Translate this page into:
Endobronchial ultrasound-guided transbronchial needle aspiration biopsy is useful evaluating mediastinal lymphadenopathy in a cancer center
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Endobronchial ultrasound-guided tra0nsbronchial needle aspiration (EBUS-TBNA) biopsy is used to stage mediastinal lymph nodes in cancer patients to optimize treatment strategies. In this retrospective study, the authors determined the utility of EBUS-TBNA biopsy in the evaluation of mediastinal lymphadenopathy at a high-volume cancer center.
Materials and Methods:
The pathology database was searched for all patients who had undergone EBUS-TBNA biopsy of mediastinal lymph nodes over a one-year period. Cytologic diagnoses were correlated with clinical histories, subsequent resection, and clinical follow-up data.
Results:
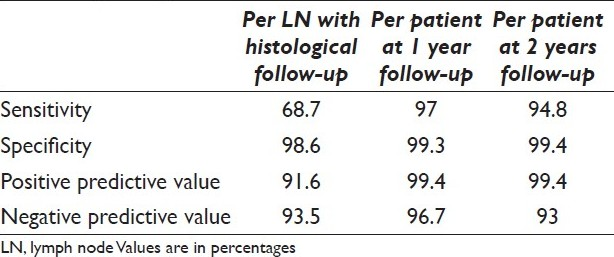
Of 928 lymph node samples, 226 (24%) were diagnosed as malignant, 4 (0.4%) were suspicious for malignancy, 9 (1%) were atypical, 640 (69%) were benign, and 47 (5%) were insufficient for evaluation. In 89 (9.6%) cases, the patients had surgical resection. There was one false positive, in which the primary tumor contained infiltrating lymphocytes, had been sampled. There were five false-negative cases, which resulted from sampling errors, including two with micrometastases. The sensitivity, specificity, and positive and negative predictive value rates for EBUS-TBNA biopsy in the evaluation of mediastinal lymph nodes were 68.7% and 98.6% and 91.6% and 93.5%, respectively on a per lymph node basis. The overall clinical sensitivity, specificity, and positive and negative predictive value rates after one year clinical/radiological and histologic follow-up were 97%, 99.3%, 96.7% and 99.4%, respectively.
Conclusions:
EBUS-TBNA biopsy is a sensitive and specific method for evaluating mediastinal lymphadenopathy in patients with lung and other primary tumors.
Keywords
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA)
lymphadenopathy
lung cancer
lymph node metastasis
mediastinum
staging
INTRODUCTION
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy is a relatively new modality for sampling mediastinal lymph nodes. An endobronchial ultrasound probe is used to visualize the target lesion and the surrounding structures and simultaneously sample the target lesion under direct sonographic guidance. This technique allows sampling of upper and lower paratracheal, retrotracheal, subcarinal, hilar, and interlobar lymph nodes[12] and can be performed under general or local anesthesia and conscious sedation in an outpatient setting.[12] Smaller sized lymph nodes (0.5–1.0 cm) can also be safely targeted by EBUS-TBNA.[3] Although mediastinoscopy is still considered the gold standard for staging mediastinal lymph nodes in lung cancer, it requires general anesthesia, is more invasive than EBUS-TBNA biopsy, and carries morbidity risks.[24] In addition to lung cancer staging, TBNA biopsy can be used for diagnosis and to evaluate other pathologic conditions of the hilar-mediastinal areas.[5]
In this retrospective study, we determined the utility of EBUS-TBNA biopsy in evaluating mediastinal lymph nodes over one year at a high-volume cancer center.
MATERIALS AND METHODS
We searched the pathology database at The University of Texas MD Anderson Cancer Center (Houston, Texas) for patients who had undergone EBUS-TBNA biopsy of mediastinal lymph nodes from November 1, 2006, to October 31, 2007. This retrospective study was approved by the institutional review board with a waiver of patient consent. The cytologic diagnoses were correlated with surgical, clinical and radiological follow-up findings within the electronic medical record database. Cytologic and histologic slides were reviewed when discrepancies existed between cytologic and surgical resection results.
All EBUS-TBNA biopsies were performed by interventional pulmonologists in the bronchoscopy suite with a team of technicians, anesthesiologists, a nurse anesthetist and a senior cytotechnologist. The procedures were performed under general anesthesia. Standard conventional flexible bronchoscopy (model BF-T160 bronchoscope; Olympus, Tokyo, Japan) was first performed to evaluate the tracheobronchial tree, and then a linear array ultrasonic bronchoscope (Olympus XBF-UC 160F) with a dedicated 22-gauge needle (NA-202C Olympus) was used to perform the ultrasonic examination and transbronchial aspiration. The regional lymph node levels of the mediastinum and hilar regions were systematically imaged and measured. All visualized lymph nodes larger than 0.5 cm were sampled using real-time ultrasonic needle guidance. When necessary, Doppler ultrasound was used to identify vessels. Each nodal level was aspirated at least twice, with immediate on-site adequacy assessment. A maximum of six passes per lymph node level was made. A senior cytotechnologist was present to smear and stain slides using both the Diff-Quik and modified Papanicolaou methods for immediate assessment to determine specimen adequacy, which was defined as the presence of lymphoid tissue or tumor. A cytopathologist was always available for assistance. It should be noted that the bronchoscopy suite was not located in close proximity to the cytology laboratory. In each case, depending on the amount of material in the rinse (RPMI-1640), a cytospin slide or a cell block was prepared. The cytologic diagnoses included negative, atypical, suspicious and positive for malignancy.
For statistical evaluation, the cytologic results were used to predict whether or not patients had mediastinal lymph node metastases, and these predictions were compared with the histological and clinical data as the gold standard. For statistical analyses, atypia and suspicious for malignancy were considered positive. We evaluated the sensitivity, specificity, positive predictive value and negative predictive value of EBUS-TBNA biopsy for cancer detection.
RESULTS
Over a one-year period, 928 EBUS-TBNA biopsy specimens had been obtained from 356 patients (170 women and 186 men; mean age, 63 years [range, 27 to 86 years]). One to six sites were performed per patient (mean, 3). The lymph node sites sampled included levels 1: Highest mediastinal (3 specimens), 2: Upper paratracheal (16 specimens), 3P: Retrotracheal (2 specimens), 4: Lower paratracheal including azygous nodes (340 specimens), 7: Subcarinal (253 specimens), 8: Paraesophageal (2 specimens), 10: Hilar (33 specimens), 11: Interlobar (269 specimens), 12: Lobar (5 specimens) and miscellaneous (3 specimens). The most common site aspirated was level 7, followed by 4R (right) and 11R.
The indications for the performance of EBUS-TBNA included mediastinal staging in patients with malignancies in 136 patients (38.2%), to rule out recurrence or metastatic disease with a prior history of malignancy in 139 patients (39%) and to rule out infection in 9 patients (2.5%). In the 72 patients (20.2%) who had no prior history of malignancy, the clinical indications were lung infiltrate/lung mass with mediastinal lymphadenopathy in 61 patients (17%), mediastinal lymphadenopathy only in 10 patients (2.8%) and lung infiltrates with pleural effusion in 1 patient (0.3%). Simultaneous targeting of a lung lesion by FNA was only performed in 2 patients; however, a lung core needle biopsy was performed in 117 patients. Of the 117 patients with core needle biopsies, 38 had diagnoses of negative for malignancy of which one was positive on repeat core biopsy.
Of the 928 lymph node aspirates, 881 were adequate and 47 were insufficient for evaluation. The cytologic diagnoses were benign in 640 (69%) cases, malignant in 228 (24%) cases, atypical in 9 (1%) cases and suspicious for malignancy in 4 (0.4%). Of the benign samples, 9 (from 4 patients) showed granulomatous inflammation [Table 1 and Figure 1]. Thirteen lymph nodes were diagnosed as either atypical or suspicious on cytology. The nine lymph nodes deemed “atypical” included 2 lymph nodes with atypical mononuclear cells in the clinical setting of Hodgkin lymphoma, 1 lymph node with atypia attributed to artifact, 5 lymph nodes atypical due to limited cells in the clinical setting of carcinoma and 1 lymph node diagnosed as atypical lymphoid population showed granulomatous inflammation with Aspergillus on resection. The 4 lymph nodes deemed “suspicious” included 3 that were suspicious for carcinoma and 1 suspicious for Hodgkin lymphoma. Of the 4 suspicious cases, only 1 was from a patient with a history of primary lung tumor.
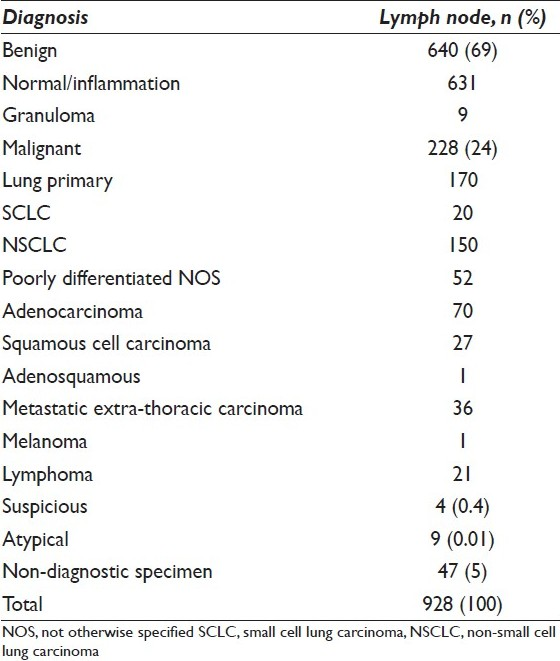
- A lymph node aspirate reveals a cluster of epithelioid histiocytes consistent with granulomatous inflammation (Papanicolaou stain)
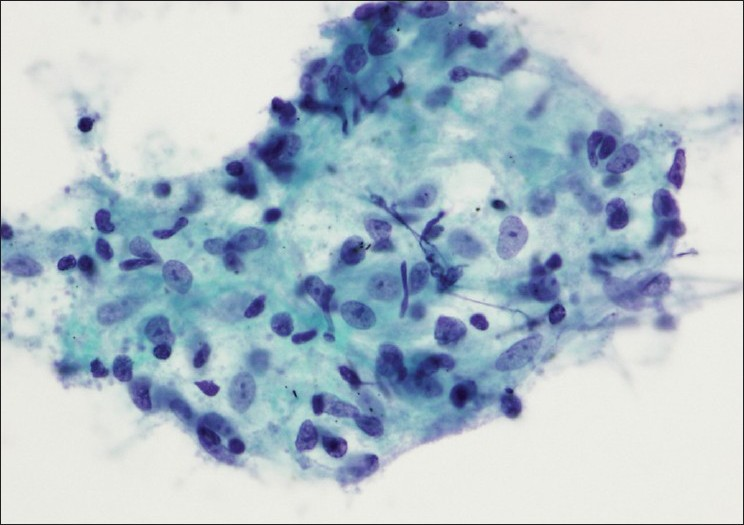
In 89 (9.6%) cases, the patients underwent subsequent surgical excision, including 80 with primary lung tumors, 5 with metastatic non-lung carcinomas or sarcomas and 4 with granulomatous inflammation. The cytologic and histologic diagnoses correlated with one another in 77 of the 89 (87%) cases and were discrepant in 12 (13%). The cytologic and histologic slides were reviewed in all discrepant cases. On initial evaluation, 2 false-positive cases were identified. In 1, abundant tumor consistent with metastatic squamous cell carcinoma was found in the aspirate of a 4R lymph node from a 54-year-old woman with bronchogenic squamous cell carcinoma. A subsequent resection 17 months later revealed no tumor; however, the lymph nodes were grossly smaller in size than initially reported at the time of EBUS-TBNA biopsy and lymphocyte depleted [Figure 2]. This patient had undergone interval treatment with carboplatin, paclitaxel and proton beam radiation therapy, with a good clinical response. She was closely followed by surveillance scans and was later found to have an enlarging irregular mass in the same site as the primary tumor, consistent with recurrence or progression of residual disease. A few mediastinal nodes, including the originally positive 4R node, appeared to be enlarged; however, subsequent EBUS-TBNA biopsies were negative or insufficient for evaluation. Two weeks after the second aspiration biopsy, she underwent lobectomy with resection of clinically suspicious mediastinal lymph nodes. The lung mass was found to be a poorly differentiated non-small cell carcinoma, but all lymph nodes (including 10 nodes in level 4R) were negative for metastatic disease. Therefore, we consider this case a true positive result, with a complete response to chemoradiation therapy.
- (a) Aspirate contains highly atypical cells and apoptotic bodies in a background of lymphocytes interpreted as metastatic carcinoma (Papanicolaou stain). (b) The resected lymph node contains granulomatous changes with lymphoid depletion and fatty replacement consistent with therapy-related changes (H and E stain)
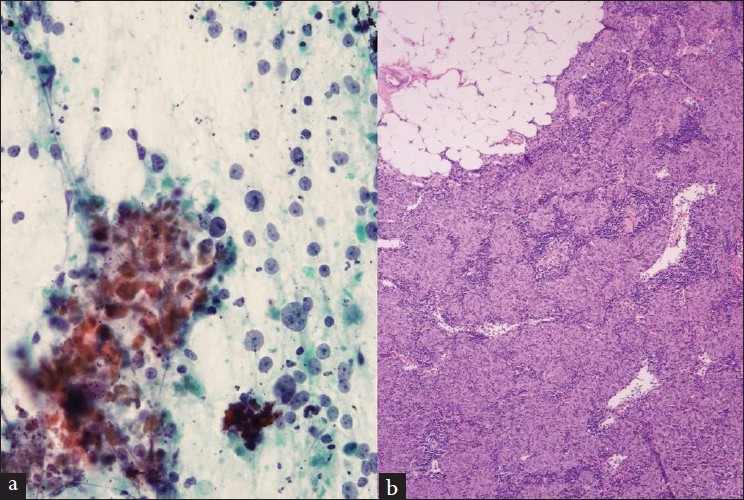
The other false-positive sample was from a 63-year-old man with bronchogenic squamous cell carcinoma. An aspirated sample from the 11L lymph node revealed numerous tumor cells admixed with lymphocytes, consistent with metastatic disease [Figure 3]. However, on surgical resection, all lymph nodes were negative for metastatic carcinoma. A histologic review revealed that the tumor was associated with an extensive lymphocytic response at the border with normal tissue. Considering the close proximity of the 11L lymph node to the hilar mass, the discrepant finding most likely represents contamination by tumor en route to the lymph node.
- (a) An aspirate from a false-positive lymph node that contains malignant cells in a background of lymphocytes (Diff-Quik stain). (b) The resected lung mass shows the malignant cells infiltrated with lymphocytes (H and E stain)
There were 5 false-negative cases in which metastatic non-small cell carcinoma was found on resection. In 2, only 1 positive lymph node was found among the 2 to 3 nodes resected. Therefore, it was difficult to determine whether the positive node had been sampled at the time of aspiration biopsy. Another case was designated level 11, with no side specified at the time of resection; however, both sides had been aspirated and were negative on cytologic evaluation. A review of the cytology slides confirmed the initial interpretation in all cases, and the corresponding histologic evaluation revealed 2 cases with only microscopic foci of metastatic disease that could have easily been missed during sampling [Figure 4]. Therefore, all the false-negatives were attributed to sampling errors. Although not a false negative, 1 case was found to contain granulomas and lymphoid tissue on resection that had been diagnosed as negative on cytology.
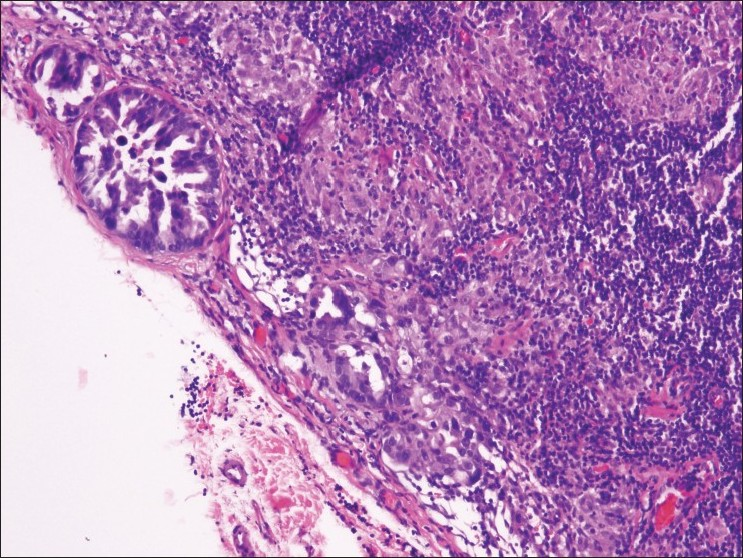
- Resected lymph node shows microscopic subcapsular foci of metastatic carcinoma that was not detected on EBUS-TBNA biopsy (H and E stain)
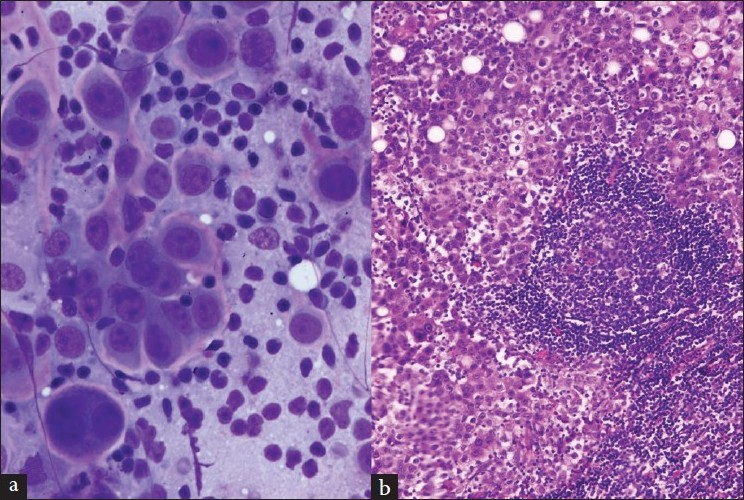
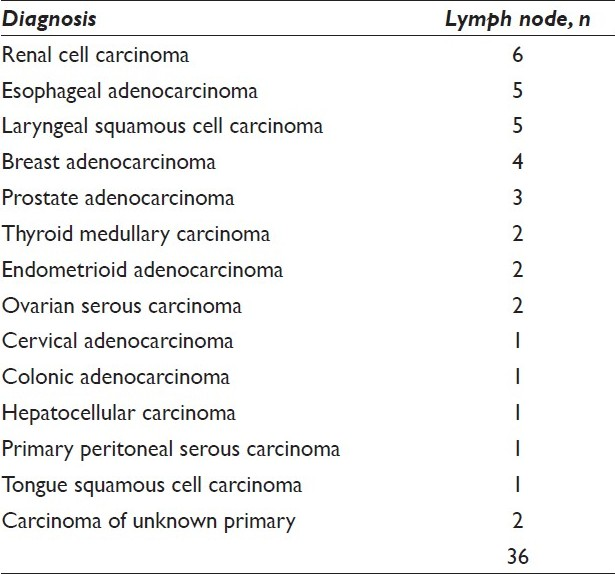
There were 36 cases [Table 2] of metastatic non-pulmonary carcinoma and 1 of metastatic melanoma [Figure 5]. Renal [Figure 6], esophageal [Figure 7], laryngeal, and breast carcinomas were the most common primary tumors that metastasized to the mediastinal lymph nodes.
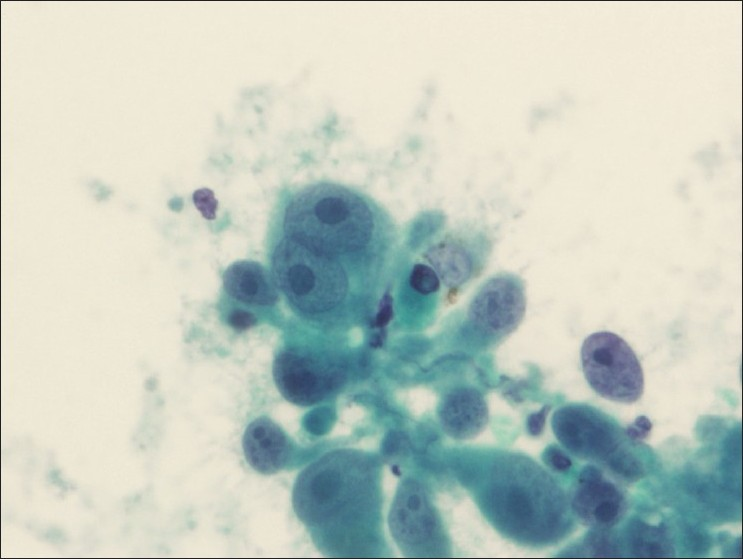
- Lymph node aspirate reveals metastatic melanoma (Papanicolaou stain)
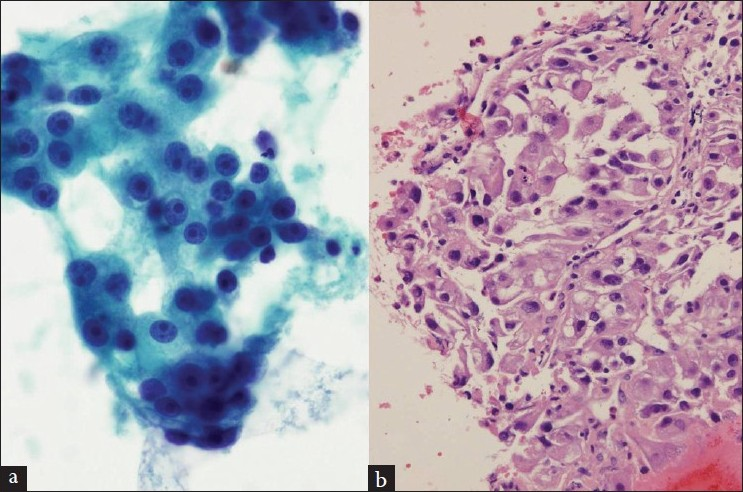
- (a and b) A lymph node aspirate, smears and cell block preparation, shows malignant cells consistent with metastasis from known renal cell carcinoma (a, Papanicolaou stain; b, H and E stain)
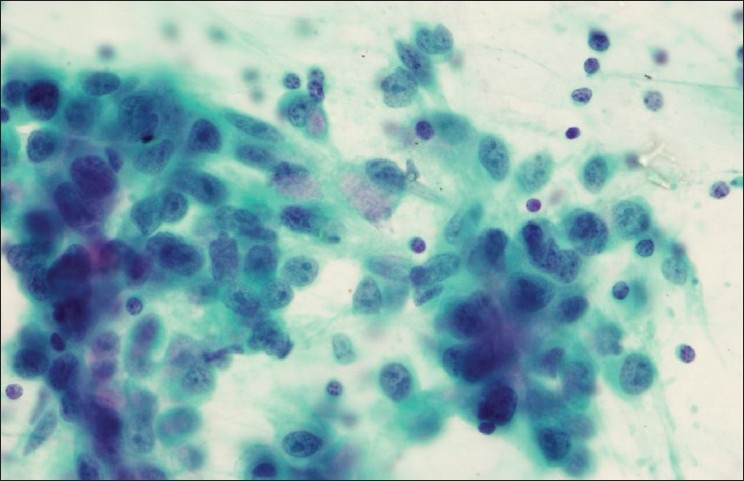
- Lymph node aspirate from a patient with a history of esophageal carcinoma reveals metastatic adenocarcinoma (Papanicolaou stain)
Lymphoma (15 non-Hodgkin and 6 Hodgkin) was diagnosed on aspirates from 21 patients (19 with a previous history of lymphoma) and subset of these have been previously reported.[6] The cytologic diagnoses of the non-Hodgkin lymphoma cases included large B-cell lymphoma (4 patients), follicular lymphoma (2 patients), small lymphocytic lymphoma (5 patients), mucosal-associated lymphoid tissue (MALT) lymphoma (1 patient), mantle cell lymphoma (1 patient), recurrent lymphoma (1 patient with a reported history of histiocytic lymphoma), and T-lymphoblastic lymphoma (1 patient). Material was submitted for immunophenotyping by flow cytometry in 8 patients and it was helpful in all cases to sub-classify the type of lymphoma. Immunomarkers were performed on cytospin and/or cell block preparations to aid the diagnosis in 5 patients.
Forty-seven (5%) aspirates were insufficient for diagnosis, containing primarily blood elements with insufficient numbers of lymphoid cells, and no germinal center aggregates or anthracotic-laden macrophages. The patients in these cases had clinical histories of poorly differentiated non-small cell carcinoma (4 cases), lung adenocarcinoma (9 cases), lung squamous cell carcinoma (11 cases), small cell carcinoma (5 cases), metastatic extrathoracic carcinoma (9 cases), adenopathy of unknown etiology or inflammatory processes (6 cases), and hematologic malignancies (3 cases). Two patients underwent subsequent surgical resections and were found to have primary lung tumors with negative lymph nodes. In addition, no tumor was present on resection in 4 patients, but one of these had granulomatous inflammation.
Cell blocks were prepared on 418 lymph node aspirates from 218 patients. The majority of cell block preparations were contributory and only 20 were non-contributory. Ancillary studies included immunostains, special stains for micro-organisms and molecular tests. A variety of immunostains were performed to subtype metastatic non-small cell carcinoma (adenocarcinoma vs. squamous carcinoma) (13 patients), confirm metastatic small cell carcinoma (7 patients), determine lymphomatous involvement (5 patients), and confirm metastases from known primaries (5 patients with colorectal carcinoma, 3 patients with breast carcinoma, and 1 patient each with prostate carcinoma, melanoma, ovarian carcinoma, and medullary thyroid carcinoma). Other ancillary studies included special stains for micro-organisms. Acid fast and Gomori methenamine silver (GMS) stains were performed in 24 patients; however, only one patient had positive results on GMS that were suggestive of histoplasmosis. An immunostain for CMV and a gram stain were negative in one patient each. Molecular studies for EGFR were performed on 2 patients.
The sensitivity and specificity rates of EBUS-TBNA biopsy on a per lymph node basis in which histologic resection was performed were 68.7% and 98.5%, respectively, and the positive and negative predictive values were 91.6% and 93.1% [Table 3]. Of the remaining cases with negative cytology on EBUS and no histologic follow-up available, 105 of the 143 cases had clinical/radiological follow-up data available at 1 year. Three of the 105 patients demonstrated progression of disease in a different mediastinal lymph node than the original FNA either by direct sampling or CT imaging. Disease progression was documented outside the mediastinum in 16 patients. After two years, no clinical/radiographic data was available in a total of 70 patients, and 75 patients remained clinically/radiologically negative for disease progression in the mediastinum. Eight of the 75 patients demonstrated disease progression outside the mediastinum and 1 patient showed metastatic disease by FNA on a different mediastinal lymph node than originally sampled. Of the 158 patients with an original positive EBUS without histological follow-up, all remained clinically/radiologically positive for mediastinal metastasis. The overall clinical sensitivity, specificity, positive predictive value and negative predictive value rate of EBUS-TBNA at 1-year follow-up were 97%, 99.3%, 96.7%, and 99.4%, respectively, and after 2 years follow-up were 94.8%, 99.4%, 99.4%, and 93%, respectively [Table 3].
DISCUSSION
Our results demonstrate that EBUS-TNA biopsy is useful for accurately staging mediastinal lymphadenopathy. Most of the procedures were performed to stage lung cancer, but other disorders were diagnosed, including metastatic carcinoma, metastatic melanoma, lymphoma, and granulomatous disease.
Accurate staging of lung carcinoma is necessary to determine the most suitable treatment plan, and during the staging process, mediastinal lymph node status is the most important factor affecting patient outcome.Even though mediastinoscopy is still considered the gold standard for sampling mediastinal lymph nodes, several limitations and disadvantages make it less than ideal. It is invasive and costly, requires general anesthesia, is associated with a low but potentially severe complication rate, and does not provide access to hilar levels.[7–10] In addition, re-mediastinoscopy can be technically difficult.[111] Although EBUS-TBNA cannot reach sub-aortic and paraesophageal lymph nodes, the addition of EUS-FNA can allow for complete evaluation of the mediastinum.[7] EBUS-TBNA biopsy has been found to be a safer, minimally invasive, and less expensive modality that can be performed under conscious sedation, with more access to hilar nodes.Moreover, it allows restaging of the mediastinum after neo-adjuvant therapy prior to surgical resection.[7]
Even though EBUS-TBNA biopsy is primarily used to evaluate mediastinal lymph nodes for staging lung cancer, it has also been reported to be useful in several other clinical settings, such as diagnosing intrapulmonary and mediastinal tumors, hilar or mediastinal adenopathies of unknown etiology such as sarcoidosis, and lymphoma.[891112] Clinical studies have shown that EBUS-TBNA biopsy has sensitivity and specificity rates of 84%–100% overall and up to 100% in lung cancer.[4713–16]
The sensitivity rate in our study was 97% on a per patient basis at 1-year follow-up and 62.5% on a per site basis with all false-negative cases representing sampling errors. In 2 cases, resection revealed only small microscopic foci of metastatic disease. This finding demonstrates that sampling errors occurred during the procedure, especially if there was partial involvement or only microscopic foci of metastatic carcinoma. In 3 of the 5 false-negative cases, the amount of lymphoid tissue was reported as “scant.” The adequacy criteria for EBUS-TBNA biopsy specimens have not been well established.[2417] Alsharif et al.[2] proposed that 40 lymphocytes per high-power field in the cellular areas of the slide or the presence of pigmented macrophage clusters are good predictors of adequate sampling. Other investigators have found these criteria difficult to apply.[4] For instance, one study showed that 50% of their samples would be rendered non-diagnostic should they apply this criteria to their study.[7] Another study suggested a lymph node aspirate would be insufficient if one of the smears did not contain an area with numerous small lymphocytes or follicle center cells.[18] At the time of this study, we did not have well-defined criteria for sampling adequacy, but in general, we required lymphoid cells that were loosely associated or in aggregates. A consensus on adequacy criteria for these specimens will help improve the false-negative rates.
The diagnostic sensitivity of EBUS-TBNA biopsy in the assessment of hilar-mediastinal lymph nodes varies greatly, according to findings reported in the medical literature, and is affected by factors such as operator experience and ability, lymph node size and location, number of aspirations performed, and the presence of rapid on-site cytologic evaluation.[19] However, some investigators report that lymph node location and size are not rate-limiting factors when performing an EBUS-TBNA biopsy, as they have no effect on the success rate of “hitting” the intended node.[20] Operator experience has been shown to improve sensitivity rates over a period of time with one study showing increased sensitivity from 44.4% after 10 cases to 94.1% after the next 46 cases, and another study showing increased sensitivity from 50% after 5 cases to 90% after 200 cases with peak diagnostic accuracy occurring after 50 cases.[1021] However, experienced bronchoscopists will also vary in their speed of learning.[22] In the year prior to this study, the pulmonologists at our institution performed EBUS-TBNA on approximately 60 patients each,[12] and therefore, they were experienced in performing this procedure.
Despite its variable sensitivity, there is uniform agreement that EBUS-TBNA biopsy has high specificity, approaching 100%, with rare cases of false positives.[2451719] Of the 2 false-positive cases in our study, one most likely represented a complete treatment effect (delayed resection after chemoradiation therapy); therefore, it is not considered a true false positive. The second case was probably a result of passing through tumor en route to the target lymph node. This scenario (passing through metaplastic or dysplastic mucosa, resulting in an atypical or even false-positive diagnosis) is an already reported pitfall of EBUS-TBNA biopsy.[22324] Other potential sources of false-positive results include atypical bronchial epithelium, reserve cell hyperplasia, activated enlarged lymphocytes and epithelioid histiocytes, and crushed lymphocytes that mimic small cell carcinoma.[27]
EBUS-TBNA biopsy was useful for diagnosing metastatic carcinomas of non-pulmonary origin as well as lymphomas in our study. The most common metastatic tumors in this series were renal cell carcinoma, esophageal adenocarcinoma, and laryngeal squamous cell carcinoma. Lymphoma cases comprised 2% of cases. With the use of on-site evaluation, specimens can be triaged for ancillary studies such as flow cytometry and immunocytochemical analyses and this technique has been reported to be a safe, minimally invasive, highly accurate alternative to more invasive procedures such as mediastinoscopy for excisional biopsy.[62526] High sensitivity rates, similar to those obtained with mediastinoscopy, have been reported, particularly for non-Hodgkin lymphoma.[12]
Of the 9 granuloma cases in this series, all of which were non-caseating, only one case showed microorganisms on special stains. In the appropriate clinical setting, the other cases could represent sarcoidosis; however, this is not a cytologic diagnosis. Sarcoidosis is diagnosed only if the clinical-radiologic findings are supported by cytohistologic findings, along with appropriate exclusion of other causes of granulomatous inflammation, such as clinical history, follow-up, a combination of negative tissue staining for acid-fast bacilli and fungal organisms, negative fungal and mycobacterial cultures, and serum fungal antibody titers.[112728] The most common sarcoidosis presentation is hilar and mediastinal lymphadenopathy, and up to 90% of patients have evidence of hilar lymph node enlargement on chest radiography.Mediastinoscopy has a high diagnostic rate; however, some lymph nodes (e.g. perihilar nodes, which are typical of stage I and II disease) are inaccessible.[28] EBUS-TBNA biopsy sensitivity rates as high as 93% have been reported in patients with suspected disease.[927–29]
Previous studies have found non-diagnostic rates of 4% to 23% for EBUS-TBNA.[430] Non-diagnostic rates have also been reported to be lower in EBUS-TBNA (8.7%) when compared to conventional TBNA (28.3%) in one study.[30] In our study, only 5% of specimens were insufficient for diagnosis, which is among the lowest rates reported in the medical literature.[25] The presence of an on-site cytopathologist has been reported to reduce the rate of non-diagnostic samples and improve sensitivity.[24] In addition, on-site evaluation allows for proper triaging of specimens and requesting further sampling when additional material is needed for ancillary studies, such as cell block and flow cytometry analyses.[4] Likewise, at our institution, the low non-diagnostic rate is largely attributed to immediate assessment. Although diagnostic pitfalls can occur with rapid on-site cytologic evaluation including misinterpretation of bronchial epithelial cells as malignant cells, cells with discohesive cells or bland neoplasms as benign, and mucinous metaplasia of goblet cells as metastatic signet ring carcinoma, awareness of these diagnostic dilemmas can avoid confusion at the time of immediate assessment.[3132] Overall, despite these drawbacks, on-site immediate assessment seems to significantly improve diagnostic yield, especially for clinicians who are at the beginning of their learning curve for EBUS procedures.[31]
In summary, our findings confirm that EBUS-TBNA biopsy is highly sensitive and specific for diagnosing mediastinal lymph nodes and illustrate factors that contribute to false-positive results, such as sampling primary tumors that contain lymphocyte infiltrates. Therefore, the cytology results should be correlated with the clinical and radiographic findings.
COMPETING INTERESTS
The author(s) declare that they do not have competing commercial interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and reviewers are blinded for authors) through automatic online system.
The authors thank Cheryl Conner, Bertina Gaines, and Christopher Castro for their administrative assistance and Ann Sutton for her editorial assistance.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/10/82022
REFERENCES
- Staging and diagnosis of non-small cell lung cancer: Invasive modalities. 2007. Respirology. 12:173-83. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17298448
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial fine-needle aspiration: The University of Minnesota experience, with emphasis on usefulness, adequacy assessment, and diagnostic difficulties. 2008. Am J Clin Pathol. 130:434-43. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=18701418
- [Google Scholar]
- EBUS-TBNA: An opportunity for clinicians, cytopathologists and patients to gain from multidisciplinary collaboration. 2010. Cytopathology. 21:3-5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20070790
- [Google Scholar]
- Cytology of endobronchial ultrasound-guided transbronchial needle aspiration: A retrospective study with histology correlation. 2009. Cancer Cytopathol. 117:482-90. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=19834962
- [Google Scholar]
- Transbronchial and transoesophageal (ultrasound-guided) needle aspirations for the analysis of mediastinal lesions. 2006. Eur Respir J. 28:1264-75. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17138681
- [Google Scholar]
- The utility of endobronchial ultrasound-guided transbronchial needle aspiration biopsy in the diagnosis of mediastinal lymphoproliferative disorders. 2011. Cancer Cytopathol. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=21308997
- [Google Scholar]
- The diagnostic value of endobronchial ultrasound-guided needle biopsy in lung cancer and mediastinal adenopathy. 2010. Diagn Cytopathol. 38:337-42. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=19890836
- [Google Scholar]
- Role of endobronchial ultrasound-guided transbronchial needle aspiration in the management of lung cancer. 2008. Gen Thorac Cardiovasc Surg. 56:268-76. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=18563521
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA): Applications in chest disease. 2010. Respirology. 15:71-9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=19895387
- [Google Scholar]
- Endobronchial ultrasound-guided fine-needle aspiration of mediastinal lymph nodes: A single institution's early learning curve. 2008. Ann Thorac Surg. 86:1104-9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=18805141
- [Google Scholar]
- Clinical implications of granulomatous inflammation detected by endobronchial ultrasound transbronchial needle aspiration in patients with suspected cancer recurrence in the mediastinum. 2008. J Cardiothorac Surg. 3:8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=18298864
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration in the diagnosis of lymphoma. 2008. Thorax. 63:360-5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17965071
- [Google Scholar]
- The value of mediastinal staging with endobronchial ultrasound-guided transbronchial needle aspiration in patients with lung cancer. 2009. Eur J Cardiothorac Surg. 36:465-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=19502074
- [Google Scholar]
- Evaluation of mediastinal lymph nodes with endobronchial ultrasound: The thoracic surgeon's perspective. 2010. J Thorac Cardiovasc Surg. 139:578-82. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20176204
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration for mediastinal lymph node staging in non-small cell lung cancer. 2008. Semin Thorac Cardiovasc Surg. 20:274-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=19251164
- [Google Scholar]
- Cytologic accuracy of samples obtained by endobronchial ultrasound-guided transbronchial needle aspiration at Thomas Jefferson University Hospital. 2008. Acta Cytol. 52:687-90. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=19068672
- [Google Scholar]
- Cytopathologic diagnoses of fine-needle aspirations from endoscopic ultrasound of the mediastinum: Reproducibility of the diagnoses and representativeness of aspirates from lymph nodes. 2007. Cancer. 111:234-41. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17570515
- [Google Scholar]
- Analysis of cytological specimens from mediastinal lesions obtained by endoscopic ultrasound-guided fine-needle aspiration. 2006. Cancer. 108:206-11. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=16752408
- [Google Scholar]
- Evolving role of interventional pulmonology in the interdisciplinary approach to the staging and management of lung cancer: bronchoscopic mediastinal staging of lung cancer. 2006. Clin Lung Cancer. 8:110-5. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17026811
- [Google Scholar]
- Ultrasound-guided transbronchial needle aspiration: An experience in 242 patients. 2003. Chest. 123:604. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=12576386
- [Google Scholar]
- Bronchoscopic evaluation of the mediastinum using endobronchial ultrasound - A description of the first 216 cases performed at an Australian tertiary hospital. Intern Med J 2009 Available from
- [Google Scholar]
- Learning curves for endobronchial ultrasound using cusum analysis. Thorax. 65:534-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20522852
- [Google Scholar]
- False-positive interpretations in respiratory cytopathology: Exemplary cases and literature review. 2008. Diagn Cytopathol. 36:13-9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=18064688
- [Google Scholar]
- Cytological pitfalls in bronchopulmonary tumors. 1997. Diagn Cytopathol. 17:412-6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=9407200
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration cytology: A state of the art review. 2010. Cytopathology. 21:6-26. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20015257
- [Google Scholar]
- Endobronchial ultrasound-guided transbronchial needle aspiration for the evaluation of suspected lymphoma. 2010. J Thorac Oncol. 5:804-9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20521347
- [Google Scholar]
- Endobronchial ultrasound-guided miniforceps biopsy in the biopsy of subcarinal masses in patients with low likelihood of non-small cell lung cancer. 2008. Ann Thorac Surg. 85:1874-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=18498786
- [Google Scholar]
- Endobronchial ultrasound: New insight for the diagnosis of sarcoidosis. 2007. Eur Respir J. 29:1182-6. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17331972
- [Google Scholar]
- Real-time endobronchial ultrasound-guided transbronchial needle aspiration is useful for diagnosing sarcoidosis. 2007. Respirology. 12:863-8. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=17986115
- [Google Scholar]
- Cytology of endobronchial ultrasound-guided transbronchial needle aspiration versus conventional transbronchial needle aspiration. 2010. Cancer Cytopathol. 118:278-86. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20740503
- [Google Scholar]
- The role of endobronchial ultrasound guided transbronchial needle aspiration cytology in the investigation of mediastinal lymphadenopathy and masses, the North Tees experience. J Clin Pathol. 63:445-51. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20299387
- [Google Scholar]
- Diagnostic difficulties and pitfalls in rapid on-site evaluation of endobronchial ultrasound guided fine needle aspiration. 2010. Cytojournal. 7:9. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieveanddb=PubMedanddopt=Citationandlist_uids=20607094
- [Google Scholar]








