedicated FNA pass for flow cytometry analysis. In addition, a dedicated pass was dedicated for cell-block for immunocharacterization and potential molecular studies. On Diff-quick slide examination, the finding of monotonous large lymphocytes with atypical features (abnormal nuclei, necrosis, and increased mitotic activity) is indicative of a lymphomatous process. Demonstration of a lineage specific CD marker/s (CD19; B-cells; and CD3; T-cells) is indicated for proper typing of lymphoma into general categories of B- and T- Cell Lymphoma.
The main differential here would be DLBCL and Follicular lymphoma, Grade III. DLBCL, NOS is the most common lymphoma affecting adults. DLBCL is subdivided into morphologic variants, molecular subtypes, and even distinct disease entities on the basis of biologic, morphologic, and clinical studies. The interpretation of Large B-Cell Lymphoma in this case was based on the presence of large cells (morphology) and expression of CD20 (CB-IHC). However, a full panel of immunostains and a FISH panel (IGH, BCL-2, and BCL-6) were performed in efforts to further subtype the lymphoma. The large lymphocytes are immunoreactive for CD20, Pax-5 (confirming B-lineage), and CD10, BCL-6 (indicating Germinal Center-subtype). The negative expression of BCL-2 should trigger the suspicion of BL; however, the large cells are nonimmunoreactive with C-MYC, essentially excluding BL. One of the major deferential diagnoses is a B-lymphoblastic leukemia/lymphoma which is ruled out based on the negative immature blast markers (TdT and CD34).
Due to the high proliferation index Ki-67, molecular studies were performed to exclude a more aggressive entity high-grade B cell lymphoma, NOS, defined as an aggressive mature B cell lymphoma with BCL2, and/or BCL6 rearrangement and lacking MYC rearrangement. FISH analysis for BCL6, BCL2, and MYC failed to demonstrate rearrangements in IGH/BCL-2, BCL-6, or C-MYC. Given the tendency of high grade follicular lymphoma (grade III) to be negative for the conventional I GH/BCL-2 rearrangement, follicular lymphoma could not be excluded, leading to the final interpretation of Large B-Cell Lymphoma with Germinal Center immunophenotype and high Ki-67.
Acknowledgment
The CytoJournal thanks Kedar Inamdar, MD, Director, Div. of Hematopathology, Department of Pathology, Henry Ford Hospital, Detroit, MI 48202 for conducting an editorial review as Academic editor of this article in CytoJournal Monograph Related Review Series.
The authors thank Dr. Mir Yousuf Khan for contributing images for some figures. The authors also thank Janavi Kolpekwar for her copy-editing support.
LIST OF ABBREVIATIONS (In Alphabetic Order)
ABC - Activated B-cell-like
ALCL - Anaplastic large
TCL ALL - Acute lymphoblastic lymphoma
AML - Acute myeloid leukemia
BL - Burkitt’s lymphoma
FISH - Fluorescent situ hybridization
CAP - College of American Pathologists
cHL - Classic Hodgkin lymphoma
CISH - Chromogenic situ hybridization
CLL - Chronic lymphocytic leukemia
CLL/SLL - chronic lymphocytic leukemia/lymphoma
CT - Computed Tomography
DHL - “double/triple hit” lymphoma DLBCL - Diffuse large
B-cell lymphoma
DQ - Diff Quik
FFPE - Formalin Fixed Paraffin Embedded
FL - follicular lymphoma
FNA - Fine-needle aspiration
GC - Germinal center
GCB - germinal center B-cells
HCL - Hairy cell leukemia
HGBCL - High-grade B-cell Lymphoma
HIV – Human Immunodeficiency Virus
HL - Hodgkin lymphoma
IHC – Immunohistochemistry
ISMCL - in situ mantle cell neoplasia
LBL - Lymphoblastic lymphoma
LPL - Lymphoplasmacytic lymphoma
LNs - lymph nodes
MALT – Mucosa-associated lymphoid tissue
MCL - Mantle cell lymphoma
MDS - Myelodysplasic syndrome
MPO – Myeloperoxidase
MZL - Marginal zone lymphoma
NCB - Needle core biopsy
NGS - Next generation sequencing
NHBCL - Non-Hodgkin B cell lymphoma
NHBL - on-Hodgkin B-cell lymphoma
NHL - non-HL
NOS - Not otherwise specified
NOS - Not otherwise specified
PBL - Plasmablastic lymphoma
PCN - Plasma cell neoplasm
PCR - polymerase chain reaction PEL - Primary effusion lymphoma
PET - Positron emission tomography
PMBL - Primary mediastinal large B-cell lymphoma
PTCL - Peripheral TCL
ROSE - Rapid On-Site Evaluation
RPMI - Roswell Park Memorial Institute
RS - Reed-Sternberg
SCIP approach - Subtractive Coordinate Immunoreactivity Pattern approach
TCL - T-cell lymphoma
TCR - T cell receptor
TFH - T-follicular helper
THRLBCL - T-cell/histiocyte-rich large B-cell lymphoma
WHO - World Health Organization
References
- Unexplained lymphadenopathy: Evaluation and differential diagnosis. Am Family Phys. 2016;94:896-903.
- [Google Scholar]
- Systematic review of the effectiveness of fine-needle aspiration and/or core needle biopsy for subclassifying lymphoma. Arch Pathol Lab Med. 2015;139:245-51.
- [CrossRef] [PubMed] [Google Scholar]
- The use of fine needle aspiration biopsy in the evaluation of lymphadenopathy. Semin Diagn Pathol. 2001;18:110-23.
- [Google Scholar]
- Value of fine needle aspiration cell blocks in the diagnosis and classification of lymphoma. Int J Clin Exp Pathol. 2014;7:7717.
- [Google Scholar]
- Flow cytometric immunophenotyping and cell block immunocytochemistry in the diagnosis of primary NonHodgkin's Lymphoma by fine-needle aspiration: Experience from a tertiary care center. J Cytol. 2014;31:123.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology and flow cytometry in the diagnosis and subclassification of non-Hodgkin's lymphoma based on the World Health Organization classification. Acta Cytol. 2007;51:390-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology and flow cytometry in the diagnosis and subclassification of non-Hodgkin's lymphoma based on the World Health Organization classification. Acta Cytol. 2007;51:390-8.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology versus histopathology in diagnosing lymph node lesions. EMHJ-Eastern Mediterr Health J. 1996;2:320-5.
- [Google Scholar]
- Accuracy of fine needle aspiration in the diagnosis of peripheral lymph node enlargements, Lagos University Teaching Hospital, Nigeria. Nig Q J Hosp Med. 2011;21:59-63.
- [Google Scholar]
- Diffuse large B-cell lymphoma (DLBCL): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v116-25.
- [CrossRef] [PubMed] [Google Scholar]
- Immunoflowcytometry and cell block immunohistochemistry in the FNA diagnosis of lymphoma: A review of 73 consecutive cases. J Clin Pathol. 2000;53:451-7.
- [CrossRef] [PubMed] [Google Scholar]
- Laboratory workup of lymphoma in adults. Am J Clin Pathol. 2021;155:12-37.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle cytology: Technical procedures and ancillary techniques. Monogr Clin Cytol. 2018;23:4-13.
- [CrossRef] [PubMed] [Google Scholar]
- A proposal for the performance, classification, and reporting of lymph node fine-needle aspiration cytopathology: The Sydney system. Acta Cytol. 2020;64:306-22.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology in the diagnosis of lymphoma: The next step. Am J Clin Pathol. 2000;113:623-27.
- [CrossRef] [PubMed] [Google Scholar]
- Isotonic Medium S™, AV BioInnovation USA. Available from: https://www.avbioinnovation.com/product/isotonic-medium-s [Last accessed on 2021 Feb 20]
- [Google Scholar]
- Combining fine-needle aspiration and flow cytometric immunophenotyping in evaluation of nodal and extranodal sites for possible lymphoma: A retrospective review. Diagn Cytopathol. 1997;16:200-6.
- [CrossRef] [Google Scholar]
- Effectiveness of the cell block technique in diagnostic cytopathology. J Cytol. 2012;29:177.
- [CrossRef] [PubMed] [Google Scholar]
- Cytology: Diagnostic Principles and Clinical Correlates Philadelphia, PA: Elsevier/Saunders; 2014.
- [Google Scholar]
- Diagnosis and subclassification of follicle center and mantle cell lymphomas on fine-needle aspirates: A cytologic and immunocytochemical approach based on the Revised European-American Lymphoma (REAL) classification. Cancer. 1999;87:216-23.
- [CrossRef] [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to the SCIP approach In: Shidham VB, Atkinson BF, eds. Cytopathologic Diagnosis of Serous Fluids (1st ed). WB Saunders Company: Elsevier; 2007. p. :55-78. Ch. 5
- [Google Scholar]
- Dual color multiplex TTF-1 + Napsin A and p63 + CK5 immunostaining for subcategorizing of poorly differentiated pulmonary non-small carcinomas into adenocarcinoma and squamous cell carcinoma in fine needle aspiration specimens. CytoJournal. 2012;9:10.
- [CrossRef] [PubMed] [Google Scholar]
- Dual-color immunocytochemistry (Ki-67 with LCA) for precise grading of pancreatic neuroendocrine tumors with applicability to small biopsies and cell blocks. CytoJournal. 2020;17:6.
- [CrossRef] [PubMed] [Google Scholar]
- Flow cytometric immunophenotyping for hematologic neoplasms. Blood. 2008;111:3941-67.
- [CrossRef] [PubMed] [Google Scholar]
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-90.
- [CrossRef] [PubMed] [Google Scholar]
- Utility of fine-needle aspiration as a diagnostic technique in lymphoma. J Clin Oncol. 2004;22:3046-52.
- [CrossRef] [PubMed] [Google Scholar]
- A rare case of HHV-8-positive/HIV-negative/EBV-negative primary effusion lymphoma in a renal transplant recipient. Cytopathology. 2012;23:137-9.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of the algorithms classifying the ABC and GCB subtypes in diffuse large B-cell lymphoma. Oncol Lett. 2018;15:6903-12.
- [Google Scholar]
- Cell-blocks and other ancillary studies (including molecular pathology and proteomics) Cytojournal. 2021;18:4.
- [CrossRef] [Google Scholar]
- Applicazionedella PCR nelladiagnosi di linfomi non Hodgkin di tipo B sucampionicitologici da agoaspirazione [Application of PCR in the diagnosis of B-type non-Hodgkin's lymphomas in cytological specimens from fine-needle aspiration] Pathologica. 2000;92:172-6.
- [Google Scholar]
- High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31:37-42.
- [CrossRef] [PubMed] [Google Scholar]
- Lymph nodes: Cytomorphology and flow cytometry In: Comprehensive Cytopathology. Amsterdam: Elsevier Saunders; 2008. p. :671-711.
- [CrossRef] [Google Scholar]
- CellBlockistry: Chemistry and art of cell-block making-A detailed review of various historical options with recent advances (Review) CytoJournal. 2019;16:12.
- [CrossRef] [PubMed] [Google Scholar]
- CellBlockistry: Science of cell-block making as ancillary cytopathology component in the era of minimally invasive techniques with increasing role of molecular pathology (short communication) Clin Surg. 2019;4:2510.
- [Google Scholar]
- AV BioInnovation, USA. Available from: http://www.avbioinnovation.com [Last accessed on 2021 Feb 20]
- [Google Scholar]
- Cytology of plasma cell rich effusion in cases of plasma cell neoplasm. J Cytol2016;. ;33:150.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of pericardial effusions in patients with leukemia. Cancer. 2010;116:2366-371.
- [Google Scholar]








