Translate this page into:
Comparative evaluation of six parametric Robinson and three parametric Howell's modification of Scarf-BloomRichardson grading method on breast aspirates with histopathology: A prospective study
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Fine needle aspiration cytology (FNAC) is a quick method to assess the tumor grade before its removal which will help clinicians to decide on the appropriate neo adjuvant therapy. This is essentially true in developing countries where core needle biopsy still is not used as a standard practice to sample breast carcinoma. Assessment of biological aggressiveness by cytological grading (CG) without removing the would be of immense value. The National Cancer Institute, Bethesda, sponsored conference had recommended that tumor grading on FNA material should be incorporated in cytology reports for prognostication.
Aim:
The present study was carried out to evaluate which among the two, five parametric Robinson or three parametric Scarf–BloomRichardson (SBR) cytology grading method corresponds better with the histological grading (HG) in breast carcinoma.
Materials and Methods:
FNAC of 150 cases of ductal carcinoma breast with subsequent histological confirmation was studied to assess the tumor grade on cytology by two distinct methods Robinson and Howell's modification of SBRmethod and then correlated with histologic grade.
Results:
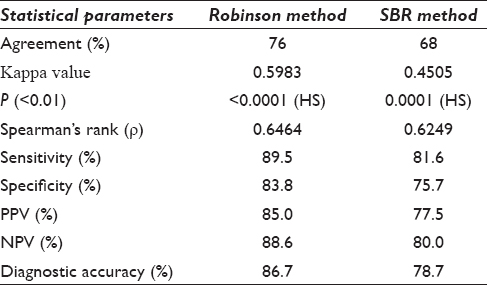
Comparative analysis revealed concordance of 76% by Robinson and 68% by SBR with Kappa value of 0.6683 and 0.4505 and diagnostic accuracy of 86.7% and 78.7%, respectively.
Conclusions:
We conclude that Robinson method showed a better correlation and higher kappa value of agreement in comparison with SBR method. Robinson method of CG is simpler, objective, and easily reproducible for grading breast carcinomas.
Keywords
Breast carcinoma
cytological grade
histological grade
Robinson grading system
Scarff–Bloom–Richardson grading system
INTRODUCTION
Breast carcinoma is one of the most common cancers in women worldwide.[1] Among Indian females, it is second most common next to cervical carcinoma. The cumulative incidence in females until 64 years of age is 1%–2%.[2] Apart from clinical stage, many factors such as tumor type, histological grading (HG), hormone receptor status, DNA ploidy, cell proliferation markers, and expression of different oncogenes determine the prognosis in a given patient.[1] Nottingham method described by Elston and Ellis for HGof breast carcinoma is a widely accepted grading system with good prognostic correlation.[23] The recognition of HG as an important prognostic factor has led to the methods being devised for assessing tumor grade on cytology with the aim of obtaining prognostic information preoperatively.[3] The additional information which FNA can provide about the intrinsic tumor features are nuclear grading, mitotic index, hormone receptor status, and DNA contents,[45] which are of prognostic value. Nuclear grading on cytological specimen has been shown to correlate well with the HG unlike other parameters such as tubule formation and mitosis.[678] The grading of tumour before its removal would help clinicians to decide on the appropriate neoadjuvant therapy as low-grade tumors can be treated with tamoxifen while high-grade call for preoperative chemotherapy. This is essentially true in developing countries where core needle biopsy still is not used as a standard practice to sample breast carcinoma cases. Assessment of biological aggressiveness by cytological grading (CG) without removing the tumor, therefore, would be of immense value.[910] Nuclear grading of breast carcinoma is easy to perform, and hence, it should be included as a fundamental cytologic parameter in the fine-needle aspiration cytology (FNAC) report whenever possible.[1112] The National Cancer Institute, Bethesda, sponsored conference had recommended that tumor grading on FNA material should be incorporated in cytology reports for prognostication.[1314] However, the most reliable method for CG that closely reflects the most widely used HG is yet to be determined.[2] Despite having many CG systems, there is still no agreement among the pathologists to accept which of them as a standard method for routine reporting. Majority of them are based on nuclear criteria which are well appreciated on cytology. Robinson method uses six parameters and has been shown to give reproducible results. On the other hand, method proposed by Howell's modification of Scarff–Bloom–Richardson (SBR) evaluates the applicability of three histological parameters to aspiration cytology, and very few studies have compared the two grading systems. Thus, to weigh the outcome of six parametric Robinson methods and three parametric Howell's methods, we conducted the present study with the aims and objectives of:
-
To study the CG of carcinoma breast by Robinson and Howell modification of SBR method
-
To compare the two CG systems and correlate them with modified Bloom–Richardson (MBR) HG.
MATERIALS AND METHODS
We included 150 breast carcinoma cases diagnosed on FNAC (preoperative) and subsequently confirmed on histology as Infiltrating Ductal Carcinoma (NOS). The exclusion criteria were: (1) breast carcinoma patients diagnosed on cytology but not confirmed by histology and (2) patients having a history of preoperative chemotherapy or radiotherapy for breast carcinoma. FNAC was performed using 10 ml syringe with 22–23-gauge needle under all aseptic precautions.[15] Small and vague lumps were preferably sampled under ultrasonography guidance. Usually, 3–4 smears prepared were immediately fixed in 95% ethanol and stained with hematoxylin and eosin (H and E) and Papanicolaou stains by standard procedure. The cytological and subsequent HG was done by two observers independently and then correlated. The discrepant findings were discussed, and the final consensus was reached on Nikon multithreaded microscope (field diameter 0.44 mm).[16]
Cytology grading was done by two methods
Robinson grading system
Six different cytological parameters were evaluated. A score of 1–3 was given to each of these parameters, and the tumor was graded by adding up the scores. Cancers that were scored in the range of 6–11 were Grade I, score of 12–14 Grade II, and Grade III for a score ranging from 15 to 18.
Howell's modification of Scarff–Bloom–Richardson cytological grading method
Like HG (Nottingham MBR grading)[171819] in SBR method, three criteria were used for grading on cytology, tubule formation, nuclear pleomorphism, and mitotic count and were scaled as 1, 2, or 3, and the final SBR score ranged between 3 and 9, which was divided into three grades (I-III). For Grade I, the score varied from 3 to 5; for Grade II, the score was 6–7; and for Grade III, the score was 8–9.[131416]
Histopathology grading
The surgical specimens in the form of lumpectomy or mastectomy received were immediately and adequately fixed in 10% formalin for 12–48 h. The tissues were processed and sections were subsequently stained with the H and E stain. Histological typing was done according to the World Health Organization2003. The Nottingham MBR’sgrading was used for HG. Three parameters were used for grading on histology, tubule formation, nuclear pleomorphism, and mitotic count and were scaled as 1, 2, or 3, and the final MBR score ranged between 3 and 9, which was divided into three grades (I-III). For Grade I, the score varied from 3 to 5; for Grade II, the score was 6–7; and for Grade III, the score was 8–9.[131718] Mitotic figures were counted only at the peripheral invasive margin of the tumor in the most mitotically active area per 10 hpf (×40) using Nikon microscope (field diameter 0.44 mm).
Statistical analysis
Association between Robinson and SBR with MBR histopathological grading was assessed by Chi-square test. Agreement and discordance between cytological and HGwas assessed by kappa measure of agreement and Spearman rank correlation test. The probability value P < 0.0001 was considered significant. Statistical software STATA version 13.0 was used for statistical analysis. STATA Corp, US.
RESULTS
The present prospective, cross-sectional study included a total of 150 cases. Among these, 5 (3.33%) female patients had a positive family history in first-degree relative. The maximum patients, 55 (36.7%), were in the age group of 41–50 years. According to Robinson CG, maximum cases, 70 (46.7%), belonged to Grade I. The Grade II tumors were maximum 71 (47.3%) as per SBR cytology grading method. According to MBR HGmaximum cases, 74 (49.3%), belonged to Grade I which correlated with Grade I of Robinson's method [Table 1]. The overall concordance between Robinson and SBR CG methods is 102 (68.0%) with highest concordance of 53 cases in Grade I. Concordance between Robinson CG and MBR HG is highest 56 (75.67%) in Grade I ductal carcinomas, followed by43 (75.43%) for Grade II and 15 (78.94%) for Grade III carcinomas [Table 2]. The highest concordance of 56 (75.67%) was observed between SBR and MBR grading method for Grade I ductal carcinoma, followed by40 (70.17%) for Grade II and 6 (31.57%) for Grade III [Table 3].

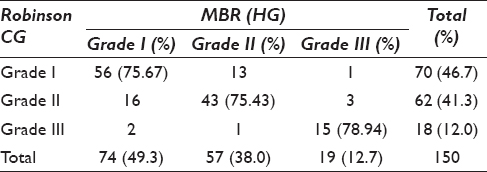

There is an agreement of 76% between Robinson CG and MBR HG with a kappa (k) value of 0.5983 and rho (ρ) value of 0.6464 with a statistically significant P < 0.0001 [Table 4]. To statistically evaluate which of the two CG methods more closely corresponded to the HG the Grade I cases were considered as “low grade” and both Grade II and III cases were clubbed as “high grade” in both cytological and HG methods. These two categories were then separately compared with the corresponding HG. In the present study, the diagnostic accuracy of 86.7% and 78.7% was observed [Table 4], respectively, by Robinson and SBR methods. Figures 1-3 show cytomorphological spectrum of Robinson Grade I, II, and III ductal carcinomas while Figures 4-6 eveals SBR cytomorphological features for Grade I, II, and III, respectively.
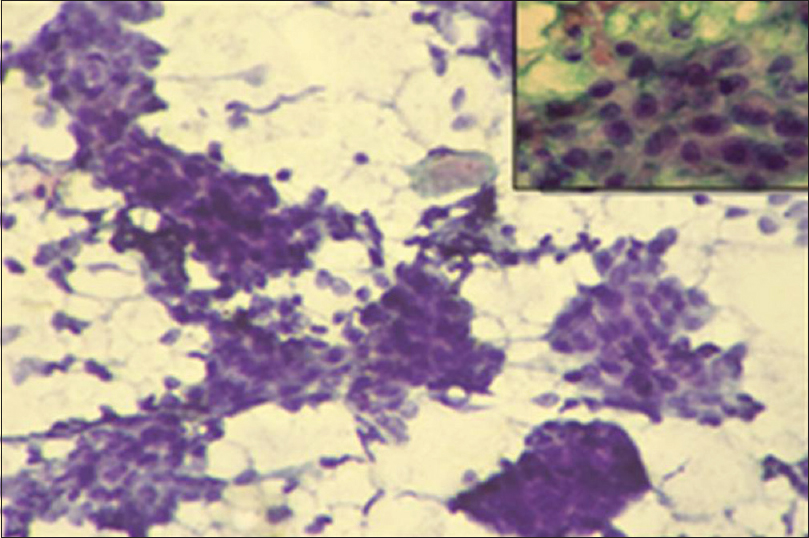
- Robinson cytology Grade I: Cohesive cell clusters having cell size about 1–2 times of red blood cell. Monomorphic cells with vesicular nuclei, smooth margin and indistinct nucleoli. (PAP ×20); Inset: (Pap ×40)

- Robinson cytology Grade II: Loosely cohesive size cell clusters with size about –3-4 times of red blood cell, with mild nuclear pleomorphism and noticeable nucleoli. (PAP ×20); Inset (Pap ×40)
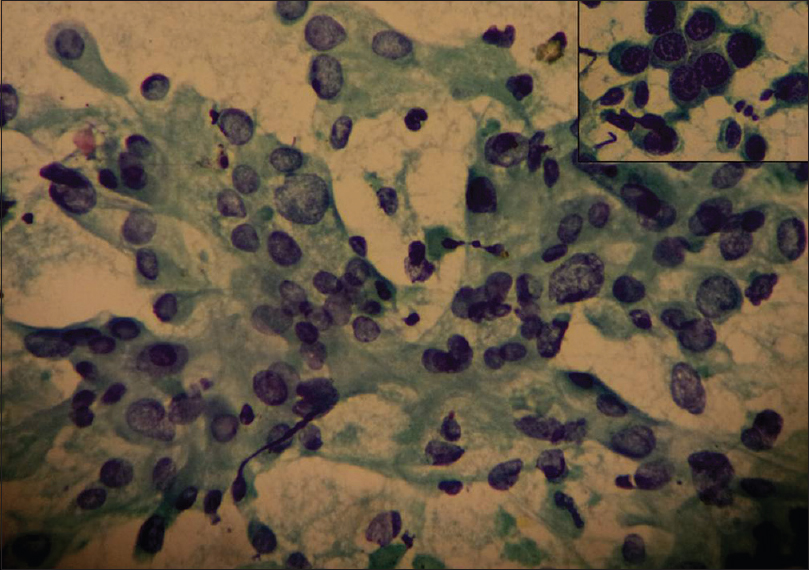
- Robinson cytology Grade III: Dissociated cells with size more than five times of red blood cell, with marked nuclear pleomorphism, irregular nuclear margin, clumped chromatin and prominent nucleoli. (Pap stain, ×20); inset (Pap ×40)
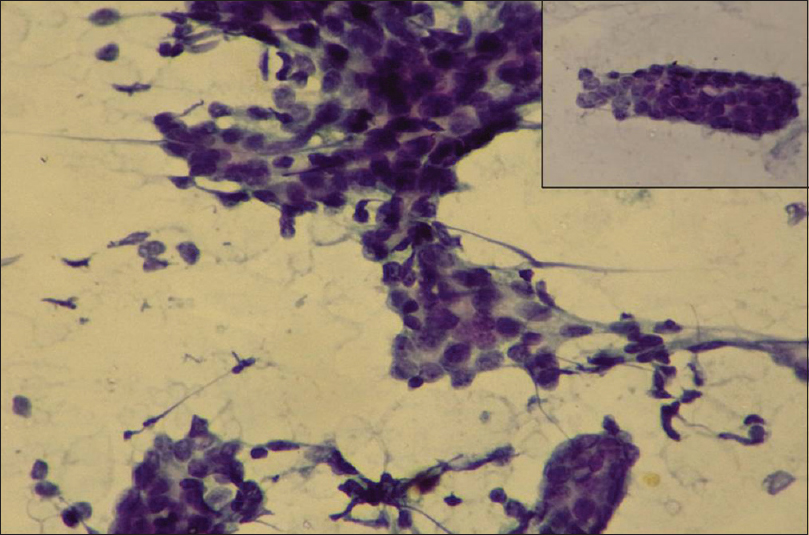
- Howell's modification Scarff–Bloom–Richardson cytology Grade I: Cohesive cell clusters forming the tubular structures with mild nuclear pleomorphism. (Pap ×20); inset (Pap ×40)
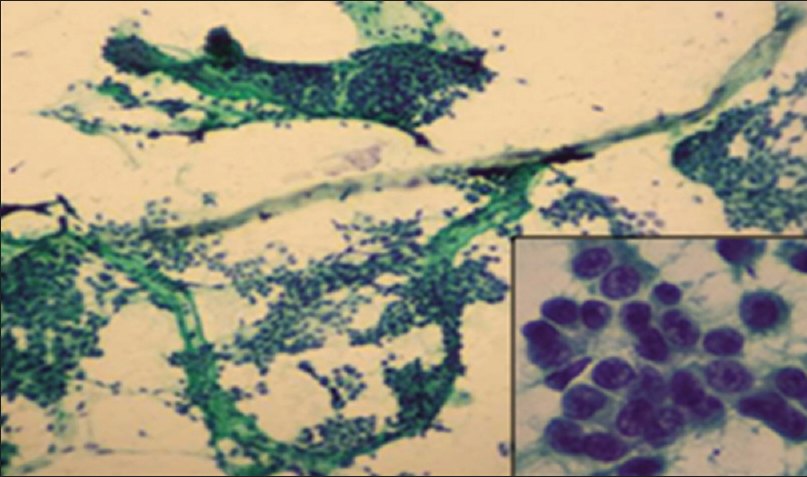
- Howell's modification Scarff–Bloom–Richardson cytology Grade II: Cohesive cell clusters having tubule formation in some areas with moderate nuclear pleomorphism, granular chromatin with occasional mitosis. (Pap ×10); inset (Pap ×40)
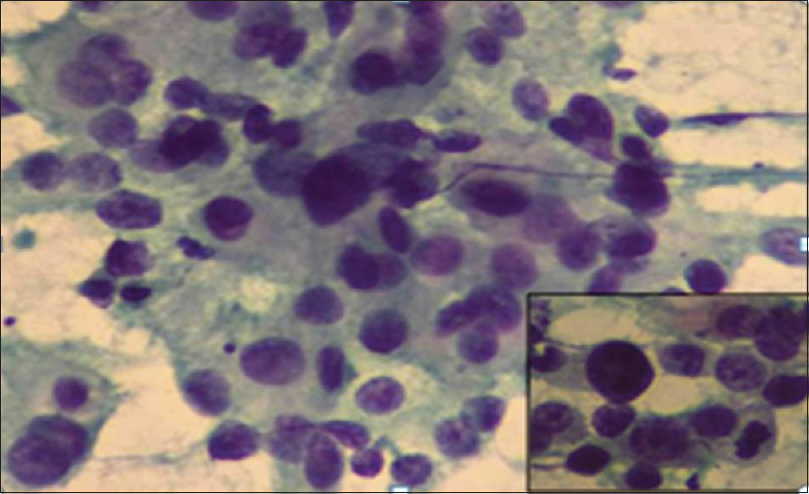
- Howell's modification Scarff–Bloom–Richardson cytology Grade III: Predominantly dissociated cells with marked nuclear pleomorphism. Mitosis figures along with tumor giant cell. (Pap ×20); inset (Pap ×40)
DISCUSSION
The aim of cytology grading is to assess a tumour in situ so that the most suitable treatment could be selected immediately. Histological concordance gives the cytopathologist a feedback which helps in increasing the efficiency of his work.[20] We studied CG of 150 cases of ductal carcinoma by six parametric Robinson and three parametric SBR methods. Various authors quoted that Robinson grading system is found to be better because of its simplicity, specificity, and reproducibility.[1913] Most researchers have attempted CG based on nuclear morphology which have not taken into account the pattern of cell distribution and mitosis.[1469] To overcome this deficiency, we selected one more CG method Scarff modification of Bloom Richardson (SBR) which evaluates all the three histological parameters on cytology.[131415] A complete agreement of 67.3% is observed between Robinson and SBR method [Table 1]. All Grade I cases by Robinson were similarly graded by SBR method. Discordant cases were in both Grade II and III. There are very few studies in the literature regarding comparative evaluation of CG by these two methods.
Our study revealed overall concordance of 76% (114/150) by Robinson method and was comparable with the published literature which was in the range of 70%–80%[2122] [Table 2]. The highest concordance was 56/74 (75.67%) for Grade I ductal carcinomas, followed by43/57 (75.43%) in Grade II and 15/19 (78.94%) in Grade III category. This suggested that Robinson method is a reasonably reliable for grading breast carcinomas on FNA smears. Das et al.,[1] in their study of 52 cases, revealed an absolute concordance between Robinson and histopathology grading in 71.2% cases. Pandey et al.[10] studied 30 cases which showed highest concordance (83.3%). Out of 36 discordance cases [Table 1] in our study, 33 cases revealed one-grade difference; among them, 16 cases were overgraded and 17 were undergraded. There were three cases with two grade differences, out of which two were under graded, and 1 case was over graded. HG was based on the degree of tubule formation, nuclear pleomorphism, and mitosis.[623] Tubule formation is difficult to assess on cytology, so also mitotic count is not included in the parameters by Robinson method. These might be the factors responsible for discordance observed between cytological and HG systems in our cases.
On comparing SBR CG with MBR histology grade [Table 3], the highest concordance was observed 56 (75.67%) in Grade I ductal carcinomas, followed by 40 (70.17%) in Grade II and 6 (31.57%) in Grade III. Its overall concordance is 68%. Our observations were comparable with study by Einstien et al.[14] and Saha et al.[13] High concordance observed in study of Bansal et al.[16] may be because they sampled peripheral portion of breast lump. All the 48 discordant cases in our study [Table 3] had one-grade difference, 27 cases were over graded, while 21 were under graded.
Association between Robinson CG and MBR HG was studied by Pearson's Chi-square test. Spearman's rank correlation was also used to correlate cytological and HG The agreement observed by various studies includes 77.19% by Saha et al.,[13] 77.7% by Einstien et al.,[14] 74.57% by Pandya and Shah[24] 72.2% by Phukan et al.,[20] and 68.97% by Sood et al.[21] The agreement in our study was 76.0% with kappa (k) 0.5983, which suggests moderate agreement and is comparable various studies [Table 5]. The correlation coefficient (ρ) of 0.6464 indicates moderate positive correlation and the P values are found to be highly significant (<0.001). A study by Saha et al.[13] and Einstien et al.[14] showed a strong positive correlation with a rho value slightly on the higher side [Table 5]. The diagnostic accuracy of 86.7%, sensitivity of 89.5%, and specificity of 83.8% were observed by us and were higher as compared to Das et al.[1] and Khan et al.[25].
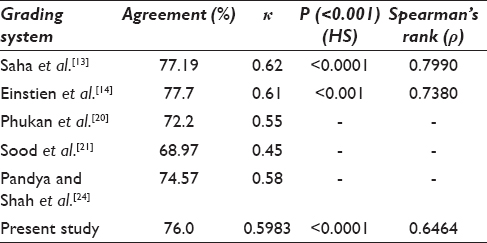
Association of SBR (CG) with MBR (HG) studied showed concordance of 68.0% that was comparable with the studies in the literature. Saha et al.[13] had 63.16% concordance, 69.4% by Einstien et al.,[14] and 93% by Bansal et al.[16] [Table 6] The k 0.4505 suggests a moderate degree of agreement between the two methods and the correlation coefficient (ρ) of 0.6249 indicating moderate positive correlation. We observed the sensitivity of 81.6%, specificity 75.7%, and diagnostic accuracy 78.7% by SBR method [Table 4]. In the present study, the diagnostic accuracy was 86.7% and 78.7% [Table 4], respectively, by Robinson and SBR methods which suggested that the former method is better than the later one for CG of breast carcinoma.
CONCLUSIONS
CG of breast carcinoma is possible using both Robinson and Howell's modification of SBR methods with nearly similar concordance. However, Robinson method showed a better Spearman correlation and higher kappa value of agreement in comparison with SBR method. Robinson method of CG is simpler, objective, and easily reproducible. The parameters of SBR method are representative of modified histological criteria such as tubule formation and mitotic count which are difficult to find on aspiration smears, resulting in lowering of the tumor grade. Due to these reasons, Robinson method has an edge over SBR method and is recommended for routine use on cytology smears for grading purpose.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Author 1: (a) Substantial contributions to conception and design, acquisition of data,
Analysis and interpretation of data;
(b) Drafting of the article and revising it critically for important intellectual
content and statistical analysis.
(c) Final approval of the version to be published.
Author 2: (a) Substantial contributions to acquisition of data, statistical analysis and
Interpretation of data;
(b) Drafting of the article for important intellectual content.
All authors read and approved the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
All authors agree to state that: (1) The work done has not been published elsewhere. (2) All authors have been actively involved in substantive work leading to the manuscript and are jointly responsible for its content. (3) The work done is approved by the institutional ethics committee, GMC Nagpur. INDIA.
LIST OF ABBREVIATIONS (In alphabetic order)
CG - Cytology grade
HG - Histology grade
H and E - Hematoxylin and eosin method
MBR - Modified Bloom–Richardson's histology grading method
MGG - May-Grunwald Giemsa stain
PAP - Papanicolaou stain
SBR – Howell's modification of Scarff–Bloom–Richardson's cytology grading method.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Comparative evaluation of grading of breast carcinomas in fine needle aspirates by two methods. Indian J Med. 2003;118:247-50.
- [Google Scholar]
- Critical appraisal of cytological nuclear grading in carcinoma of the breast and its correlation with ER/PR expression. J Cytol. 2008;25:58-61.
- [Google Scholar]
- Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long. term follow-up. Histopathology. 1991;19:403-10.
- [Google Scholar]
- Robinson cytologic grading of invasive ductal breast carcinoma: Correlation with histologic grading and regional lymph node metastasis. Acta Cytol. 2005;49:149-53.
- [Google Scholar]
- A comparative and evaluative study of cytological and histological grading system profile in malignant neoplasm of breast – An important prognostic factor. Indian J Pathol Microbiol. 2006;49:199-202.
- [Google Scholar]
- Prognostic value of cytological grading of fine-needle aspirates from breast carcinomas. Lancet. 1994;343:947-9.
- [Google Scholar]
- Cytopathological grading, as a predictor of histopathological grade, in ductal carcinoma (NOS) of breast, on air-dried Diff-Quik smears. Diagn Cytopathol. 2003;29:185-93.
- [Google Scholar]
- Prognostication factors from the fine needle aspirates: Breast carcinoma nuclear grade. Diagn Cytopathol. 1994;10:203-4.
- [Google Scholar]
- Cytological grading of breast cancers and comparative evaluation of two grading systems. J Cytol. 2010;27:55-8.
- [Google Scholar]
- A comparative and evaluative study of two cytological grading systems in breast carcinoma with histological grading: An important prognostic factor. Anal Cell Pathol (Amst). 2014;2014:767215.
- [Google Scholar]
- Cytological grading of breast neoplasia and its correlation with histological grading. Indian J Pathol Microbiol. 2006;49:208-13.
- [Google Scholar]
- Histologic grading of breast carcinoma. A reproducibility study. Cancer. 1994;73:2765-70.
- [Google Scholar]
- Comparative evaluation of six cytological grading systems in breast carcinoma. J Cytol. 2013;30:87-93.
- [Google Scholar]
- Comparison of 3-Tier cytological grading systems for breast carcinoma. ISRN Oncol. 2014;2014:252103.
- [Google Scholar]
- Fine Needle Aspiration Cytology. (5th ed). London: Churchill Livingstone; 2012. p. :1-8.
- [Google Scholar]
- Comparative evaluation of the modified scarff-bloom-richardson grading system on breast carcinoma aspirates and histopathology. Cytojournal. 2012;9:4.
- [Google Scholar]
- Breast. In: Rosai J, ed. Rosai and Ackerman‘s Surgical Pathology Vol 2. (10th ed). Edinburgh: Mosby Elsevier; 2012. p. :1659-70.
- [Google Scholar]
- (4th ed). Lyon: IARC; 2012. p. :14-38.
- Application of the Scarff-Bloom-Richardson tumor grading system to fine-needle aspirates of the breast. Am J Clin Pathol. 1994;101:262-5.
- [Google Scholar]
- Cytological grading of breast carcinoma on fine needle aspirates and its relation with histological grading. South Asian J Cancer. 2015;4:32-4.
- [Google Scholar]
- Comparative study of cytomorphological Robinson's grading for breast carcinoma with modified Bloom-Richardson histopathological grading. Patholog Res Int. 2013;2013:146542.
- [Google Scholar]
- The cytological grading of malignant neoplasms of the breast and its correlation with the histological grading. J Clin Diagn Res. 2013;7:1035-9.
- [Google Scholar]
- Value of cytoprognostic classification in breast carcinomas. J Clin Pathol. 1986;39:489-96.
- [Google Scholar]
- Comparative evaluation of Robinson's cytological grading with Elston and Ellis’ Nottingham modification of Bloom Richardson histopathology grading for breast carcinoma. Natl J Community Med. 2012;3:491-5.
- [Google Scholar]
- Role of cytological grading in prognostication of invasive breast carcinoma. J Cytol. 2009;26:65-8.
- [Google Scholar]








