Translate this page into:
Diagnostic utility of p16 immunocytochemistry for Trichomonas in urine cytology
*Corresponding author
-
Received: ,
Accepted: ,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We present a case in which p16 immunocytochemistry helped establish the diagnosis ofTrichomonasin urine from a male patient. Based on this finding, we recommend p16 immunocytochemistry as a diagnostic tool for unexpected patients or specimen types in which potential trichomonads are identified following routine cytologic evaluation.
Article
We received 50 ml of voided urine from a 84-year-old diabetic male who presented with hematuria. Routine urine cultures yielded no growth after 24 hours. A Papanicolaou-stained ThinPrep[TM] slide was prepared which revealed benign urothelial cells, neutrophils including "cannonballs" (i.e. neutrophils aggregated around epithelial cells), red blood cells and numerousTrichomonasorganisms Figure 1. The diagnosis ofTrichomonaswas based upon the presence of a discernible nucleus and cytoplasmic granules that were identified in several of the trichomonads. A visible nucleus and well-defined cytoplasmic granules at 40x magnification are specified as important morphological features required for a confident diagnosis ofTrichomonasin liquid-based Pap tests 1. Although we did not identify flagella in the trichomonads in our case, the finding of flagella, while helpful, is not always required to make a diagnosis ofTrichomonas1. In fact, the morphologic identification ofTrichomonason liquid-based Pap tests has been shown to be highly accurate 2. Nevertheless, exfoliated cells including microorganisms in urine are often degenerated, which makes the identification ofTrichomonasin these specimens by morphology alone difficult. Therefore, confirmatory testing may be needed. However, traditional methods to detectTrichomonasincluding culture and wet-mount microscopy, as well as molecular studies, may not always be readily available, particularly on fixed samples received in liquid-based vials for cytologic evaluation.
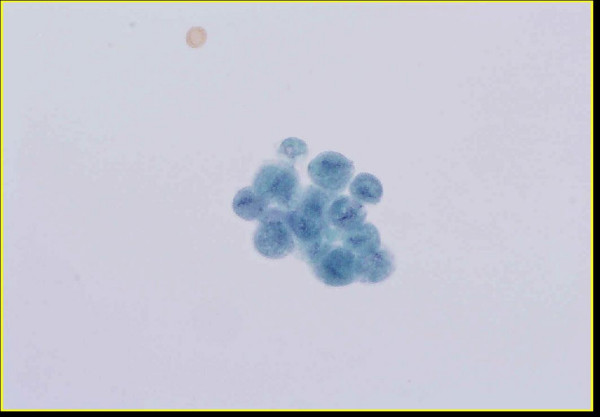
- Group of Trichomonas organisms present in urine (Thin-
Prep™, Papanicolaou stain).
In order to differentiate parasites from degenerated urothelial cells in our case we prepared additional ThinPrep[TM] slides from the residual specimen to perform immunocytochemistry for cytokeratin (using a cocktail of high- and low-molecular weight keratins) and p16 (using primary purified mouse anti-human p16 antibody, clone G175-405, supplied by BD Biosciences Pharmingen, San Diego, CA, USA). We included p16 based upon recent published data by our group indicating thatTrichomonas vaginalisorganisms in cervicovaginal specimens are immunoreactive for p16 3. In all ten of these cervicovaginal specimensT. vaginaliswere p16 positive and demonstrated strong cytoplasmic staining. Immunocytochemical staining for p16, a proven biomarker for high grade dysplasia associated with Human Papillomavirus (HPV) infection, has been previously applied successfully to cervicovaginal cytology specimens 4. However, p16 immunoreactivity is not specific for HPV-infected epithelium, as immunoreactivity has previously been documented with inflammatory cells, multinucleated giant cells, bacteria and mucus in cervicovaginal specimens [4-6]. It is unclear if p16 staining ofTrichomonasorganisms reflects specific immunoreactivity (to unknown epitopes) or may be non-specific. In our case, urothelial and squamous cells were strongly immunoreactive for cytokeratin Figure 2 but were negative for p16, whereas trichomonads demonstrated strong p16 immunoreactivity Figure 3 and Figure 4 and failed to react with cytokeratin. Appropriate controls were included in this study (data not shown). Following the diagnosis ofTrichomonas , our patient was treated with a course of metronidazole.
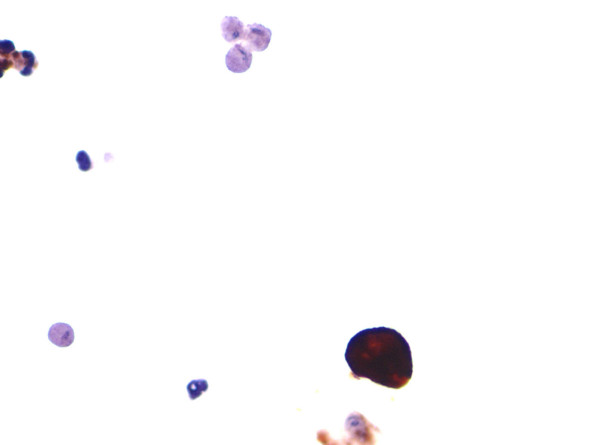
- Cytokeratin immunocytochemistry. A single urothelial cell
demonstrates strong cytokeratin immunoreactivity whereas
surrounding trichomonads are negative. Degenerated inflammatory
cells are also present in this field.
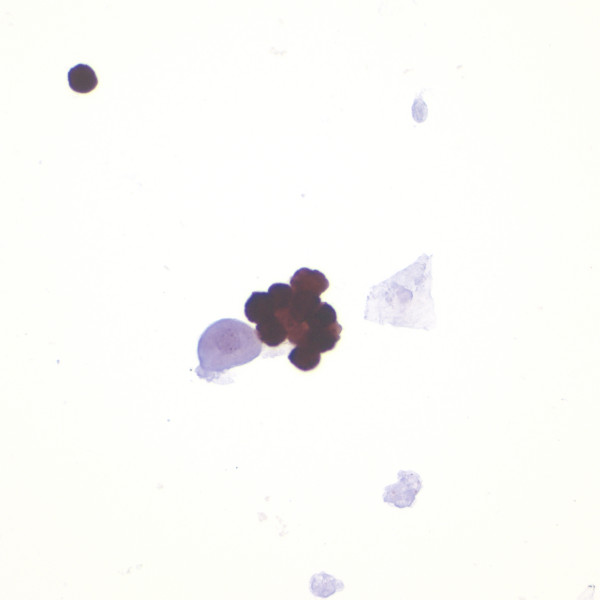
- p16 immunocytochemistry. A group of trichomonads demonstrate
strong p16 immunoreactivity whereas an adjacent
degenerated urothelial cell and squamous cell are negative.
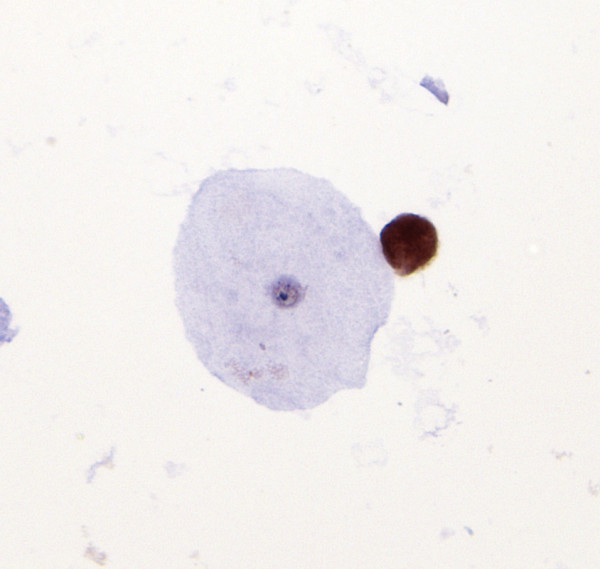
- A single trichomonad, adjacent to an unstained exfoliated
squamous epithelial cell, is shown to be p16 immunoreactive.
Trichomonasinfection in male patients may be asymptomatic or associated with non-gonococcal urethritis, prostatitis, and urethral strictures. Protozoa in men may be harbored in the uncircumcised prepuce, urethra, seminal vesicles, prostate gland or bladder. As shown in the present case, it is often difficult to establish the diagnosis ofTrichomonasin men, particularly when the diagnosis is based solely on the cytological evaluation of poorly preserved organisms in a urine specimen. The diagnosis may be especially problematic when the patient denies sexual contact, or when a sexually transmitted disease in a particular patient, such as an infant 7 or the elderly, seems implausible. Adding to the problem is the fact that when in urine, trichomonads usually assume variable shapes 8. Parasite variation in size and shape may be further exaggerated in air-dried urine smears 9. In male patients, the organisms also tend to be somewhat smaller than their counterpart in female patients 10. In addition to the present patient, our laboratory has diagnosedTrichomonasin Papanicolaou-stained urine specimens in eight men of mean age 59 years (range 47-82 years), over a 15 year period. This finding is in keeping with published data suggesting that higher organism loads occur in older men 11. We do not know if any of these individuals also had diabetes.
In conclusion, as illustrated in the case presented, we recommend the use of p16 immunocytochemistry to help establish the diagnosis ofTrichomonasin unexpected patients or specimen types in which potential trichomonads are identified following routine cytologic evaluation.
References
- Diagn Cytopathol 2005, 32: 253-259
- Diagn Cytopathol 2005, 32: 341-344
- Modern Pathology 2005, 18: 73A-74A
- Acta Cytol 2002, 46: 25-29
- Diagn Cytopathol 2002, 27: 365-370
- Int J Cancer 2004, 108: 871-876
- Pediatr Infect Dis 1982, 1: 340-341
- Pol Tyg Lek 1991, 46: 997-999
- Acta Cytol 2000, 44: 484-485
- J Urol 1972, 107: 840-842
- Sex Transm Infect 2003, 79: 151-153







