Translate this page into:
Comparative evaluation of conventional cytology and a low-cost liquid-based cytology technique, EziPREP™, for cervicovaginal smear reporting: A split sample study
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Wolters Kluwer - Medknow and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Liquid-based cytology (LBC) for cervical cancer screening offers several advantages over conventional cytology. However, the extremely high cost of the current approved devices precludes widespread application of LBC technique in resource-constrained countries. This study aimed to evaluate the performance of an indigenous low-cost LBC technique, EziPREP™ (EP), against conventional preparations (CPs) for cervical cancer screening.
Materials and Methods:
A cross-sectional split-sample study with consecutive cervical sampling was conducted on 515 women attending the clinic at our institute. CP smears were prepared as per the standard technique using spatula and endocervical brush followed by detaching the head of brush into the fixative vial of EP. The EP samples were processed as per the manufacturer's protocol. Both CP and EP smears were stained using standard Papanicolaou stain protocol. Both sets of smears were evaluated for staining quality, morphologic details, and cytologic diagnoses. Cytologic diagnoses were correlated with cervical biopsy findings, wherever available. Performance characteristics of the two techniques were calculated.
Results:
The unsatisfactory rate for CP was 1.0%, while on EP, 1.3% smears had inadequate cellular material. The staining quality and morphological details were comparable in both sets of smears. The detection of infections and epithelial cell abnormality was more, though not statistically significant in EP smears. There was a 98% concordance in cytologic diagnosis between CP and EP smears. Cytohistologic concordance was observed in 96% of cases for both CP and EP smears. Although the time taken for processing and staining of smears was higher for EP (2.5 min for EP per smear and 1.6 min for CP per smear), the screening time reduced from 6.5 min per smear for CP to 2.2 min in EP smears.
Conclusion:
EP provides monolayered cervical smears with vivid morphological details, leading to reduced screening time and relatively higher pick-up of infections and low-grade cervical lesions as compared to conventional smears. The availability of such low-cost devices may enable wider application of cytology-based cervical cancer screening in low-resource countries.
Keywords
Cervical cancer
conventional cytology
EziPREP™
histopathology
liquid-based cytology
Papanicolaou smear
screening
INTRODUCTION
Cervical cancer continues to afflict a large number of women worldwide, especially in the low-resource countries, despite being a preventable disease through screening and detection of precancerous lesions and more recently using human papillomavirus (HPV) vaccination.[1] Organized cervical screening programs utilizing cytology as a screening modality have successfully reduced incidence as well as mortality due to cervical cancer in most of the developed nations.[2] However, the sensitivity of conventionally prepared cervical smears has been reported to be as low as 50%, attributable to the factors such as presence of obscuring hemorrhage, inflammation, mucus, or marked overlapping of cells precluding proper interpretation of morphologic features and leading to false-negative reports.[34] As an attempt to overcome these limitations, liquid-based cytology (LBC) was introduced for the preparation of monolayered smears avoiding the obscuring factors and reducing the rate of unsatisfactory smears.[5] Various studies have confirmed the reduction in unsatisfactory samples using LBC technology with variable results, regarding the rate of detection of clinically significant cervical lesions. However, the currently available approved devices for LBC use expensive automated equipment with high running costs, which is a deterrent in wide applicability of this technology for cervical cancer screening in resource-constrained nations.[6]
The present study aimed to evaluate a low-cost indigenous LBC technique vis-a-vis the conventional technique for cervical cancer screening in a low-resource setting.
MATERIALS AND METHODS
A cross-sectional split-sample study was carried out at a cancer research center over 6 months (June–December 2018) to evaluate the comparative performance of conventional cytology and EziPREP™ (EP) technique. Colposcopy-directed biopsies were the gold standard in this study. The study was approved by the institutional ethics committee.
Sample collection
The study population included females attending the gynecology outpatient department of our collaborating hospitals under an opportunistic cervical cancer screening program. After obtaining informed consent, cervical samples were collected using the Ayre's spatula and endocervical brush. Initially, conventional smears were prepared by spreading the cells from both the collection devices onto the prenumbered slides and immediately fixed in 95% ethanol. This was followed by breaking the head of the Cervex-Brush in the EP vial containing 20 ml of fixative solution. The samples were then submitted to the cytopathology laboratory for further processing and evaluation.
Processing of conventional smears
The conventional smears were stained by the standard Papanicolaou (Pap) stain using manual staining method followed in our laboratory.
EziPREP™ liquid-based cytology
EP is a semi-automated technology using a proprietary separator solution for filtering out the mucus and blood from cervical specimens. The samples are processed in the EP Nanocyt Neo processor that utilizes a filter-less technology for the preparation of monolayered smears. The equipment has inbuilt programs with multiple sample testing capacity and provision of making double smears on a single precoated slide.
Processing of EziPREP™ samples
The samples were kept at room temperature until processing, which was usually done on the same day. The steps of preparation, as per the manufacturer's protocol, included layering 7 ml of the sample after vortexing over 5 ml of separator solution followed by centrifugation at 1500 rpm for 5 min. The supernatant was discarded and the pellet was vortexed and loaded (50–75 μl) in the Nanocyt chamber followed by centrifugation in the Nanocyt Neo processor at 1500 rpm for 2 min with a precoated slide. This yielded double smears on a single slide. The slides were fixed in 95% ethanol and stained by routine Pap staining method.
Evaluation of the slides
The conventional preparations (CPs) as well as EP-processed slides were evaluated in a blinded fashion by two experienced cytopathologists (SG and RG) for staining quality and cytoplasmic and nuclear details. The smears were reported as per the current Bethesda system (2014) of reporting cervicovaginal cytology.[7] Post-evaluation in a blinded manner, the smears were decoded and paired. Cases with discordance between the CP and EP diagnosis were reviewed by a third pathologist (RM) whose opinion was considered as final. A comparison was made between the two techniques using the Chi-square test.
Cervical biopsy findings of the included cases, wherever available, were correlated with both the CP and EP diagnoses. Cervical biopsies were reported in accordance with the cervical intraepithelial neoplasia (CIN) classification.[8] Cytologic-histologic correlation was performed as per the American Society of Cytopathology (2017) guidelines using the discrepancy assessment grid.[9] Sensitivity, specificity, and positive predictive value (PPV) to diagnose CIN2+ lesions were calculated for both CP and EP at cytology threshold of ASC-US.
RESULTS
The age of females included in the study ranged from 22 to 80 years (mean: 40.5 years). Of the 515 females, a majority (86%) presented with minor complaints such as vaginal discharge, low back pain, or abdominal pain.
Cytological evaluation
Of the included cases, 5 (1.0%) were unsatisfactory for the evaluation on CP, while on EP, 7 (1.3%) were unsatisfactory, mainly due to inadequate cellular material. The overall staining quality of both CP and EP smears was satisfactory with comparable morphological details [Table 1].
| Parameter | CP | EP | P (Chi-square test) |
|---|---|---|---|
| Cell borders | |||
| Distinct | 428 (83.1) | 437 (84.8) | 0.44 |
| Indistinct | 87 (16.9) | 78 (15.2) | |
| Cytoplasmic staining | |||
| Excellent | 103 (20) | 118 (22.9) | 0.12 |
| Satisfactory | 322 (62.5) | 329 (63.8) | |
| Unsatisfactory | 90 (17.4) | 68 (13.2) | |
| Nuclear borders | |||
| Distinct | 430 (83.5) | 433 (84.1) | 0.79 |
| Indistinct | 85 (16.5) | 82 (15.9) | |
| Chromatin | |||
| Crisp | 397 (77.1) | 388 (75.3) | 0.51 |
| Hazy | 118 (22.9) | 127 (24.7) |
CP: Conventional preparations, EP: EziPREP™
In both techniques, nuclear chromatin appeared crisp and easily interpretable and nuclear membranes were well demarcated. Cytoplasmic staining quality was also equally maintained in both CP and EP smears, as shown in Figures 1 and 2.
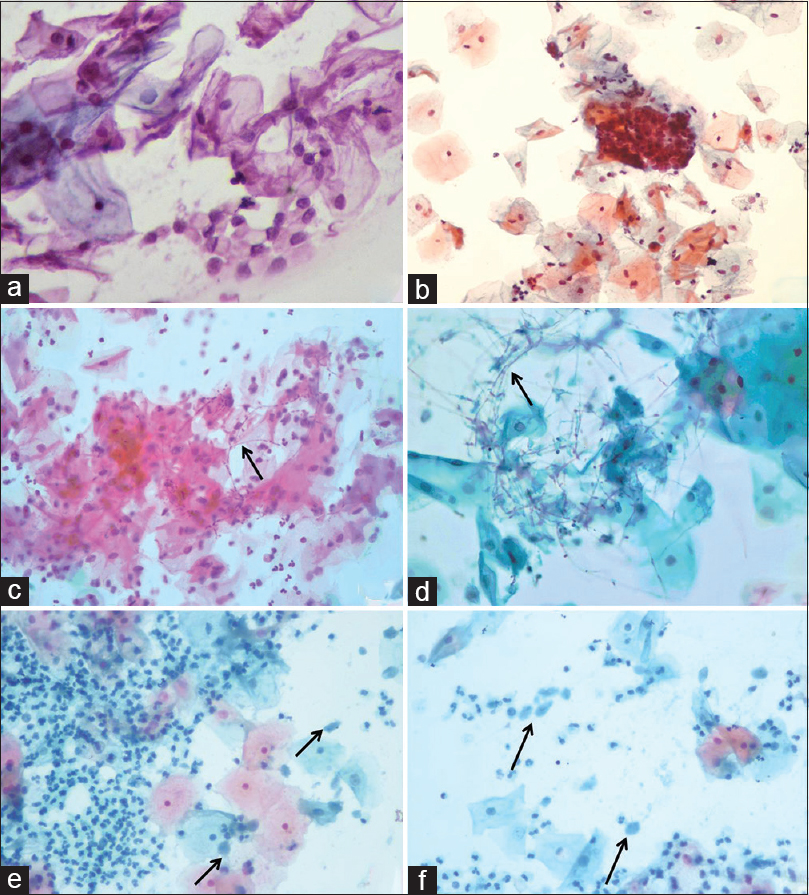
- A panel of photomicrographs demonstrating conventional (a) and corresponding EziPREP™ smear (b) appearance of no intraepithelial lesion or malignancy or NILM. Conventional smear shows few fungal hyphae in a case of candidiasis (c) while the hyphae are seen more clearly in EziPREP™ smear in the same case (d). Only few scattered trophozoites of Trichomonas vaginalis are seen in conventional smear (e) compared to EziPREP™ smear showing numerous easily discernible trophozoites (f) (a-f: Pap, ×400)
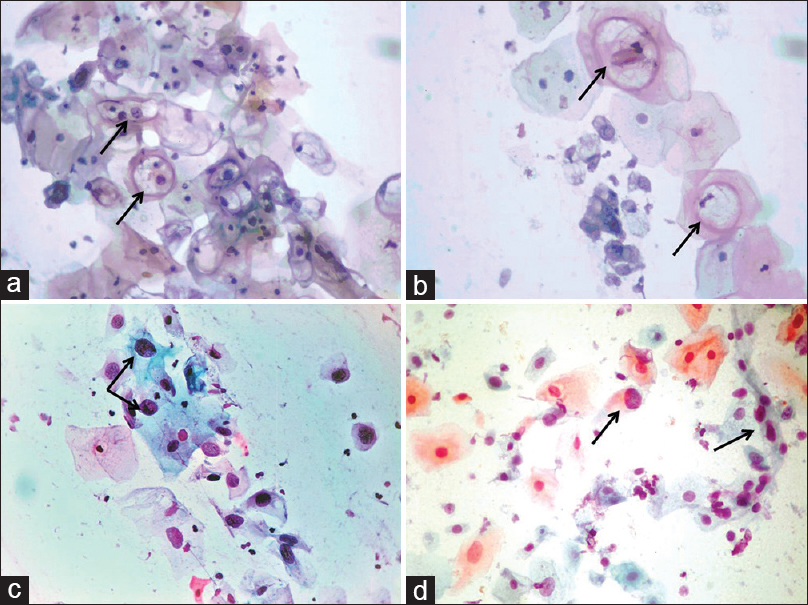
- Corresponding conventional (a) and EziPREP™ smears (b) showing similar features in a case of low-grade squamous intraepithelial lesion with HPV changes. A case of high-grade squamous intraepithelial lesion with atypical cells in conventional (c) and EziPREP™ smear (d) (a-d: Pap, ×400)
EP technique yielded monolayer smears over a diameter of 16 mm. Cellular overlapping and crowding were minimal in EP smears, whereas this was a frequent feature in CP slides. The background of smears prepared by EP technique was clear with polymorphs aggregating to form clearly identifiable clusters, and mucus was also significantly less in these smears. Red blood cells were few in EP smears and where present were seen as ghost images. On the other hand, corresponding CP smears showed polymorphs spread all over the smear, sometimes obscuring cellular details. RBCs were more in number and at times interfered with smear interpretation. The uniform thin EP smears with an absence of obscuring factors were relatively easier to screen and report. The average time taken for screening and reporting a CP smear was 6.5 min compared to 2.2 min for an EP smear.
The frequency of various cytologic diagnoses in the 508 cases that were adequate by both CP and EP techniques is given in Table 2.
| Cytologic diagnosis | Conventional smear | EP smear | P |
|---|---|---|---|
| NILM | 488 | 483 | 0.36 |
| Candida | 7 | 11 | 0.33 |
| Shift in flora (bacterial vaginosis) | 58 | 65 | |
| Trichomonas vaginalis | 5 | 7 | |
| Epithelial cell abnormality (%) | 20 (3.9) | 25 (4.9) | 0.44 |
| ASC-US | 5 | 8 | |
| ASC-H | 2 | 2 | |
| LSIL | 3 | 5 | |
| HSIL | 5 | 5 | |
| Malignant | 3 | 3 | |
| AGC-NOS | 2 | 2 |
LSIL: Low-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion, EP: EziPREP™, ASC-US: Atypical squamous cells of undetermined significance, NILM: Negative for intraepithelial malignancy, ASC-H: Atypical squamous cells – cannot exclude HSIL, AGC-NOS: Atypical glandular cells – not otherwise specified
Concordance between two techniques
Concordance was evaluated among these 508 smears for morphological diagnosis on CP and EP smears [Table 3]. Of the 508 cases, 481 (94.6%) were negative by both techniques. On CP, 20 (3.9%) were reported as ASC-US and above lesion while ASC-US+ diagnosis was given in 25 smears (4.9%) prepared by EP. The overall concordance rate between CP and EP was 98% (498 of 508 cases). The concordance was 98.7% for negative for intraepithelial lesion or malignancy (NILM), 40% for ASC-US, 100% for low grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), carcinoma, and atypical glandular cells – not otherwise specified (AGC-NOS), taking CP diagnosis as the denominator. Of the six cases diagnosed as ASC-US on EP and NILM on CP, a repeat smear performed after 4–6 weeks showed resolution of the abnormality in three while persistent ASC-US was seen in three cases. A review of CP smears in these cases failed to reveal cells which could be labeled as ASC-US. One case showed discordance between CP and EP in being called NILM in the former and LSIL in the latter. Conventional smear review, in this case, revealed few scattered cells with morphological features consistent with LSIL that had been overlooked during the initial screening.
| EP smear | Conventional smear | ||||||
|---|---|---|---|---|---|---|---|
| NILM | ASC-US | ASC-H | LSIL | HSIL | Malignant | AGC-NOS | |
| NILM | 481 | 2 | 0 | 0 | 0 | 0 | 0 |
| ASC-US | 6 | 2 | 0 | 0 | 0 | 0 | 0 |
| ASC-H | 0 | 0 | 1 | 0 | 1 | 0 | 0 |
| LSIL | 1 | 1 | 0 | 3 | 0 | 0 | 0 |
| HSIL | 0 | 0 | 1 | 0 | 4 | 0 | 0 |
| Malignant | 0 | 0 | 0 | 0 | 0 | 3 | 0 |
| AGC-NOS | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
EP: EziPREP™, LSIL: Low-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion, NILM: Negative for intraepithelial malignancy, ASC-US: Atypical squamous cells of undetermined significance, ASC-H: Atypical squamous cells – cannot exclude HSIL, AGC-NOS: Atypical glandular cells – not otherwise specified
Cytologic-histologic concordance
Histologic findings were available in 307 of the 508 cases (60.4%), as given in Table 4. On correlation of cytologic diagnosis with cervical biopsy, an agreement was seen in 296 (96.4%) cases in CP and 96.1% (295 cases) in EP smears. Of note is the finding that three cases of CIN1 and one case of CIN2 were missed on CP, being called NILM. One case of CIN2 escaped detection on EP smears also and was labeled as NILM. Major discordance, i.e., atypical squamous cells – cannot exclude HSIL (ASC-H)/HSIL on cytology and less than CIN2 in biopsy or CIN2+ on cervical biopsy with less than HSIL on cervical smear, was seen in one case (0.3% of 307 cases with biopsy correlation) on both CP and EP smears. In both the CP and EP groups, a minor discrepancy (one grade higher or lower) was noted in 10 cases (3.2%).
| Histologic diagnosis | Cytologic diagnosis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NILM | ASC-US | ASC-H | LSIL | HSIL | Malignant | AGC-NOS | ||||||||
| CP | EP | CP | EP | CP | EP | CP | EP | CP | EP | CP | EP | CP | EP | |
| Negative | 283 | 281 | 3 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| CIN 1 | 3 | 0 | 1 | 2 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| CIN 2-3 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 5 | 4 | 0 | 0 | 0 | 0 |
| Carcinoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | 3 | 0 | 0 |
EP: EziPREP™, LSIL: Low-grade squamous intraepithelial lesion, HSIL: High-grade squamous intraepithelial lesion, NILM: Negative for intraepithelial malignancy, ASC-US: Atypical squamous cells of undetermined significance, ASC-H: Atypical squamous cells – cannot exclude HSIL, AGC-NOS: Atypical glandular cells – not otherwise specified
Test characteristics
Sensitivity, specificity, and PPV for CP and EP were comparable at ASC-US threshold [Table 5] taking CIN2+ lesion on biopsy as the gold standard cutoff for positivity.
| Cytologic threshold | Type of preparation | Sensitivity (%) | Specificity (%) | PPV (%) |
|---|---|---|---|---|
| ASC-US | CP | 93.3 | 98.6 | 77.7 |
| EP | 94.1 | 96.8 | 64 |
CP: Conventional preparations, EP: EziPREP™, ASC-US: Atypical squamous cells of undetermined significance, PPV: Positive predictive value
DISCUSSION
Our study compares a low-cost LBC technique with conventional smear preparation for cervical cytology. Although the reported sensitivity and specificity of LBC in the detection of high-grade lesions have been shown to be more or less similar in comparison studies, the reduced rates of unsatisfactory samples, clarity of the background, and small area to be screened with resultant improved efficiency have led to a switch to LBC technique in most of the western countries.[10] However, the high cost of the currently available approved devices for the preparation of LBC samples precludes their widespread application in resource-constrained countries. Hence, efforts are being undertaken to devise low-cost methods or devices providing comparable monolayer with a clear background as seen in the commercially available equipment. Manual methods of preparing LBC smear have been evaluated in a few studies with variable results.[1112] Other low-cost LBC techniques such as Liqui-PREP™ (centrifugation–cellular base addition – centrifugation and slide preparation by pipetting), Pap Spin® (cytospin-based smear preparation), Turbitec® (centrifugation onto a polylysine slide), and cytoscreen (centrifuge-based) have been compared with the conventional Pap smear for morphologic parameters and pick-up rate of intraepithelial lesions. Majority of these studies have shown lower rates of unsatisfactory samples, better morphological details, and improved cytologic-histologic concordance in the LBC techniques.[31314] The present study has evaluated one such indigenous technique for its utility in the detection of cervical precancerous lesions as well as assessed its processing and staining characteristics to allow for easy adaptability of the cytoscreeners and cytopathologists. A comparison of conventional cervical cytology and one of the LBC techniques can be conducted using “split-sample” or “direct-to-vial” study designs. Both these have merits and demerits. The “split-sample” procedure allows for comparison among the same study population. On the other hand, “direct-to-vial” study design includes comparison with conventional smears of an identical but not the same population since the sample of an individual is processed with either of the techniques but not both.[15] We undertook a split-sample study to evaluate the performance characteristics of an indigenous low-cost LBC machine, EP vis-á-vis conventional cytology. The gold standard was colposcopy-directed biopsies.
Earlier reports comparing conventional and liquid-based preparations for cervical cytology have shown a significant reduction in unsatisfactory rate to <2% in LBC.[1016] However, the unsatisfactory rate on CP was appreciably low in our study since the cervical samples are collected by trained medical social workers. In the present study, the unsatisfactory rate was marginally higher for EP smears, mainly due to inadequate cellularity. This may have happened since the conventional smears were prepared before placing the same collection device into EP vial. None of the EP smears showed marked obscuring by blood or inflammatory cells. This notwithstanding, LBC does contribute to better smear preparation and reduced unsatisfactory rates for the majority of the laboratories practicing gynecologic cytology.
Infectious pathologies such as Candida, shift in vaginal flora, Trichomonas vaginalis were appreciated more clearly in EP smears, though the difference in pick-up rate did not reach statistical significance. The clearer background in EP made it easier for the screeners and pathologists to detect candidal hyphae. In one case, the CP smear showed small foci of necrosis and scattered epithelioid-like cells while the EP revealed well-formed granuloma assisting in making a presumptive diagnosis of tubercular cervicitis on cytology. Few earlier studies have also highlighted the ease in the detection of infectious etiologies on LBC preparations,[1017] similar to the present study.
The rate of reporting ASC-US was higher in EP smears than CP. This finding is in consonance with earlier studies comparing LBC and CP.[18] The histologic correlation of these increased number of cases of ASC-US in the present study showed two cases, each being diagnosed as CIN1 and CIN2 on cervical biopsy. Although the sensitivity and specificity of both CP and EP to detect CIN2+ lesions were found to be similar in the present study, larger studies are required to delineate the clinical significance of increased detection of low-grade lesions on EP smears. The frequency of LSIL was also found to be higher on EP as compared to CP, although the difference was not statistically significant. The higher detection of low-grade lesions in EP, similar to earlier studies comparing LBC and CP, has been ascribed to the better cellular morphology in a cleaner background, with minimal cell overlapping allowing for a more precise diagnosis.[18] However, the detection rate of HSIL and higher lesions was similar in both the techniques (CP and EP). Previous studies have reported variable results pertaining to the advantage of LBC techniques in the detection of cervical precancerous lesions. Some authors have shown LBC to be more sensitive and specific in detecting cervical lesions compared to CP.[19] Other studies, including a systematic review, have found no appreciable difference in the proportion of high-grade lesions detected by two methods.[2021] The one uniform finding reported in most of the studies is the reduction in unsatisfactory rates in LBC, leading to uncompromised reporting. Of the three additional ASC-US lesions detected on EP in the present study, two were confirmed as CIN2 on biopsy. Among the LSIL category, one of the additional cases picked up on EP was reported as CIN2 on biopsy. Hence, the detection rate of a clinically significant cervical lesion was higher on EP compared to CP. The test characteristics of both the techniques were comparable in our study with slightly higher sensitivity and lower specificity and PPV for EP compared to CP. This may be attributable to the higher number of ASC-US lesions detected in EP smears contributing to false positivity.
The EP system evaluated in the present study employs a simple technique for the preparation of monolayer of cells without the use of expensive equipment that are required for the currently available United States Food and Drug Administration (USFDA)-approved devices. This is the first study assessing the utility of EP in cervical cancer screening. Adaptation by the cytopathologists to the smear prepared by EP required only 2–3 weeks after which there was a significant reduction in the time taken for the screening of cervical smears. The time involved in processing of the samples and preparation of a stained smear in the EP method in the present study was about 30 min for a batch of 12 samples (2.5 min per sample) compared to approximately 40 min for a batch of 25 conventional smears stained manually (1.6 min per slide). However, this is somewhat less than the technician time required for commercial liquid-based systems.[22] Smear reading time is reduced significantly from 6.5 min in CP to 2.2 min in EP smear (monolayer in a 16 mm diameter circle), which can result in reporting more number of smears in the same time with less fatigue of both cytoscreeners and cytopathologists and thereby improve laboratory efficiency. An option of double smear from the same sample, each in a 16-mm diameter circle, is also available, thereby increasing the number of cells screened and improving the accuracy of cervical cancer screening. Although we did not perform HPV testing on the residual sample in EP vials, the technical brochure of the product does mention that the material is suitable for use in HPV DNA testing.[23]
A detailed cost-analysis was beyond the scope of this paper. However, a brief comparison of the reagent cost revealed that the cost per test for the EP kits was about 1.5 times lower compared to those of the commercially available USFDA-approved LBC processors. In addition, the initial equipment cost for EP was much less than the commercially available semi-automated LBC equipment. At the same time, the time taken for preparation of cervical smears using semi-automated techniques such as EP vis-á-vis the commercially available approved fully automated LBC processors need to be factored into the cost analysis.
CONCLUSION
This low-cost LBC device holds promise in providing monolayered cervical smears with a clear background and better cellular morphology than CP. Detection of infectious pathologies, as well as low-grade lesions, was seen to be better, though not statistically significant in the liquid-based smears as compared to conventional smears. Wider availability and multicentric evaluation of the performance of such low-cost LBC devices should be undertaken. If found superior to conventional cytology, it may pave the way for programmatic inclusion of cytology-based cervical cancer screening on a larger scale in resource-limited countries with a provision of the performance of HPV-based testing on the residual sample.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author.
Each author has participated sufficiently in the work and taken public responsibility for appropriate portions of the content of this article.
RG conceived and designed the study, participated in sign-outs and comparative evaluation and cyto-histo correlation, and wrote and revised the manuscript. RY made the EziPREP preparations including staining. AS and DK were involved in conventional smear preparations, primary screening of cervical smears. Sandeep carried out the histopathology of cervical biopsies and assisted in cytohistocorrelation. RM was involved in comparative evaluation and helped in draft and critical review of the manuscript. SG conceptualized and designed the study, signed out the cases, performed comparative evaluation and cyto-histo correlations, and helped in draft and critical review of the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board of the institution associated with this study. Authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
AGC-NOS – Atypical glandular cells – not otherwise specified
ASC-US – Atypical squamous cells – undetermined significance
ASC-H – Atypical squamous cells – cannot exclude HSIL
CIN – Cervical intraepithelial neoplasia
CP – Conventional preparation
DNA – Deoxyribonucleic acid
EP – EziPREP™
HPV – Human papilloma virus
HSIL – High grade squamous intraepithelial lesion
LBC – Liquid based cytology
LSIL – Low grade squamous intraepipthelial lesion
NILM – Negative for intraepithelial lesion or malignancy
Pap – Papanicolaou
PPV – Positive predictive value
TBS – The Bethesda System
USFDA – United States Food and Drug Administration.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
The Nanocyt Autoprocessor machine used in this study was on loan from LBC India, Chennai, India. The company was not involved in the design of the study, collection of the data, analysis of the results, or preparation of the manuscript.
REFERENCES
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424.
- [Google Scholar]
- Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26(Suppl 10):K29-41.
- [Google Scholar]
- Conventional Pap smear and liquid based cytology for cervical cancer screening – A comparative study. J Cytol. 2007;24:167-72.
- [Google Scholar]
- Manual liquid based cytology in primary screening for cervical cancer – A cost effective preposition for scarce resource settings. Asian Pac J Cancer Prev. 2012;13:3645-51.
- [Google Scholar]
- Acomparison of liquid-based cytology and pap smear as a screening method for cervical cancer. Oncol Rep. 2007;18:157-60.
- [Google Scholar]
- Validation of a low-cost, liquid-based screening method for cervical intraepithelial neoplasia. Am J Obstet Gynecol. 2006;195:965-70.
- [Google Scholar]
- Nayar R, Wilbur DC, eds. The Bethesda System for Reporting Cervical Cytology: Definitions, Criteria, and Explanatory Notes (3rd ed). New York: Springer; 2015.
- Tumors of the Cervix, Vagina and Vulva. Washington DC: Armed Forces Institute of Pathology; 1992.
- Gynaecologic Cytology-Histology Correlation Guideline. J Am Soc Cytopathol. 2017;6:8-13.
- [Google Scholar]
- Liquid-based cytology versus conventional cytology for evaluation of cervical pap smears: Experience from the first 1000 split samples. Indian J Pathol Microbiol. 2015;58:17-21.
- [Google Scholar]
- Study of a manual method of liquid-based cervical cytology. Indian J Pathol Microbiol. 2008;51:190-4.
- [Google Scholar]
- Comparison of Manual Liquid Based Cytology and Conventional Pap Smear in Cervical Cancer Screening. Natl J Lab Med. 2017;6:PO32-7.
- [Google Scholar]
- Clinical utility of Liqui-PREP™ cytology system for primary cervical cancer screening in a large urban hospital setting in China. J Cytol. 2009;26:20-5.
- [Google Scholar]
- Efficiency of an inexpensive liquid-based cytology performed by cytocentrifugations: A comparative study using the histology as reference standard. Cytojournal. 2005;2:15.
- [Google Scholar]
- Comparison between siriraj liquid-based and conventional cytology for detection of abnormal cervicovaginal smears: A split-sample study. Asian Pac J Cancer Prev. 2008;9:575-80.
- [Google Scholar]
- 2003. Guidance on the use of Liquid-Based Cytology for Cervical Screening. NICE Technology Appraisal Guidance No. TA69. London: National Institute for Clinical Excellence. Available from: https://www.nice.org.uk/guidance/ta69/resources/guidance-on-the-useof-liquidbased-cytology-for-cervical-screening-pdf-2294706450373
- Significance of a diagnosis of microorganisms on Pap smear. J Low Genit Tract Dis. 2008;12:40-51.
- [Google Scholar]
- Clinical results of the liquid-based cervical cytology tool, liqui-PREP, in comparison with conventional smears for detection of squamous cell abnormalities. Asian Pac J Cancer Prev. 2009;10:399-402.
- [Google Scholar]
- Performance of thinPrep liquid-based cervical cytology in comparison with conventionally prepared papanicolaou smears: A quantitative survey. Gynecol Oncol. 2003;90:137-44.
- [Google Scholar]
- Effect of study design and quality on unsatisfactory rates, cytology classifications, and accuracy in liquid-based versus conventional cervical cytology: A systematic review. Lancet. 2006;367:122-32.
- [Google Scholar]
- Accuracy of liquid based versus conventional cytology: Overall results of new technologies for cervical cancer screening: Randomised controlled trial. BMJ. 2007;335:28.
- [Google Scholar]
- SpinThin, a simple, inexpensive technique for preparation of thin-layer cervical cytology from liquid-based specimens: Data on 791 cases. Cancer. 2000;90:135-42.
- [Google Scholar]
- Eziprep Liquid Based Cytology Kita: Eziprep Gyne Kit. Available from: http://www.eziprep.in/products.php








