Translate this page into:
Cytologic features, immunocytochemical findings, and DNA ploidy in four rare cases of epithelioid hemangioendothelioma involving effusions
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Epithelioid hemangioendothelioma (EHE) involving serous effusion is extremely rare, and the diagnosis can be challenging. DNA ploidy quantitation of EHE in effusion fluids has not been previously described in the English-language literature.
Methods:
Specimens of cytological diagnosed with EHE in effusion fluids between 2002 and 2009 were retrieved from the pathology files at MD Anderson Cancer Center. A total of four cases of EHE involving or arising from effusion fluids were found, and we reviewed cytospin, smears, cell block sections, and immunostained slides. DNA image analysis for ploidy and proliferation evaluation was performed on a destained, papanicolaou-stained slide from each case.
Results:
The tumor cells were epithelioid with prominent cytoplasmic vacuolization and intracytoplasmic inclusions, which could resemble reactive mesothelial cells, mesothelioma, or adenocarcinoma. The tumor cells were positive for endothelial markers. DNA image analysis in three of the four cases revealed predominantly diploid and tetraploid subpopulations, with few aneuploid cells and fairly low proliferation indices, and these patients had fairly prolonged survival.
Conclusions:
DNA image analysis is useful for differentiating EHE from reactive mesothelial cells and high-grade carcinoma. For accurate diagnosis of EHE in effusion fluids, cytologic features should be considered together with clinical history and ancillary studies.
Keywords
Cytology
DNA ploidy
effusion
epithelioid
hemangioendothelioma
immunocytochemistry
proliferation index
INTRODUCTION
Epithelioid hemangioendothelioma (EHE) is an unusual endothelial tumor with unpredictable biologic behavior[1] that theoretically can involve every organ and anatomic site. Common sites of occurrence include the lung, liver, bone, and soft tissue. We have previously described the cytologic and immunohistochemical features of EHE in a series of 14 cases[2] from multiple sites. However, EHE involving or arising from the serous cavity is extremely rare;[3] the cytologic features of EHE in effusions have been described in the literature only in individual case reports.[456789]
Although the biologic behavior of EHE has been shown to be unpredictable, a WWTR1-CAMTA1 gene fusion was recently shown to differentiate between low-and intermediate-grade EHE and high-grade EHE.[1011] DNA image analysis has been shown to be a sensitive and reliable technique for evaluating DNA content and proliferation of tumors.[1213141516171819] However, abnormal DNA ploidy patterns have not been previously described in EHE. The presence of aneuploid cells may be an indicator of malignancy in serous fluid because background mesothelial cells are usually diploid.
In the present series, we focus on four cases of EHE detected in serous cavity effusions, three metastatic to the serous cavities, and one primary peritoneal EHE. We sought to determine whether EHE demonstrated characteristic abnormal DNA image analysis features, – i.e., DNA ploidy and proliferation as measured by Feulgen image analysis –that when aligned with biologic behavior and clinical outcome, could aid in the diagnosis of this rare entity, which can be challenging with cytologic analysis alone.
METHODS
Internal approval for conducting the study was obtained from the Institutional Review Board at The University of Texas MD Anderson Cancer Center. Cytology specimens diagnosed with EHE in effusion fluids between 2002 and 2009 were retrieved from the pathology files at MD Anderson Cancer Center. Cytospin, smears, cell block sections, and immunostained slides were retrospectively reviewed. DNA image analysis was performed on one papanicolaou-stained slide from each case. Information regarding clinical presentation, diagnostic procedures, treatment, and follow-up was obtained through chart review from the institutional patient-care database.
Specimens
Smears and cytospin preparations were air-dried and stained with Diff-Quik or fixed in Carnoy's solution (a 3:1 ratio of 95% ethanol to glacial acetic acid) and then stained using the Papanicolaou method. Cell block sections were available in all cases and stained with hematoxylin and eosin. Immunoperoxidase stains were used on cell block sections.
DNA image analysis
Papanicolaou-stained smears with representative cells were destained, stained with Feulgen, and then, digitized interactively by a cytotechnologist using a SAMBA 4000 image analyzer (Samba Technologies, Meylan, France). The smears were then analyzed as described previously.[14] At least, 100 of the most atypical cells per slide were selected. Lymphocytes and normal mouse hepatocytes were used as internal and external controls. The resulting DNA histograms were classified according to Auer et al., Krishnamurthy et al., and Sun et al.[151617] For each analysis, the following variables were automatically calculated by the image analysis software: DNA index, ploidy balance, proliferation index, degree of hyperploidy (percentage of cells with a DNA content of >5c), and degree of aneuploidy. According to this classification, there are usually four types of histograms: diploid, low proliferation, and no cells >5c (low grade); broad diploid (diploid or tetraploid), low proliferation, and no more than 10 cells >5c (intermediate grade); aneuploid (diploid or aneuploid; can be multiple aneuploidy stem lines), high proliferation, and numerous cells >5c (high grade); and polyploid, (diploid, tetraploid, and octaploid stem lines, and low proliferation).
RESULTS
Clinical information
The medical history, primary location, effusion type, current lesions, and primary cytologic diagnosis for each case are summarized in Table 1. All four patients were women aged 20–65 years. The patients in cases 1 and 4 had malignancies other than EHE. Each case is described in detail below.

Case 1
A 33-year-old woman was admitted for dyspnea. Nine years before admission, she had a carcinoid tumor in the appendix resected and then received chemotherapy. On admission, she was found to have abdominal/pelvic ascites with multiple nodules coating all peritoneal surfaces and in the liver and bilateral lungs. Because an immunoperoxidase stain for synaptophysin was negative in the ascitic fluid cell block section, the cytopathologist reported “no malignant cells identified, reactive mesothelial cells, acute and chronic inflammation.” Later, the patient underwent peritoneal biopsy, which yielded malignant cells with strong immunoreactivity for endothelial-associated antigens, leading to a diagnosis of primary EHE in the peritoneum. Retrospective immunocytochemical analysis was positive for CD31 and FLI-1, confirming the presence of single EHE cells in the ascitic fluid smear. The patient died 43 months after her initial diagnosis.
Case 2
A 20-year-old woman with a history of EHE in the left maxilla 3 years earlier (treated with excision of the primary tumor, radiation, and chemotherapy) presented with progressive abdominal distention of 2-month duration. A computed tomography scan revealed a large amount of ascitic fluid. The patient was alive 60 months after the initial diagnosis and then lost to follow-up.
Case 3
A 65-year-old woman with a history of EHE in the right lung 2 years earlier presented with chest pain and shortness of breath of 1-month duration. A computed tomography scan revealed a large amount of the left plural effusion. Two years earlier, the intraoperative frozen section of the lung nodule was interpreted as adenocarcinoma with signet ring cell features, probably metastatic in origin. Paraffin section showed that the cells were negative for cytokeratin and positive for the endothelial markers (CD31 and CD34). The primary EHE in the lung had been removed, and the patient had been treated with multiple chemotherapies. The patient died of the disease 48 months after the initial diagnosis.
Case 4
A 64-year-old woman with a complicated history presented with shortness of breath of 4-month duration. Twenty-six years earlier, she had been diagnosed with well-differentiated lymphocytic lymphoma in the stomach and received a partial gastrectomy followed by radiation therapy. Three years later, she underwent the left thyroid lobectomy. Pathologic analysis revealed an occult, 0.3-cm papillary carcinoma. Eight months before presentation, she was diagnosed with a Stage I papillary renal cell carcinoma, 2.0 cm × 1.5 cm × 1.0 cm, for which she underwent a left-sided radical nephrectomy. Four months later, she developed shortness of breath. A positron emission tomography/computed tomography scan showed a large mediastinal mass. A mediastinal fine-needle aspiration biopsy was performed with endoscopic ultrasound guidance, and the pleural fluid was subsequently acquired for cytologic evaluation. The initial diagnosis was metastatic carcinoma consistent with her history of primary renal cancer, but this was later revised to EHE after clinical features were considered, along with additional positive immunohistochemical staining for several endothelial markers. In addition, the patient's background lymphocytes were shown to be lymphoma by flow cytometry. The patient died of disease 4 months after the initial diagnosis of EHE.
Cytologic findings
Cytologic features of the four cases were somewhat similar. The smears were highly cellular, with numerous single epithelioid cells and small aggregates of these cells in a background of mesothelial cells and blood elements. The epithelioid cells were round to oval, variable in size and had more nuclear atypia and a higher nuclear/cytoplasmic ratio than reactive mesothelial cells in the same smear did [Figure 1]. The smaller tumor cells had delicate cytoplasm whereas the larger tumor cells showed moderately abundant cytoplasm. The nuclei were eccentrically located, and these cells resembled plasmacytoid or signet ring cells [Figure 2]. The chromatin pattern was finely granular, numerous binucleated and multinucleated cells were present, and cytoplasmic intranuclear pseudoinclusions were infrequent. Cell-in-cell engulfment was seldom seen. Careful review showed that a few cells in each case had a single large cytoplasmic vacuole, and tumor cells with intracytoplasmic lumina were rare. A signet ring appearance and intracytoplasmic lumina were prominent on cell block sections [Figure 3]. Mitotic figures were infrequent.
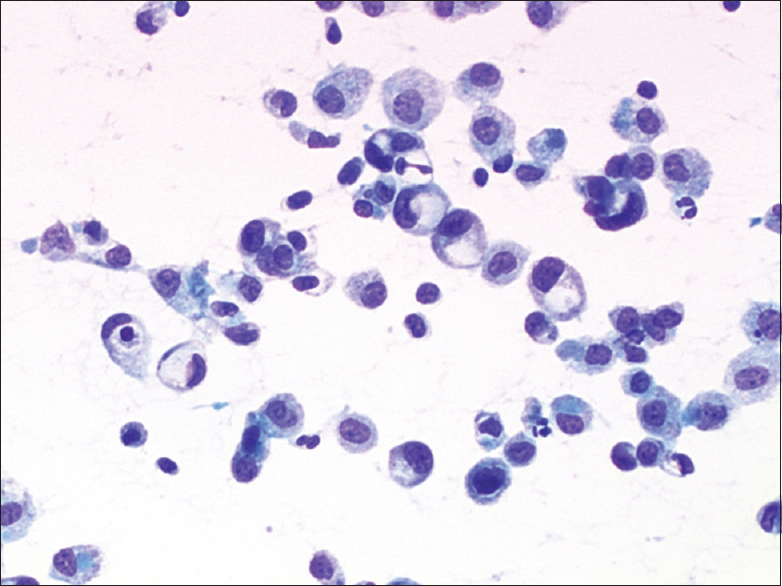
- Cytologic features of epithelioid hemangioendothelioma in effusion. Case 2. Loosely cohesive and single plasmacytoid tumor cells exhibited more nuclear atypia and had a higher nuclear/cytoplasmic ratio than reactive mesothelial cells did (direct smear, Papanicolaou stain, ×400)
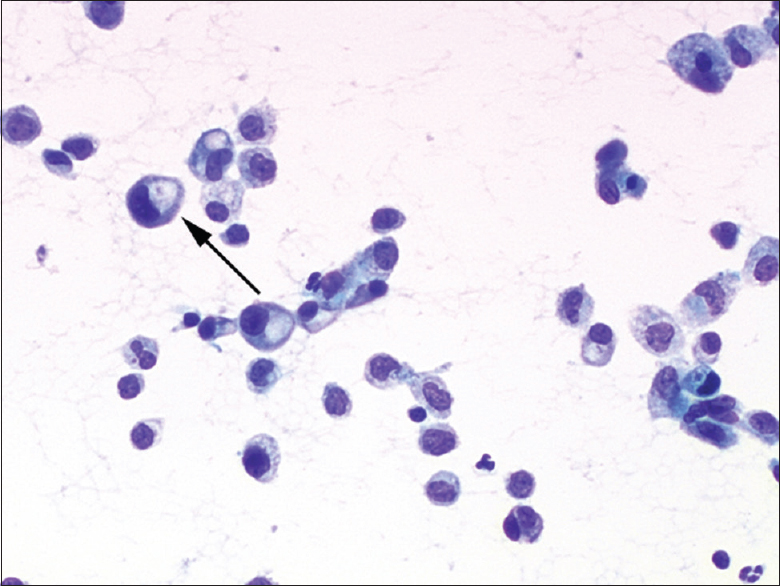
- Cytologic features of epithelioid hemangioendothelioma in effusion. Case 2. The nuclei were eccentrically located, and these cells resembled plasmacytoid or signet ring cells. Few cells exhibited an intracytoplasmic lumina (arrow; direct smear, Papanicolaou stain, ×400)

- Cytologic features of epithelioid hemangioendothelioma in effusion. Case 2. Demarcated cytoplasmic vacuoles contained degenerated blood cells (arrow; cell block, hematoxylin and eosin stain, ×200)
Immunocytochemistry
Immunocytochemical results are summarized in Table 2. Malignant cells were positive for CD31 [Figure 4], CD34 [Figure 5], FLI-1 [Figure 6], and factor VIII, however, they were negative for keratin 7, calretinin, and CD68. Malignant cells showed weak-to-moderate focal pancytokeratin staining in case 4. Tumor cells in the peritoneum biopsy specimen from case 1 were positive for several mesothelial markers, including podoplanin/D2-40 but negative for calretinin and CK5/6.

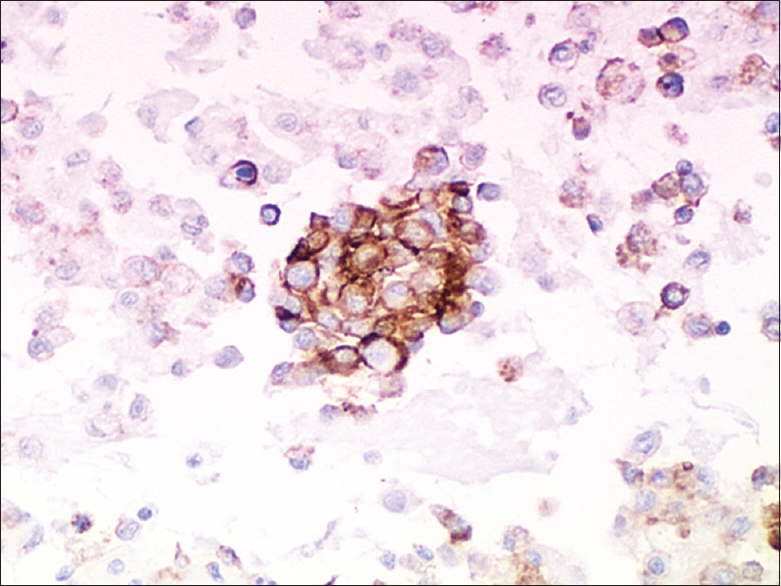
- Immunohistologic findings. Case 2. Tumor cells were CD31-positive (strong, diffuse) (cell blocks, ×200)

- Immunohistologic findings. Case 2. Tumor cells were CD34-positive (strong, diffuse) (cell blocks, ×200)

- Immunohistologic findings. Case 2. Tumor cells were FLI-1-positive (focal) (cell blocks, ×200)
DNA ploidy
Common to all four cases, DNA image analysis revealed predominantly diploid and tetraploid or near tetraploid subpopulations of cells, with the degree of hyperploidy (or cells >5c) ranging from 0.6% to 11.1% [Table 2 and Figures 7-10]. The proliferation index was evaluable in three cases and ranged from 1.5% to 7.0%, with a mean of 4.01% (the proliferation index could not be evaluated in case 3 owing to overlapping DNA stem lines). Interestingly, the patient who remained alive at 60 months had the lowest proliferation index (1.5%).

- DNA image analysis revealed predominantly diploid and tetraploid or near tetraploid subpopulations of cells in all cases. Case 1: diploid and near tetraploid DNA index; proliferation inde × 7.0%; 10.9% of cells with DNA content >5c; degree of aneuploidy 45.7% (patient died of disease after 43 months)
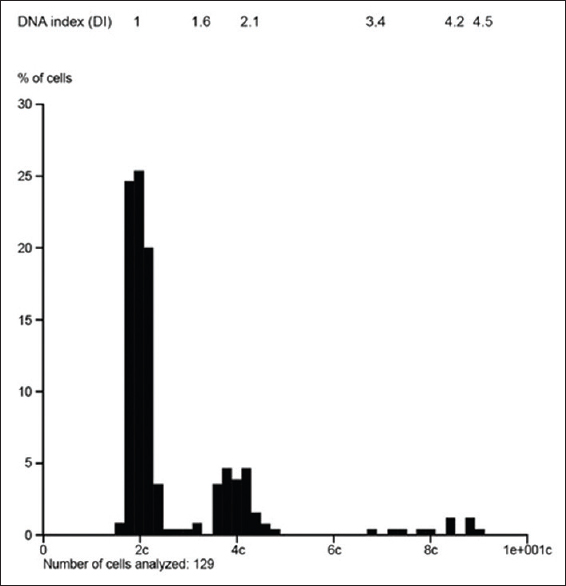
- DNA image analysis revealed predominantly diploid and tetraploid or near tetraploid subpopulations of cells in all cases. Case 2: diploid, tetraploid, and octaploid subpopulation (polyploid pattern); low-proliferation index (1.5%); 4.6% cells with DNA content >5c; degree of aneuploidy 13.1% (patient alive at 60 months)
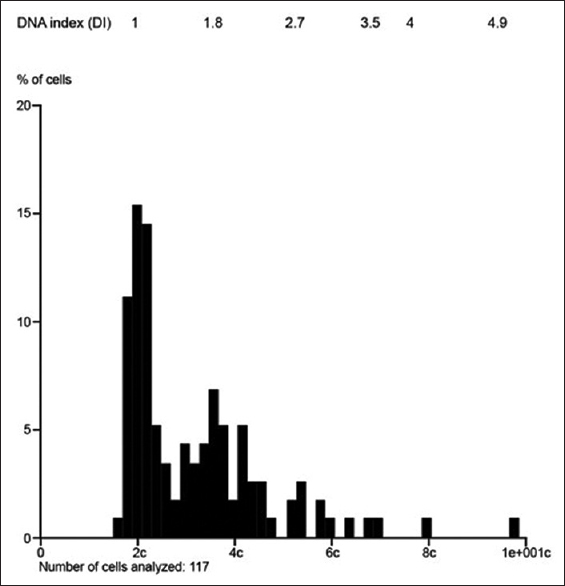
- DNA image analysis revealed predominantly diploid and tetraploid or near tetraploid subpopulations of cells in all cases. Case 3: predominantly diploid and triploid stem lines; proliferation index not able to be evaluated due to overlapping DNA stemlines; 11.1% of cells with DNA content >5c; degree of aneuploidy 43.6% (patient died of disease after 48 months)

- DNA image analysis revealed predominantly diploid and tetraploid or near tetraploid subpopulations of cells in all cases. Case 4: diploid and tetraploid DNA indices; low proliferation index (3.7%); one cell with DNA content >5c; degree of aneuploidy 11.0% (patient died 4 months following the diagnosis of effusion from other comorbidities not related to epithelioid hemangioendothelioma)
DISCUSSION
EHE is a rare endothelial tumor with an estimated prevalence of <1 in 1 million.[20] In 1982, Weiss and Enzinger described EHE as an angiocentric vascular tumor with metastatic potential.[21] The neoplasm may appear at any age and affects both sexes with similar frequency although all of the patients reported in the present series were women. Histologically, EHE is composed of epithelioid endothelial cells, which have been referred to as “epithelioid” or “histiocytoid” cells, arranged in short cords and nests set in a distinctive myxohyaline stroma. These cells display endothelial differentiation and have intracytoplasmic lumina (vacuoles) containing erythrocytes that distort or blister the cells' contours.
The diagnosis of EHE in effusion specimens is challenging without a previous history of EHE not only because some of the diagnostic features may be equivocal but also because the tumor shares many morphologic features with more common findings in effusion, such as reactive mesothelial cells, adenocarcinoma, and mesothelioma. Another important differential diagnosis is epithelioid angiosarcoma, a rare subtype of angiosarcoma. Misdiagnosis has been reported previously in several cytologic studies.[89] It is important to recognize EHE in effusion specimens because the diagnosis has important prognostic and treatment implications.
A review of the literature showed only a few case reports describing the cytologic findings of EHE in effusion fluids.[456789] The cytologic features in the present series were somewhat similar to those described in these previous cases. Intracytoplasmic lumina are considered to be a specific feature of EHE but are not present in every case of EHE in effusion fluids.
Clinical information is vital in the differential diagnosis of EHE because this diagnosis is rarely thought of de novo. For example, in the present series, the patient in case 4 had a misleading and complicated disease history, which made the cytopathologic diagnosis of EHE more challenging. The patient had a cytopathologic diagnosis of metastatic carcinoma, which the clinician challenged for two reasons. First, the patient's papillary renal cell carcinoma was only 2.0 cm and limited to the kidney. Second, her papillary thyroid carcinoma was both occult and remote, characteristics that excluded the possibility of metastatic disease from these two sites. Of interest, this patient's history of low-grade B-cell lymphoma did not cause any differential diagnostic problems.
Case 1 also exemplified how a misleading or complicated disease history can make the cytopathologic diagnosis of EHE more challenging. This patient had a carcinoid tumor in the appendix before presenting with ascitic fluid. The smear had low cellularity and very few large cells, which had a low nuclear/cytoplasmic ratio and abundant dense cytoplasm and could not be definitely distinguished from atypical mesothelial cells. Because an immunoperoxidase stain for synaptophysin was negative, the cytopathologist reported “no malignant cells identified.” Later, the peritoneal biopsy showed malignant cells with strong immunoreactivity for endothelial-associated antigens, thereby establishing the basis for the diagnosis of primary EHE in the peritoneum. The cytopathologist then retrospectively performed immunocytochemical analysis of CD31 and FLI-1, and the positive staining confirmed the presence of single EHE cells in the ascitic fluid smear.
Immunohistochemical evaluation is very important for diagnosing EHE in effusions. Since all of the four cases we reported had a history of EHE or had been diagnosed by biopsy or surgical excision, and cytological materials were limited in some cases, we chose to do some selected immunohistochemical markers in these cases. A review of the literature showed only a few case reports describing the cytologic findings of EHE in effusion fluids, and most of them had only limited immunocytochemical markers.[456789] Initially, to diagnose EHE in effusions, one needs to perform immunopanels (vimentin, pancytokeratin, calretinin, WT-1, LCA (CD45), and CD68) which will identify the background mesothelial and inflammatory cells, to create a basic map for confirmation of a “second-foreign” population by a subtractive coordinate immunoreactivity pattern (SCIP) approach.[22] Once the “map” has been created, immunohistochemical staining for a panel of endothelial markers can be added to further characterize the second population. EHE cells characteristically display all endothelial markers, namely, CD31, CD34, and factor VIII-related antigen, along with the relatively new endothelial markers FLI-1 and ERG. FLI-1 and ERG are members of the erythroblast transformation-specific family of transcription factors, which are expressed in endothelial cells. Recent studies have shown that these two markers are helpful in highlighting the endothelial nature of EHE.[2324] Cytokeratin is focally expressed in 20%–30% of EHE cases.[25] In the present series, pan-cytokeratin was expressed in one case. Special stains such as the mucicarmine stain may also be helpful in differentiating adenocarcinomas from EHEs because adenocarcinomas may have vacuoles containing mucin whereas EHEs may have vacuoles but lack intracellular mucin.[26] In previous reports, EHE did not express any mesothelial markers.[27] In the present series, tumor cells in the peritoneal biopsy specimen in case 1 were positive for several mesothelial markers, including podoplanin/D2-40 but negative for calretinin and CK5/6. Recently, some authors reported podoplanin/D2-40 expression in a variety of lymphovascular neoplasms, including lymphangioma, Kaposi sarcoma, and hemangioendothelioma, and posited that podoplanin/D2-40 would be useful as a diagnostic marker for EHE in the liver.[2829]
Epithelioid angiosarcoma is also positive for CD31, CD34, and factor VIII. Sirgi et al.[30] reported that antitumor-associated glycoprotein-72 reactivity was selective for epithelioid angiosarcoma and that immunohistochemical analysis revealed cMYC positivity in angiosarcomas. In contrast to EHE, epithelioid angiosarcoma is a mitotically active, highly atypical neoplasm with high-grade nuclear features and is usually associated with aneuploidy.[31] Cytoplasmic intranuclear inclusion bodies in these tumors have not been described.[32]
Primary peritoneal EHE is extremely rare. One recent report summarized 10 cases; some of the patients presented with a diffuse peritoneal tumor with ascites, a case similar to that of case 1 in the present series.[3]
The evaluation of body fluid cytology specimens is common in cytology laboratories; however, EHE in the serous cavity is extremely rare. Detection of WWTR1-CAMTA1 and YAP1-TFE3 fusion genes could be used to ensure correct diagnosis as well as grading of tumors.[1011] However, fluorescence in situ hybridization is expensive and the probes are not commercially available. Another modality that has been used to identify neoplastic cells in effusion is DNA ploidy analysis. The previous studies showed that reactive mesothelial cells were diploid and were not aneuploid.[3334] In the present series, we showed that EHE cells were predominantly diploid and tetraploid with low-proliferation indices and generally not highly aneuploid. The DNA index of the aneuploid subpopulations ranged from 1.5 to 2.1. Motherby et al.[35] measured nuclear DNA content in 108 malignant mesotheliomas as well as 102 metastatic carcinomas and 100 reactive effusions. They found that 72% of the mesotheliomas had aneuploid subpopulations with a DNA index within a range of 1.80–2.20c, which is quite similar to our findings with EHE. Reactive effusions do not usually show aneuploidy or cells with DNA content >5c. Thus, DNA image analysis is useful for differentiating EHE from reactive mesothelial cells. In contrast, DNA aneuploidy is common in effusions of metastatic carcinomas, and adenocarcinoma showed a higher DNA index than we found with EHE, ranging from 1.11 to 3.6.[3637] We have performed DNA ploidy analysis on angiosarcoma (unpublished data) and found aneuploidy patterns with DNA indices that were diploid and aneuploid with high proliferative index consistent with a tumor of high biological grade. Future studies comparing the DNA ploidy and proliferation patterns of fluids containing EHE with adenocarcinoma and angiosarcoma may help in the differential diagnosis if the “intermediate” DNA ploidy pattern is confirmed. This same intermediate ploidy and proliferation pattern have also been seen in urothelial carcinomas of low-to-intermediate grades.[38]
The biologic behavior of EHE may range from indolent to aggressive.[1] Metastatic disease at the time of diagnosis does not necessarily correspond with reduced survival. DNA image analysis in three of the four cases in the present series revealed predominantly diploid and tetraploid subpopulations, with few aneuploid cells and fairly low-proliferation indices, consistent with an “intermediate” or “broad diploid” ploidy pattern and were generally associated with fairly prolonged survival. The proliferation index may be important in estimating survival duration, as illustrated by the fact that the patient who remained alive at 60 months had the lowest proliferation index (1.5%). These cytometric features, especially the low proliferation index, reflected the fairly indolent clinical course of disease in these cases. The relationship between DNA ploidy and prognosis of EHE remains to be further studied.
CONCLUSIONS
To the best of our knowledge, DNA ploidy quantitation on EHE in effusion fluids has not been previously described in the English-language literature. This technique may be useful for differentiating EHE from reactive mesothelial cells, high-grade sarcomas, and carcinomas and provide a novel insight into the biological behavior of the tumor as well as assisting in the correct classification. The cases reported in the present series indicate that an accurate diagnosis of EHE in effusion fluids by cytopathology is possible, provided that cytologic features are considered in conjunction with clinical history and ancillary studies.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
There are no competing interests as reported by all authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Ying Chen: reviewed the slides, collected the clinical information and wrote the paper.
Abha Khanna: performed the experiments of DNA image analysis for ploidy.
Jie Qing Chen: reviewed the slides and took pictures.
Hua-Zhong Zhang: performed the experiments of DNA image analysis for ploidy.
Nancy P. Caraway: reviewed and edited the manuscript.
Ruth L. Katz: conceived and designed the study, reviewed and edited the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
All authors have followed the ethical guidelines for Human subjects research according to the declaration of Helsinki and institutional review board.
LIST OF ABBREVIATIONS (In alphabetic Order)
DA – Degree of aneuploidy
DH – Degree of hyperploidy
DI – DNA index
DOD – Died of disease
EHE – Epithelioid hemangioendothelioma
PI – Proliferation index.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Clinical patterns and outcome in epithelioid hemangioendothelioma with or without pulmonary involvement: Insights from an internet registry in the study of a rare cancer. Chest. 2011;140:1312-8.
- [Google Scholar]
- Epithelioid hemangioendothelioma: A study of 14 cytopathology cases. J Am Soc Cytopathol. 2015;4:148-59.
- [Google Scholar]
- Primary peritoneal epithelioid hemangioendothelioma. Int J Surg Pathol. 2006;14:257-67.
- [Google Scholar]
- Epithelioid hemangioendothelioma of the lung: Pleural effusion cytology, ultrastructure, and brief literature review. Diagn Cytopathol. 1995;13:54-60.
- [Google Scholar]
- Metastatic epithelioid hemangioendothelioma in a pleural effusion: Diagnosis by cytology. Diagn Cytopathol. 1994;11:64-7.
- [Google Scholar]
- Epithelioid hemangioendothelioma in pleural fluid. Diagn Cytopathol. 1997;16:372-4.
- [Google Scholar]
- Epithelioid hemangioendothelioma: Cytomorphology and histological features of a case. Diagn Cytopathol. 1989;5:75-8.
- [Google Scholar]
- Cytomorphological features of epithelioid hemangioendothelioma in ascitic fluid with radiological, clinical and histopathological correlations. Acta Cytol. 2014;58:211-6.
- [Google Scholar]
- Epithelioid hemangioendothelioma in pleural effusion. Diagn Cytopathol. 2015;43:751-5.
- [Google Scholar]
- Thoracic epithelioid malignant vascular tumors: A clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol. 2015;39:132-9.
- [Google Scholar]
- A novel WWTR1-CAMTA1 gene fusion is a consistent abnormality in epithelioid hemangioendothelioma of different anatomic sites. Genes Chromosomes Cancer. 2011;50:644-53.
- [Google Scholar]
- DNA ploidy abnormalities in basal and superficial regions of the crypts in barrett's esophagus and associated neoplastic lesions. Am J Surg Pathol. 2008;32:1327-35.
- [Google Scholar]
- Chromosomal alterations in the clonal evolution to the metastatic stage of squamous cell carcinomas of the lung. Br J Cancer. 2000;82:65-73.
- [Google Scholar]
- Image analysis of cellular DNA content in peritoneal fluid of patients with ovarian tumors of low malignant potential and invasive epithelial ovarian cancer. Gynecol Oncol. 1996;61:204-9.
- [Google Scholar]
- DNA image analysis combined with routine cytology improves diagnostic sensitivity of common bile duct brushing. Cancer. 2001;93:229-35.
- [Google Scholar]
- Grading follicular lymphoma on fine needle aspiration specimens. Comparison with proliferative index by DNA image analysis and ki-67 labeling index. Acta Cytol. 2004;48:119-26.
- [Google Scholar]
- Relationship between DNA ploidy level, nuclear size, and survival in large cell lymphoma. Am J Clin Pathol. 1995;103:568-73.
- [Google Scholar]
- Image analysis of papillary thyroid carcinoma fine-needle aspirates: Significant association between aneuploidy and death from disease. Cancer. 1999;87:155-60.
- [Google Scholar]
- Hepatic malignant epithelioid hemangioendothelioma: A case report and review of the literature. Am Surg. 2008;74:64-8.
- [Google Scholar]
- Epithelioid hemangioendothelioma: A vascular tumor often mistaken for a carcinoma. Cancer. 1982;50:970-81.
- [Google Scholar]
- Immunocytochemistry of effusion fluids: Introduction to the SCIP approach. In: Cytopathologic Diagnosis of Serous Fluids (1st ed). Philadelphia, USA: Elsevier, W. B. Saunders Company; 2007. p. :55-78.:55-78.
- [Google Scholar]
- Expression of fli-1, a nuclear transcription factor, distinguishes vascular neoplasms from potential mimics. Am J Surg Pathol. 2001;25:1061-6.
- [Google Scholar]
- ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol. 2011;35:432-41.
- [Google Scholar]
- Epithelioid hemangioendothelioma of the liver: A clinicopathologic study of 137 cases. Cancer. 1999;85:562-82.
- [Google Scholar]
- Primary pleural epithelioid hemangioendothelioma with rhabdoid phenotype: Report and review of the literature. Diagn Cytopathol. 2007;35:203-8.
- [Google Scholar]
- Pulmonary epithelioid hemangioendothelioma mimicking mesothelioma. Pathol Int. 2008;58:730-4.
- [Google Scholar]
- Podoplanin is a useful diagnostic marker for epithelioid hemangioendothelioma of the liver. Mod Pathol. 2008;21:125-30.
- [Google Scholar]
- B72.3 and CD34 immunoreactivity in malignant epithelioid soft tissue tumors. Adjuncts in the recognition of endothelial neoplasms. Am J Surg Pathol. 1993;17:179-85.
- [Google Scholar]
- Flow cytometric DNA analysis of vascular soft tissue tumors, including african endemic-type Kaposi's sarcoma. Hum Pathol. 1992;23:1055-60.
- [Google Scholar]
- Characteristic cytologic findings of epithelioid hemangioendothelioma: A case report and review of literature. Diagn Cytopathol. 2011;39:124-7.
- [Google Scholar]
- Complementary value of DNA flow cytometry and image morphometry in detection of malignant cells in effusion fluids. Malays J Pathol. 2014;36:83-90.
- [Google Scholar]
- Image cytometry: An aid for cytological diagnosis of pleural effusions. Diagn Cytopathol. 2005;32:173-6.
- [Google Scholar]
- Static DNA cytometry as a diagnostic aid in effusion cytology: I. DNA aneuploidy for identification and differentiation of primary and secondary tumors of the serous membranes. Anal Quant Cytol Histol. 1998;20:153-61.
- [Google Scholar]
- Epithelioid pleural mesotheliomas and pulmonary adenocarcinomas: A comparative DNA flow cytometric study. Hum Pathol. 1991;22:972-8.
- [Google Scholar]
- Evaluation and prognostic value of DNA content and of morphometric parameters in malignant mesothelioma using digital image analysis. Lung Cancer. 1996;14:229-37.
- [Google Scholar]
- Combination of cytologic evaluation and quantitative digital cytometry is reliable in detecting recurrent disease in patients with urinary diversions. Cancer. 2007;111:323-9.
- [Google Scholar]








