Translate this page into:
Encounter with unusual tumor having classic cytomorphology presenting as neck mass

*Corresponding author: Vaishali Atmaram Walke, Department of Pathology and Lab Medicine, All India Institute of Medical Sciences, Bhopal, Madhya Pradesh, India. drvaishaliw@yahoo.com
-
Received: ,
Accepted: ,
How to cite this article: Walke VA, Agale SV, Patil PK. Encounter with unusual tumor having classic cytomorphology presenting as neck mass. CytoJournal 2021;18:6.
HTML of this article is available FREE at: http://dx.doi.org/10.25259/Cytojournal_20_2020
CASE REPORT
A 50-year-old woman complained of the right-sided neck swelling of 6 months duration. It was a gradually increasing 4x3 cm, soft-firm, non-tender, slightly mobile mass. On ultrasonogram (USG) neck, there was massive, necrotic lymphadenopathy which suggested the possibility of tuberculosis and a small (0.9 × 0.7 cm size) cystic lesion in the thyroid gland. The patient revealed a history of similar neck swelling 3 years back, which regressed completely after aspiration, reports of which were not available. The fine-needle aspiration cytology of neck mass yielded approximately 5 ml, thin, hemorrhagic fluid; the swelling regressed partially after aspiration. The procedure was repeated from the residual mass. The FNA smears from neck nodes are provided.
Q1: What is the possible diagnosis?
Metastasis of papillary thyroid carcinoma
Nasopharyngeal carcinoma
Follicular dendritic cell sarcoma
Lymphoma
Ectopic meningioma
Answer: (c) Follicular dendritic cell sarcoma
The literature search reveals that follicular dendritic cell (FDC) sarcoma usually display cellular smears presenting as thick syncytial groups, whorls, fascicles, or single cells intimately admixed with mature lymphocytes and plasma cells. The cytological features are better highlighted on May-Grunwald Giemsa (MGG) stain [Figure 1a]. The cells are round to oval, with mild-to-moderate pale wispy cytoplasm; indistinct cell borders [Figure 1b], round to oval to plump nuclei with fine chromatin, prominent nucleoli, and many stripped nuclei. Nuclear atypia is variable. Bilobed and multinucleated tumor cells can be seen. Nuclear grooves and intranuclear inclusions have also been reported [Figure 1c].[1-4] Our case presented majority of the above described cytoarchitectural features. In addition, microfollicular arrangement and vague papilloid structures were also seen. The nuclei were hyperchromatic, however, prominent nucleoli were not observed [Figure 1d].
![(a) FNA from cervical LN: Tumor cells seen as thick fragments and singly placed cells, intimately admixed with lymphocytes and plasma cells (May-Grunwald Giemsa [MGG], ×20). (b) Oval, plump to elongated cells with indistinct outline, moderate pale cytoplasm (Papanicolaou [PAP], ×20). (c) Nuclei are round, oval to plump with fine chromatin and prominent nuclear intranuclear inclusion and nuclear grooves (arrow) (Papanicolaou, ×40). (d) Vague microfollicular pattern and dissociated tumor cells (PAP, ×20).](/content/105/2021/18/1/img/Cytojournal-18-6-g001.png)
- (a) FNA from cervical LN: Tumor cells seen as thick fragments and singly placed cells, intimately admixed with lymphocytes and plasma cells (May-Grunwald Giemsa [MGG], ×20). (b) Oval, plump to elongated cells with indistinct outline, moderate pale cytoplasm (Papanicolaou [PAP], ×20). (c) Nuclei are round, oval to plump with fine chromatin and prominent nuclear intranuclear inclusion and nuclear grooves (arrow) (Papanicolaou, ×40). (d) Vague microfollicular pattern and dissociated tumor cells (PAP, ×20).
Considering the primary presentation of enlarged neck nodes, USG favoring solitary hypoechoic lesion in thyroid and cytomorphology of vague papillary structures, microfollicular pattern, [Figure 1d] nuclear grooves, and intranuclear inclusions highlighted under MGG stain [Figure 1c]; metastasis of papillary thyroid carcinoma is the most probable diagnosis. However, the classical features such as papillary structures with core, metaplastic cytoplasm, and stringy colloid were absent
The other differential diagnosis can be nasopharyngeal carcinoma (NPC) undifferentiated type, which it mimics clinically and presents as enlarged neck nodes. The smears reveal mostly dissociated and loose groups of cells with few enlarged pleomorphic and lobulated nuclei against a background of mature lymphocytes. The presence of nuclear grooves, inconspicuous nucleoli, and low mitotic count was against the diagnosis of NPC[4]
Possibility of lymphoma Hodgkin’s type was less likely, the pointers for lymphoma can be presence few atypical cells with pleomorphic, lobulated nuclei, and admixture with lymphocytes, plasma cells, and eosinophils. However, classical Reed–Sternberg cells were absent
Ectopic meningioma can also be a likely differential, especially when clinical presentation is neck mass. The overlapping features were oval to plump to elongated cells with bland chromatin, inconspicuous nucleoli, and intranuclear inclusions. However, the absence of whorl formation and admixture of lymphocytes with tumor cell makes the possibility of conventional meningioma less likely.[5]
Question 2: FDC sarcoma is categorized as
Low-grade malignancy
High-grade malignancy
Intermediate-grade malignancy
Benign tumor
Answer: (c) Intermediate-grade malignancy[6]
Question 3: All are common extranodal sites of this condition except
GIT
Brain
Skin
Liver
Answer: (b) Brain[7]
Question 4: Which of the following is false about poor prognostic factors of FDC sarcoma?
Tumor size >10 cm
Mitosis >5/10 hpf
Coagulation necrosis
Significant cellular atypia
Answer: (a) Tumor size more than 10 cm[7,8]
Further work up of the case
The patient was investigated, further his CT scan revealed a right cervical lymphadenopathy, seen as multiple, enlarged lymph nodes with cystic component and single 9 × 7 mm, cyst in the right lobe of thyroid. It also revealed ipsilateral enlargement of tonsil measuring 3 × 3 cm and left para pharyngeal heterogeneous, enhancing, lobulated mass favoring lymph node metastasis. FNA from parapharyngeal lymph nodes yielded similar cytomorphological features as that from cervical nodes [Figure 2]. The tonsillar biopsy showed subepithelial tumor which composed of solid nests whorls and rare foci with storiform pattern of spindle to oval to plump cells, admixed with lymphoplasmacytic infiltrate [Figure 3]. The cells have scant to moderate pale cytoplasm and central round to oval vesicular nuclei with nucleoli in some. Occasional mitotic activity was noted. The histology was suggestive of FDC sarcoma. The tumor cells expressed CD21 membranous positivity and therefore substantiated the diagnosis of FDC sarcoma [Figure 4]. The patient underwent bilateral tonsillectomy with ipsilateral radical neck dissection. The contralateral tonsil was uninvolved by tumor while the cervical lymph nodes showed evidence of metastasis. Post-surgery, the patient received radiation therapy.
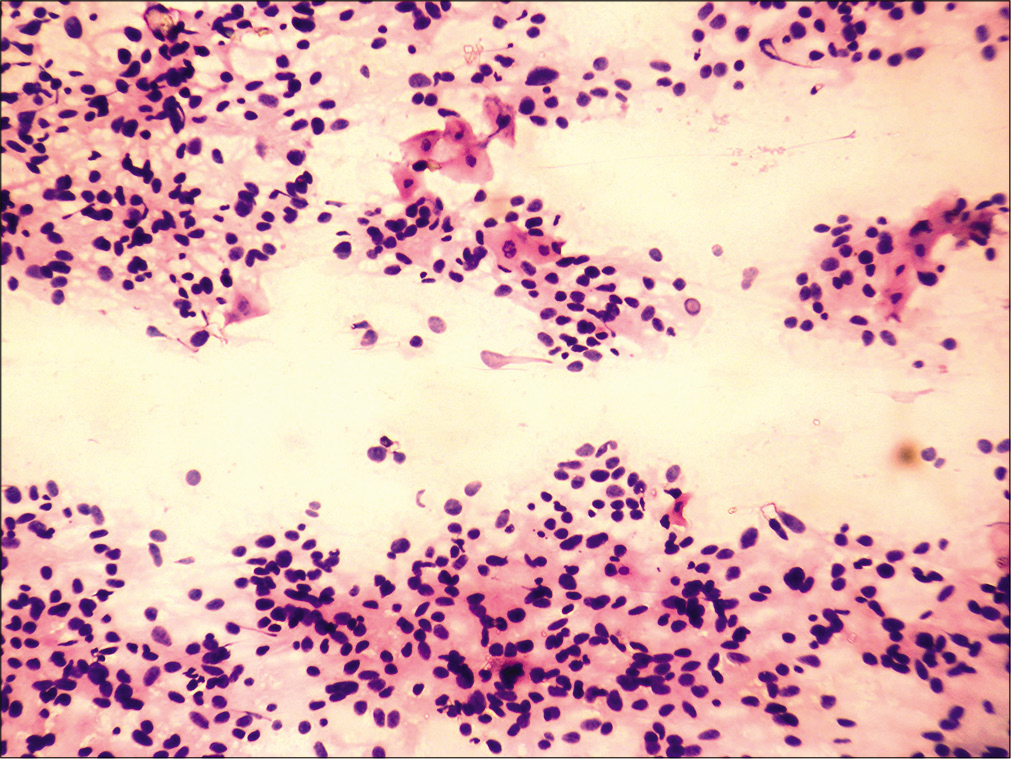
- FNA from Para pharyngeal LN mass: Tumor cells with similar morphology with few scattered mature squamous cells (Hematoxylin and eosin, ×20).
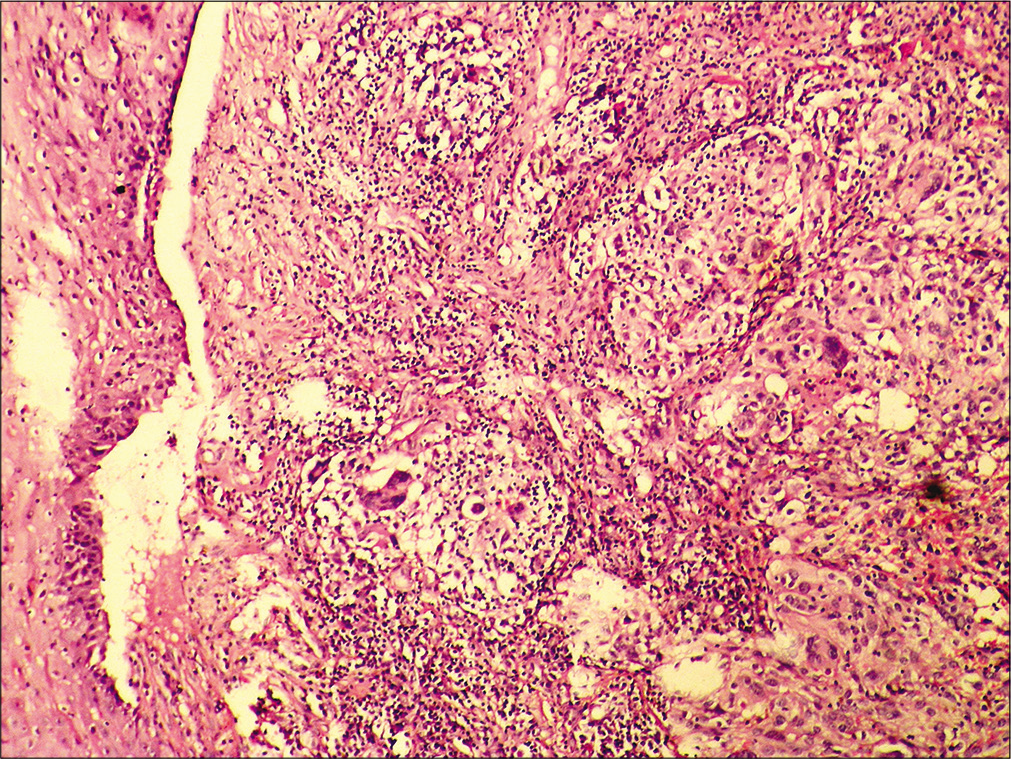
- Tonsillar mass Histopathology: Subepithelial tumor consisting of solid islands and fascicles separated by fibrous stroma having mononuclear cell infiltration. Cells are round to oval with moderate pale cytoplasm, central nuclei with vesicular chromatin, and nucleoli in some. Occasional pleomorphic cells also evident (Hematoxylin and eosin, ×20).
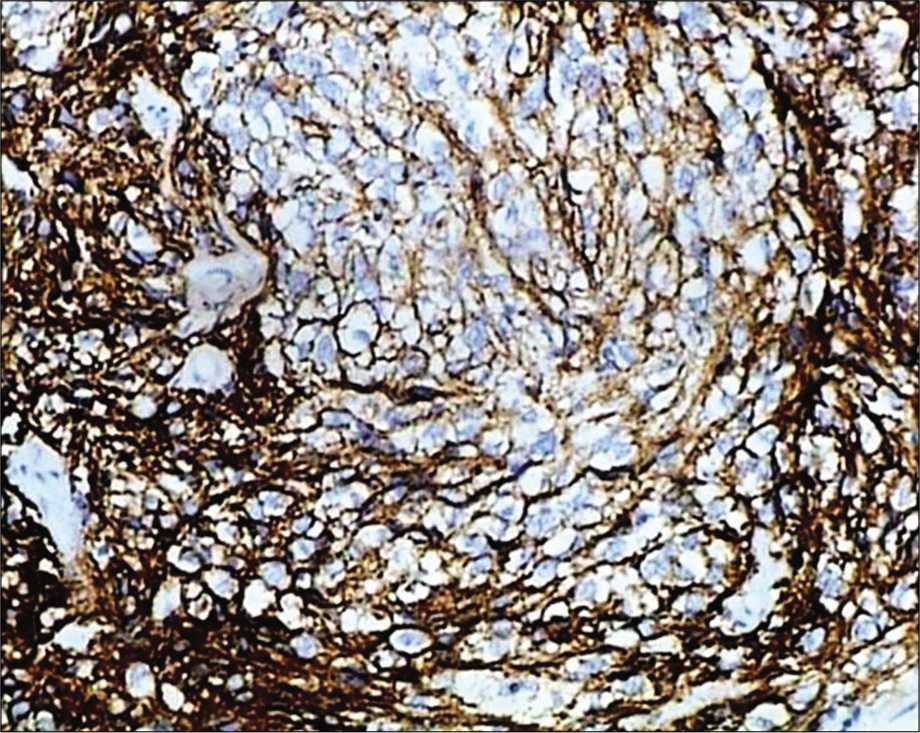
- Immunohistochemistry: Tumor cells expressing strong membranous positivity (CD21, ×20).
Brief review of the topic
FDCs also called dendritic reticulum cells form the network in germinal centers of lymph nodes. They present antigens to T lymphocytes so that memory B cells and plasma cells can be generated.[9] FDC sarcoma is a rare and under-recognized intermediate grade malignancy. Chan et al. reported recurrence in 40% while 25% metastasized and mortality rate of 16.7%.[6] It is a neoplastic proliferation of spindled to ovoid cells showing morphologic and immunophenotypic features of FDCs. Most cases occur in lymph nodes of neck, mediastinum, and axilla and often present as slow growing painless mass. Around 30% are found in extranodal sites such as palate, tonsil, pharynx, thyroid, mediastinum, soft tissue, skin, liver, spleen, and gastrointestinal tract.[7] The presentation of FDC sarcoma as a cystic swelling is a rare event.[10] Its accurate diagnosis often requires a combination of histology, immunohistochemistry (IHC), or even ultrastructural study. Although cytomorphology has been described in the literature, a pre-operative diagnosis of FDC sarcoma poses a diagnostic challenge for the cytopathologist, as most of the previously reported FNA cases were misdiagnosed as NPC, meningioma, lymphoma, or even spindle cell carcinoma depending on anatomical location of the lesion.[5] These tumors have a heterogeneous histology with spindle-shaped tumor cells that possess variable degrees of pleomorphism and are arranged in whorls, fascicular, or storiform patterns. The neoplasm displays a characteristic immunohistochemical profile, expressing CD21, CD23, and CD35 markers.[1] Adverse clinical outcome is correlated with large tumor size (>6 cm), nuclear pleomorphism, necrosis, and high mitotic count.[6,8] The line of management of FDC sarcoma is variable. If the tumor is localized to anatomical site; the choice includes excision with wide surgical margin. In cases with aggressive disease, additional radiotherapy should be considered.[8]
Summary
The high index of suspicion and knowledge about cytomorphology can allow a pre-operative recognition of FDC sarcoma. The diagnostic confirmation can further be achieved by histopathology and judicious use of IHC. Our aim is to complement the current knowledge about cytology and alert cytopathologist about this rare entity to avoid misdiagnosis.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated in this research and they have approved the final manuscript. Each author acknowledges that they have read and approved it.
ETHICS STATEMENT BY ALL AUTHORS
As this a quiz case without identifiers, our institution does not require approval from institutional ethical committee.
LIST OF ABBREVIATIONS (In alphabetic order)
CD – Cluster differentiation
CT – Computerized tomography
FDCS – Follicular dendritic cell sarcoma
FNAC – Fine-needle aspiration cytology
H and E – Hematoxylin and eosin
IHC – Immunohistochemistry
LN – Lymph node
MGG – May-Grunwald Giemsa
NPC – Nasopharyngeal carcinoma
PAP – Papanicolaou
USG – Ultrasonography.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (the authors are blinded for reviewers and vice versa) through automatic online system.
References
- Cytopathology of a primary follicular dendritic cell sarcoma of liver of the inflammatory pseudotumor-like type. Diagn Cytopathol. 2008;36:42-6.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of follicular dendritic cell sarcoma of cervical lymph node: A challenging diagnosis. J Microsc Ultrastruct. 2019;7:143-5.
- [CrossRef] [PubMed] [Google Scholar]
- Fine needle aspiration cytology of follicular dendritic cell sarcoma. A case report. Acta Cytol. 2000;44:1106-10.
- [CrossRef] [PubMed] [Google Scholar]
- Fine-needle aspiration cytology of a liver metastasis of follicular dendritic cell sarcoma. Diagn Cytopathol. 2005;32:38-43.
- [CrossRef] [PubMed] [Google Scholar]
- Cytomorphology, ultrastructural, and cytogenetic findings in follicular dendritic cell sarcoma: A case report. Acta Cytol. 2010;54:759-63.
- [Google Scholar]
- Follicular dendritic cell sarcoma, Clinicopathologic analysis of 17 cases suggesting a malignantpotential higher than currently recognized. Cancer. 1997;79:294-313.
- [CrossRef] [Google Scholar]
- Histiocytic and dendritic cell neoplasms In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Jones DM, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC; 2008. p. :354-5.
- [Google Scholar]
- Follicular dendritic cell sarcoma of neck: A case report and literature review. Auris Nasus Larynx. 2002;29:401-3.
- [CrossRef] [Google Scholar]
- Dendritic cell neoplasms: An overview. Am J Hematol. 2007;82:924-8.
- [CrossRef] [PubMed] [Google Scholar]
- Unusual presentation of follicular dendritic cell sarcoma as a cystic neck swelling. Case Rep Oncol Med. 2018;2018:4038250.
- [CrossRef] [PubMed] [Google Scholar]







