Translate this page into:
Detection of in situ and invasive endocervical adenocarcinoma on ThinPrep Pap Test: Morphologic analysis of false negative cases
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The goal of this study was to calculate the sensitivity and false negative (FN) rate of ThinPrep Pap Test (TPPT) and carefully analyze missed cases for educational purposes.
Materials and Methods:
Patients with histologically proven adenocarcinoma in-situ (AIS) or invasive endocervical adenocarcinoma (EAC) over a 17-year-period (1998-2015) were identified. The TPPT immediately preceding the histological diagnosis of AIS/ECA was designated as index Pap (IP). Paps up to 122 months before histologic diagnosis of AIS/ECA were considered for this study. All available negative and unsatisfactory TPPT were re-reviewed.
Results:
There were 78 patients with histologically-proven AIS (56) or ECA (22) with 184 TPPTs, and 95 of these TPPTs were abnormal. Of the abnormal cases, 55.7% TPPTs were diagnosed as endocervical cell abnormality (atypical endocervical cells/AIS/ECA). Notably, 44.2% of abnormal TPPTs were diagnosed as squamous cell abnormality (atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion/high grade squamous intraepithelial lesion). Including the diagnoses of squamous cell abnormality, the sensitivity of index TPPT for histologically-confirmed AIS/ECA was 88%. Eighty-eight of 184 TPPT, including 10 IP, were negative = 87, or unsatisfactory = 1. Forty-two of these slides were available for re-review. Upon review, 21 TPPT (50%) were confirmed negative and 21 TPPT (50%) were reclassified as abnormal = 20, or unsatisfactory = 1. Of the FN cases, the main difficulty in correct diagnosis was the presence of few diagnostic cell clusters which had less feathering, and consisted of smaller, rounder cells in small and tighter clusters, with nuclear overlap. In particular, nuclear overlap in three-dimensional groups precluded the accurate diagnosis. Rare FN cases showed squamous cell abnormality on re-review, and rare cases showed obscuring blood or inflammation.
Conclusion:
A significant proportion of AIS/EAC is discovered after Pap showing squamous cell abnormality. FN cases were most commonly related to nuclear overlap in tight three-dimensional clusters.
Keywords
Atypical glandular cells
cervical adenocarcinoma
false negative error
sensitivity
ThinPrep Pap test
INTRODUCTION
The Bethesda System in 2001[1] redefined endocervical cell abnormalities and introduced the term endocervical adenocarcinoma in-situ (AIS). The Pap test criteria for this entity include hyperchromatic crowded groups (HCG) with “feathering,” rosettes, atypical strips, and atypical single cells. The nuclei of AIS show enlargement, variation of sizes, crowding, and stratification. There is nuclear hyperchromasia with coarse, stippled chromatin and small or absent nucleoli. Apoptosis and mitoses are usually present. The cytoplasm is typically scant resulting in high nuclear-to-cytoplasmic ratio. The slide background is usually clean. Invasive endocervical adenocarcinoma (ECA) shows similar cytologic features to AIS including the presence of HCG, nuclear crowding, and irregular chromatin. However, in ECA prominent nucleoli and tumor diathesis are also seen.[1]
As a whole, glandular cell abnormalities are rare. They are diagnosed in <0.1% of all Pap test and constitute <5% of all abnormal diagnoses.[2] However, there is a concerning trend of steady increase in AIS/ECA incidence in the US population as well in other developed and developing countries.[345] Liquid-based preparations (LBP) including ThinPrep (Hologic Inc., Boxborough, MA, USA) and SurePath (BD Diagnostics, Burlington, NC, USA) have become the standard of care in the USA for Pap test cytology.[67] Details of endocervical glandular neoplasia (EGN) detected by LBP have previously been published.[6] Compared to conventional smears (CS), the background is cleaner, and background elements become clumped instead of being diffuse; cells also appear to form more three-dimensional groups (HCGs) which are usually darker, tighter, smaller with exaggerated crowding. Nuclear features are similar to CS except the nuclei may be smaller, and nucleoli become more obvious. Similar to CS, presence of “feathering,” strips and rosettes remain pronounced in LBP. “Feathering” of HCG remains the best criteria for diagnosing AIS with a positive predictive value of 73%, particularly, with a positive patient human papillomavirus (HPV) test.[68]
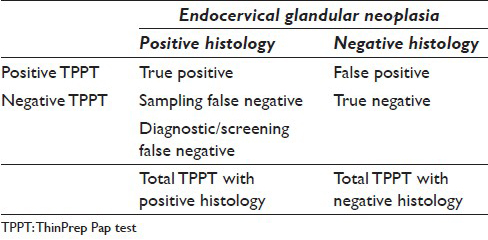
The goal of this study to determine sensitivity of the ThinPrep Pap Test (TPPT) for EGN and to determine causes for diagnostic failures. The overall sensitivity of TPPT for detection of EGN depends on two false negatives (FNs): Sampling FN, where the neoplastic cells are not adequately sampled from the endocervical canal, and diagnostic/screening FN, where the neoplastic cells are misclassified or the slide is considered unsatisfactory despite the presence of the diagnostic cells. The overall sensitivity can be calculated as sensitivity = 1 − (sampling FN + diagnostic/screening FN) [Table 1]. The estimation of sampling error may be only approximate as the true presence of EGN may not be known. In this paper, we concentrated on the evaluation of diagnostic/screening FN and uncovering diagnostic pitfalls in the identification of EGN using TPPT.
MATERIALS AND METHODS
Slide re-review
The study was approved by the Institutional Review Board. The surgical pathology files of the Weill Cornell/New York-Presbyterian Hospital were retrospectively reviewed electronically for all patients with histologically-proven AIS and ECA, over a 17-year-period, from 1998 to 2015. In identified patients, all available prior Pap diagnoses, corresponding histological diagnosis, method of diagnosis (colposcopic biopsy, cone excision, and/or hysterectomy), patient age, and diagnostic intervals were recorded. Pap immediately preceding the histologic diagnosis of AIS and ECA was defined as index Pap (IP). All cytologic diagnoses within time frame of up to 122 months preceding biopsy diagnosis of AIS or ECA were recorded. As we were specifically interested in TPPT performance, for the purpose of this study, we excluded the patients whose screening history consisted of conventional Pap smears. All study cases were prescreened with ThinPrep Imager. For the purpose of the study, only final Pap diagnoses rendered by the pathologist were available. Since HPV testing of residual TPPT sample was performed on only a small fraction of cases, we did not have HPV data that was suitable for statistical analysis or morphological comparisons.
There were 78 patients (age range, 17–78 years; mean, 38 years) with histologically proven AIS (56) or ECA (22) that underwent 184 TPPT during the study period. There were 89 TPPT that were diagnosed as negative or unsatisfactory. Forty-two of these cases were available for a re-review while the remaining 47 slides have been discarded. All available negative and unsatisfactory TPPT were re-reviewed and re-classified according to the 2001 Bethesda System criteria. In all re-reviewed cases, a histological specimen was available, and cytology-histology correlation was performed. The re-reviewed Paps were obtained between 1.5 and 61.3 months before histologic diagnosis.
Calculation of sensitivity and diagnostic false negative and false unsatisfactory rate
Our calculations followed the study by Krane et al.[9] Similar to Krane's study, we report the sensitivity of TPPT in two ways, as overall sensitivity and diagnostic sensitivity. Overall sensitivity was defined as true positive TPPT/total TPPT in patients with histologic EGN [Table 1]. All abnormal diagnoses, including squamous cell abnormalities, were considered as true positives. Furthermore, following Krane's algorithm[9] we determined diagnostic sensitivity which was defined as 100 × true positive TPPT/(true positive + diagnostic/screening FN) with the assumption that all negative TPPT unavailable for review were true negatives. In addition, following Krane et al.[9] we defined diagnostic FN and false unsatisfactory rate as 100 × diagnostic FN and false unsatisfactory TPPT/all re-reviewed negative and unsatisfactory TPPT.
RESULTS
Slide re-review
The TPPT re-review algorithm for all 78 patients is shown in Figure 1. Of the total 78 patients, 56 had histologic diagnosis of AIS (including 30 AIS with associated high-grade squamous intraepithelial lesion [HSIL]) and 22 had diagnosis of ECA (including 6 ECA with associated HSIL). Figures 2 and 3 show the review algorithm for AIS and ECA, separately.
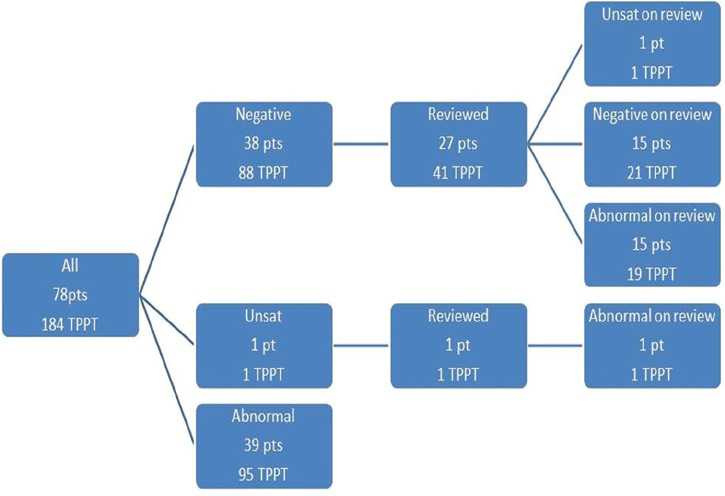
- ThinPrep Pap Test re-review algorithm for 78 patients with adenocarcinoma in situ or endocervical adenocarcinoma (184 total ThinPrep Pap Test)
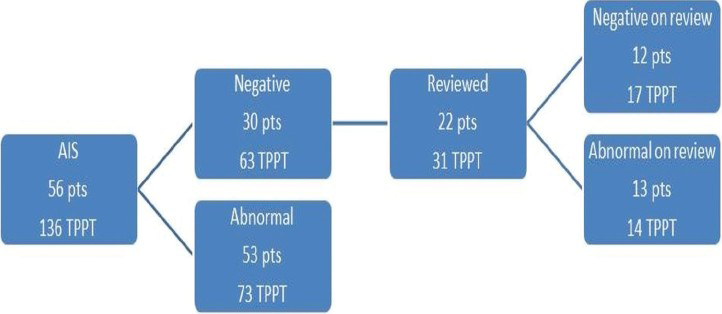
- ThinPrep Pap Test re-review algorithm for 56 patients with adenocarcinoma in situ (136 total ThinPrep Pap Test)
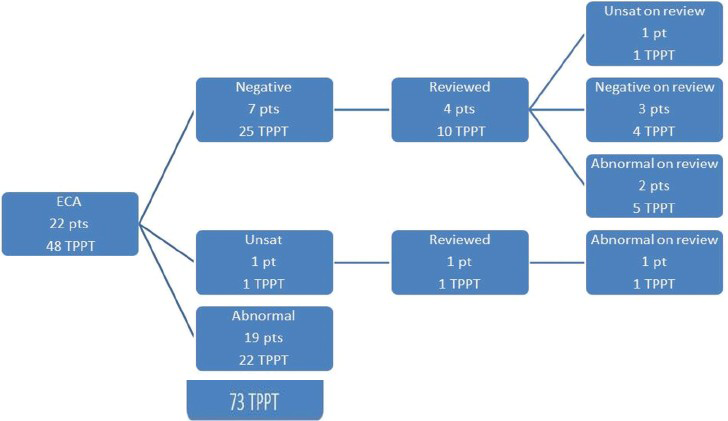
- ThinPrep Pap Test re-review algorithm for 22 patients with endocervical adenocarcinoma (48 total ThinPrep Pap Test)
From the 78 patients, there were 184 total TPPT, which included 78 IP and 106 prior TPPT. Ninety-five TPPT (52%) in 72 patients were diagnosed as abnormal, including 68 IP (88% of IP). Therefore, the overall sensitivity of TPPT was 88% for IP and 52% for all proceeding Paps considered within timeframe of the study. Of the abnormal TPPTs, 55.7% TPPTs were diagnosed as endocervical cell abnormality (atypical endocervical cells (AEC), AIS and ECA). Notably, 44.2% of abnormal TPPTs were diagnosed as squamous cell abnormality (atypical squamous cells of undetermined significance [ASCUS]/low grade squamous intraepithelial lesion [LSIL]/high grade squamous intraepithelial lesion [HSIL]). In the latter group, glandular neoplasia was subsequently detected on the biopsy or cone as an incidental finding. Original TPPT diagnoses with histologic correlations are shown in Table 2.

Eighty-nine (48%) TPPT from 38 patients were initially diagnosed as negative (Neg) (n = 88) and unsatisfactory (Unsat) (n = 1), including 10 IP. In the 10 patients with Neg/Unsat IP, colposcopic biopsies were performed because of: Previously reported HSIL and AGC, 7; positive HPV testing, 2 and postcoital bleeding, 1.
Of these 89 cases, 47 TPPT were older than 5 years and had been discarded according to CLIA′88 guidelines. These discarded TPPT preceded their corresponding histological diagnosis by an average of 47.7 months (range 0.5–122.4 months).
The remaining 42 of 89 (47%) Unsat/Neg TPPT from 27 patients (AIS, 31 TPPT from 22 patients; and ECA, 11 TPPT from 5 patients) were available for review. These 42 available Unsat/Neg TPPT preceded the histological diagnosis by an average of 21.6 months (range 1.5–61.3 months). All of the reviewed Unsat/Neg TPPT (100%) had an available corresponding histological biopsy that was also reviewed.
Upon re-review, 21/42 TPPT (50%) from 17 patients were reclassified as either abnormal = 20, or Unsat = 1. Cellular changes identified in abnormal cases are discussed below in “Classification variance” and subdivided into screening, interpretive and sampling variance. Twenty-one TPPT were confirmed as true Neg on re-review. Summary of re-review diagnoses is shown in Table 3.

The diagnostic sensitivity of TPPT, calculated in the re-reviewed slides, was 82% (100 × 95 positive TPPT/95 positive + 21 FN). The diagnostic FN rate in the reviewed TPPTs was calculated[9] by the following equation: 100 × diagnostic FN and false unsatisfactory TPPT/all reviewed negative and unsatisfactory TPPT, therefore 100 × 20/42 = 47.6%.
Classification variance
Screening variance (4 cases)
One falsely-classified Unsat was re-classified as AEC and showed abnormal HCGs that were partially obscured by blood resulting in a screening variance. Histology was ECA with AIS. Two cases re-classified as AEC were screening variance including one with HCG obscured by inflammation and other with obscuring blood. Histology of both was AIS. One initially Neg TPPT was re-classified as Unsat because of insufficient squamous cells. On histology, this was AIS/minimally invasive ECA/HSIL.
Interpretive variance (14 cases)
Four TPPT showed squamous cell abnormality [Figures 4 and 5]. Two cases showed atypical repair and few HCG and were reclassified as ASCUS. One TPPT was re-classified as LSIL and one case with pale HCGs was re-classified as ASC cannot exclude HSIL. Histology in these four cases was AIS, 1 and AIS/HSIL, 3 respectively.
- Case re-classified as atypical squamous cells of undetermined significance, atypical repair. This was an interpretation variance. The cells are arranged in elongated narrow sheets/strips. Cytoplasm is dense. Nuclei are crowded, vesicular with thick membranes and prominent nucleoli. Mitotic figures were also identified. Background shows acute inflammation (a and b). The histology of this case was adenocarcinoma in situ/high grade squamous intraepithelial lesion (Pap, ×600)
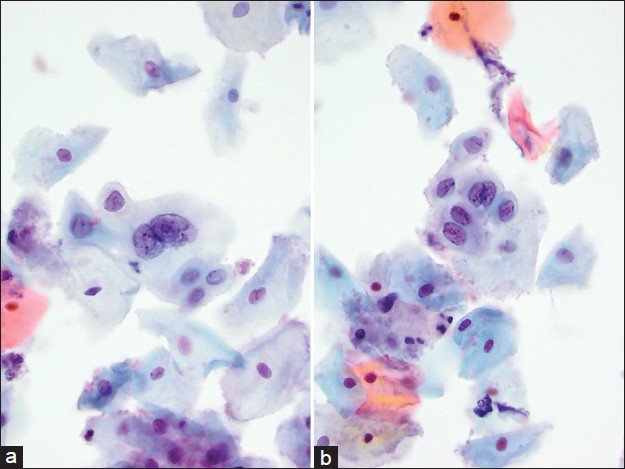
- Case re-classified as low grade squamous intraepithelial lesion. This was an interpretation variance. A bi-nucleated squamous cell with nuclear enlargement, mildly coarse chromatin, and subtle nuclear membrane irregularity (a). A small sheet of squamous cells with features of atypical squamous cells cannot exclude high grade squamous intraepithelial lesion (b). The histology of this case was adenocarcinoma in situ/high grade squamous intraepithelial lesion (Pap, ×600)
Seven cases were re-classified as AEC/AEC-favor neoplasia [Figures 6 and 7]. Originally, these cases were underdiagnosed predominantly due to fewer abnormal cells, less “feathering,” smaller, rounder cells in smaller and tighter clusters, darker attenuated nuclei, and more nuclear overlap. Histology in these seven cases was: AIS, 2; AIS/HSIL, 2; ECA, 2; ECA/AIS, 1.
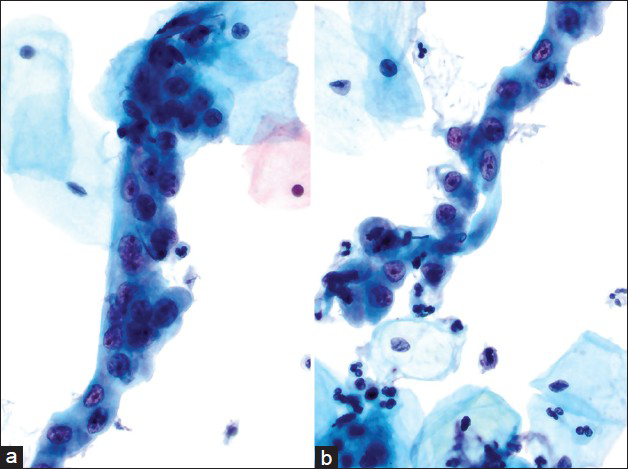
- Case re-classified as atypical endocervical cells favor neoplastic. This was an interpretation variance. The hyperchromatic crowded groups are comprised of cells with increased N/C ratios. The nuclei are elongated/oval, crowded, hyperchromatic, with overlap, coarse chromatin, and prominent nucleoli (a and b). The histology of this case was endocervical adenocarcinoma/adenocarcinoma in situ (Pap, ×600)
- Case re-classified as atypical endocervical cells. This was an interpretation variance. The hyperchromatic crowded groups show small strip and group of endocervical cells with “feathering” and high N/C ratios. Nuclear crowding, overlapping and molding is also seen (a and b). The histology in this case was endocervical adenocarcinoma (Pap, ×600)
Three cases were re-classified as AIS [Figure 8] and showed characteristic features of AIS and were considered an interpretation variance. One of these had background blood, and another had background inflammation; however, neither the blood nor inflammation was significant enough to act as obscuring factors. Histology in these three cases was: AIS, ECA/AIS, and AIS/HSIL.

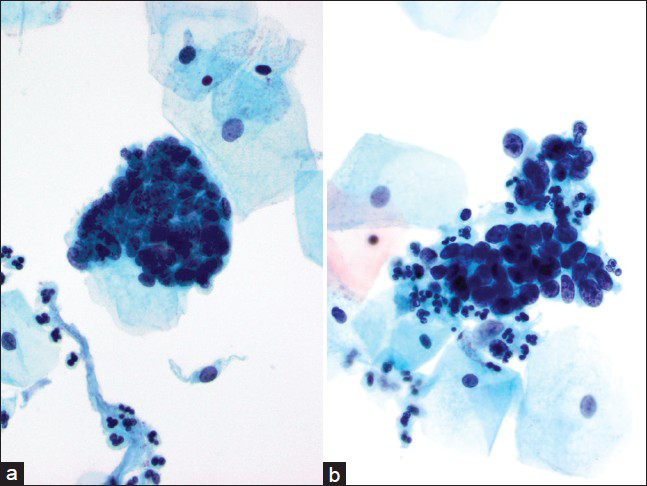
- Case re-classified as adenocarcinoma in situ. This was an interpretation variance. This case showed many hyperchromatic crowded groups with all features of adenocarcinoma in situ identified including feathering/nuclear palisading, two-dimensional sheets and rosettes. The nuclei of these groups were enlarged and hyperchromatic without nucleoli (a-d). The histology in this case was endocervical adenocarcinoma (Pap, ×600)
Sampling variance (3 cases)
Three cases were re-classified as AEC [Figure 9] had sampling variance with low cellularity. The diagnosis of AEC in these cases was possible only in retrospect after comparing with subsequent biopsies. Histology in these three cases was: AIS/HSIL, 2 and ECA/AIS.

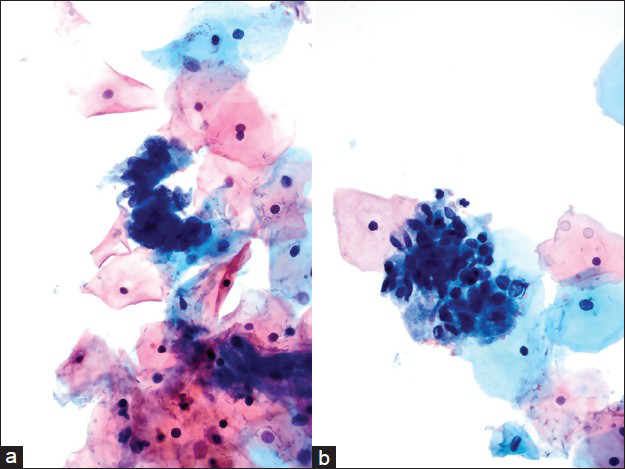
- Case re-classified as atypical endocervical cells due to sampling variance. There were few hyperchromatic crowded groups present with feathering and atypical, hyperchromatic nuclei. Occasional groups showed prominent nucleoli (a-d). The histology in this case was adenocarcinoma in situ/high grade squamous intraepithelial lesion (Pap, ×600)
DISCUSSION
The calculation of Pap sensitivity for diagnosis of AIS and ECA varies depending on study design and may be affected by the time frame of Paps included in the study. The calculated sensitivity may vary significantly depending whether only IPs were considered for the calculations or whether preexisting Paps were also included in the analysis.
In this study, we determined that the overall sensitivity of TPPT for EGN was 88% for IP, and 52% for all Paps considered within time frame of up to 122 months preceding biopsy diagnosis. The diagnostic sensitivity of TPPT, calculated based on FN error in the re-reviewed slides, was determined as 82%. The diagnostic false-negative and false-unsatisfactory rate, as defined by Krane et al.[9] in the re-reviewed slides was 47.6%. In the prior study of EGN in conventional Paps by Krane et al.[9] the overall sensitivity of all Paps included in the study was 45% (with timeframe of up to 116 months preceding biopsy diagnosis), diagnostic sensitivity was 76%, and diagnostic false-negative false-unsatisfactory rate was 50%. While the overall and diagnostic sensitivity reported by Krane for conventional Paps was just slightly lower than our results for TPPT, the diagnostic false-negative and false-unsatisfactory rate reported by Krane et al.[9] was almost identical to the results of this study.
Prior study of Roberts and Thurloe[10] reported slightly lower sensitivity of CS compared to TPPT for diagnosis of AIS (56% vs. 67%, respectively) in a study spanning 36 months before biopsy diagnosis. In comparison, the Pap sensitivity for HSIL in their study was 74% and 80% with CS and TPPT, respectively, indicating greater difficulty of detection of EGN on Pap. A similar finding was reported in a study of Renshaw et al.[11] Schoolland et al. reported that Pap sensitivity was higher for AIS plus HSIL than for AIS alone.[12] Taken together, the results of our study in TPPT and results of prior studies indicate the need for increased accuracy in EGN screening.
Mitchell et al.[13] calculated cumulative reduced incidence of invasive cervical adenocarcinoma based on the frequency of Pap screening. The reduction was estimated at 65% with yearly, 46% with biennial and 34% with triennial screening. As the recommendation for the frequency of Pap testing has changed toward increased intervals, the vigilance for detection of EGN needs to increase. With that in mind, the second focus of this study was to determine the causes of diagnostic difficulties that influence the performance of the TPPT in detecting of EGN.
Lee et al.[14] and Ruba et al.[15] analyzed cases with missed diagnosis of EGN in CS. Lee et al.[14] reported CS sensitivity for AIS as 55%. Of the slides originally diagnosed as negative, 55% were retrospectively diagnosed as abnormal upon re-review. Most often the FN diagnoses were due to small, crowded endocervical clusters possibly mistaken for endometrial cells, while other cases showed large groups in which neoplastic cells resembled reactive endocervical cells. Ruba et al.[15] reported CS sensitivity for EGN as 54% and have defined three types of errors that affect the diagnosis of adenocarcinoma in cervical specimens. These errors include sampling, screening, and diagnostic errors. The authors, however, suggested that the diagnostic errors are likely to be decreasing over time, and could be reduced with targeted educational efforts.
In this study, we identified screening variance in cases that had either a significant amount of obscuring blood or obscuring inflammation, which masked the features of AEC. Even though the design of TPPT technology efficiently removes obscuring factors, as compared to conventional Paps, excess blood still has been shown to affect the performance of TPPT.[16] In addition, when specifically addressing the common causes of unsatisfactory TPPT, it was shown that excessive lubricant and abundant blood were the two most common factors, occurring in that order.[17] Whereas unsatisfactory specimens in many instances can represent a benign condition, there are a number of women with unsatisfactory specimens that will have a subsequent histologically confirmed neoplasm, as was observed in this study. Therefore, careful attention to the HCG in TPPT that contain obscuring elements may help to prevent screening errors.
In the cases where sampling variance were identified, the TPPT showed adequate cellularity; however, few HCG were present. In all of these cases, AEC was identified on review. When HCG are present but are few in numbers, a significant diagnostic difficulty can be encountered.[1819] In such instances, glandular neoplastic lesions may be arising in the upper portion of endocervical canal, or only small neoplastic foci may be present on the mucosal surface despite diffuse involvement of glands embedded in the stroma.[20] In such cases, the lesion may be under-sampled despite the TPPT adequacy, and therefore special focus on any and all HCG must be present to reduce FN diagnoses.
Overall, the most common source of failed diagnosis was interpretive variance. In most of the cases, the interpretive variance was due to underestimation of HCG that were present in adequate numbers. In general, HCG is one of the most common sources of interpretive difficulties (and potential sources of litigation) in Pap test diagnoses, especially when they do not exhibit features fulfilling all diagnostic criteria.[1819] We have seen that many HCG comprised relatively smaller glandular cells that were present within a clean background. The interpretation of these groups had the differential diagnosis of benign lower uterine segment epithelium. In other cases, a few HCG were present and were interpreted as reactive/reparative type changes. With respect to both of these instances, neoplastic cells with small “endometrioid” type features as well as these resembling reactive endocervical cells may be mistaken for benign glandular epithelium and thus become a factor in decreased sensitivity of the TPPT.[141518] As seen in our TPPT re-classified as AIS, the presence of many benign-appearing HCG exhibiting only a few features overlapping with those of neoplastic glandular lesions may mask the presence of HCGs with overt neoplastic changes. In particular, the thick cell clusters and tissue fragments may obscure neoplastic cytologic findings. The importance of attention to subtle morphological features in the interpretation of these groups cannot be overemphasized.
Potential limitations of this study are identified. One such limitation is the retrospective unblinded review of the negative TTPT. The reviewers’ intent was to identify features of malignancy on TPPT that were purported to have been present at the time of initial screening. In doing so, this review bias places additional scrutiny on negative reviewed TPPT and may overestimate the number of FN diagnoses. In addition, HPV testing was not consistently requested in the studied cases, and therefore HPV results were not available for comparison with TPPT morphology.
CONCLUSION
There is ample opportunity to improve our performance in detecting AIS and ECA in TPPT. Minimal, poorly preserved material will always provide problems with regard to detection and diagnosis of these entities. With this in mind, a heightened awareness to the features of any HCG present should be observed when interpreting cervical TPPT.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors declare no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. MC collected the data, reviewed the slides and drafted the manuscript. ECP participated in the design of the study, performed calculations and drafted the manuscript. VJAP extracted the data from the charts for the database. ABM was involved with the chart review and extracted the data from the charts for the database. KH developed a concept for the study and edited the manuscript. RH collected the data, reviewed the slides and drafted the manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board of the institution associated with this study as applicable. This study was a retrospective study not directly involving the patients. Authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
AIS - Adenocarcinoma in-situ
AGC - Atypical glandular cells
ASCUS - Atypical squamous cells of undetermined significance
CS - Conventional smears
EAC - Endocervical adenocarcinoma
EGN - Endocervical glandular neoplasia
FN - False negatives
HSIL - High-grade squamous intraepithelial lesion
HPV - Human Papillomavirus
HCG - Hyperchromatic crowded groups
IP - Index Pap
LSIL - Low-grade squamous intraepithelial lesion
Neg - Negative
TPPT - ThinPrep Pap Test
Unsat - Unsatisfactory.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and the highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
The authors would like to thank Dr. Jeffery F. Krane for allowing us to adapt flowcharts from his publication.
REFERENCES
- The 2001 Bethesda system: Terminology for reporting results of cervical cytology. JAMA. 2002;287:2114-9.
- [Google Scholar]
- The rising incidence of adenocarcinoma relative to squamous cell carcinoma of the uterine cervix in the United States - A 24-year population-based study. Gynecol Oncol. 2000;78:97-105.
- [Google Scholar]
- Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer. 2004;100:1035-44.
- [Google Scholar]
- International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer. 1998;75:536-45.
- [Google Scholar]
- Gynecologic cytology on conventional and liquid-based preparations: A comprehensive review of similarities and differences. Diagn Cytopathol. 2013;41:257-78.
- [Google Scholar]
- Differentiating between endocervical glandular neoplasia and high grade squamous intraepithelial lesions in endocervical crypts: Cytological features in ThinPrep and SurePath cervical cytology samples. Diagn Cytopathol. 2009;37:315-9.
- [Google Scholar]
- Endocervical glandular cell abnormalities in conventional cervical smears: Evaluation of the performance of cytomorphological criteria and HPV testing in predicting neoplasia. Cytopathology. 2008;19:34-43.
- [Google Scholar]
- Papanicolaou smear sensitivity for the detection of adenocarcinoma of the cervix: A study of 49 cases. Cancer. 2001;93:8-15.
- [Google Scholar]
- Comparative sensitivities of ThinPrep and Papanicolaou smear for adenocarcinoma in situ (AIS) and combined AIS/high-grade squamous intraepithelial lesion (HSIL): Comparison with HSIL. Cancer. 2007;111:482-6.
- [Google Scholar]
- Detection of adenocarcinoma in situ of the cervix in Papanicolaou tests: Comparison of diagnostic accuracy with other high-grade lesions. Arch Pathol Lab Med. 2004;128:153-7.
- [Google Scholar]
- Improvement in protection against adenocarcinoma of the cervix resulting from participation in cervical screening. Cancer. 2003;99:336-41.
- [Google Scholar]
- Papanicolaou smear sensitivity for adenocarcinoma in situ of the cervix. A study of 34 cases. Am J Clin Pathol. 1997;107:30-5.
- [Google Scholar]
- Adenocarcinoma in situ of the uterine cervix: Screening and diagnostic errors in Papanicolaou smears. Cancer. 2004;102:280-7.
- [Google Scholar]
- Comparison of the effectiveness of two liquid-based Papanicolaou systems in the handling of adverse limiting factors, such as excessive blood. Cancer. 2006;108:27-31.
- [Google Scholar]
- The unsatisfactory ThinPrep® Pap Test™: Analysis of technical aspects, most common causes, and recommendations for improvement. Diagn Cytopathol. 2013;41:588-94.
- [Google Scholar]
- Hyperchromatic crowded groups in cervical cytology – Differing appearances and interpretations in conventional and ThinPrep preparations: A study from the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2006;130:332-6.
- [Google Scholar]
- Hyperchromatic crowded cell groups in gynaecological liquid-based cytology samples. Br J Biomed Sci. 2010;67:154-63.
- [Google Scholar]
- Obstacles to the early detection of endocervical adenocarcinoma. Int J Gynecol Pathol. 2005;24:399-403.
- [Google Scholar]








