Translate this page into:
Endoscopic ultrasound-guided fine needle aspiration of the celiac ganglion: A diagnostic pitfall
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Endoscopic ultrasound guided fine-needle aspiration (EUS-FNA) is now widely used as a primary tool in the evaluation of lymphadenopathy in both the mediastinum and abdomen. A sympathetic ganglion may be mistaken for an enlarged lymph node on endoscopic ultrasound and are rarely sampled as such. A 51-year-old female presented with a history of weight loss, vomiting for several months, and right upper quadrant discomfort. Computed tomography (CT) and magnetic resonance imaging (MRI) scans showed a dilated common bile duct (CBD) with a possible periampullary mass, paraaortic, and pericelial lymph nodes suspicious for metastatic disease. Endosonography revealed a 17 mm oval hypoechoic structure with distinct margins in the para-aortic, celiac axis region suggestive of an enlarged lymph node. An EUS-FNA was done. Cytology revealed ganglion cells with large oval epithelial-like cells with round nuclei and prominent nucleoli consistent with a benign sympathetic ganglion. It is crucial for the cytopathologist to be aware of the fact that the endoscopist might have sampled a celiac ganglion instead of a celiac lymph node and be able to distinguish the cytological features of a benign sympathetic ganglion from a malignant process.
Keywords
Celiac ganglion
endoscopic ultrasound
fine needle aspiration
INTRODUCTION
Endoscopic ultrasonography (EUS) uses the technology of endoscopy to introduce high-frequency ultrasound probes that allows for visualizations of the gastrointestinal wall and its adjacent structures. fine-needle aspiration (FNA) is a safe intervention in patients undergoing EUS that allows for biopsy of tissues and lymph nodes.[1] Endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has been widely used as a primary diagnostic modality in examining patients with pancreatic, esophageal, pulmonary, colorectal, and biliary tumor pathologies.[2–6] In patients with suspected metastatic disease to the lymph nodes, the physician will often perform a EUS-FNA with cytologic evaluation to look for the presence of malignant cells in the node. The problem arises in the sampling of the celiac lymph nodes, because the nodes are located anatomically in the same region as the celiac ganglia[7] and can be confused as such by the endoscopist. The celiac ganglia can be visualized by EUS in most patients who undergo upper gastro-intestinal EUS examinations and can usually be differentiated from lymph nodes on the basis of their endosonographic appearance alone.[8] However, it has been reported that the celiac ganglia can vary considerably in size and location.[9] In patients with anatomical variations, the celiac lymph nodes may be confused with a celiac ganglion endoscopically. Many times, cytologic evaluation can be used as a confirmatory test to determine if the sample is in fact from the celiac ganglia.[81011] We report a case of a woman with dilated common bile duct with possible periampullary mass who underwent a EUS-FNA of her celiac lymph nodes as a diagnostic and staging procedure. The cytomorphology and clinicopathologic findings in this case are described.
CASE REPORT
This case report presents a 51-year-old female with no significant past medical history with complains of weight loss, vomiting, and right upper quadrant discomfort for several months. Laboratory tests showed elevated liver enzymes with a Alanine transaminase (ALT) level of 47 U/L, aspartate aminotransferase (AST) level of 47 U/L and an alkaline phosphatase level of 274 U/L. The patient's CA-19-9 level was also elevated at 105 U/mL. Computer tomography (CT) of her abdomen and pelvis revealed biliary dilatation with a small soft tissue bulge at the ampulla. Magnetic resonance imaging (MRI) of abdomen revealed a common bile duct dilatation with narrowing just proximal to the ampulla suggestive of malignancy. It also showed local adenopathy of the para-aortic and para-celiac lymph nodes suspicious for metastasis. EUS-FNA was performed to obtain a sample of the patient's celiac lymph node for evaluation of possible metastatic tumor.
Endoscopic findings
A 17 mm oval, hypoechoic structure with distinct margins was seen in the para-aortic, celiac axis region. Color Doppler was used to ensure absence of major vessels. A 22 G needle was used to make two passes, followed by four passes made with a 25 G needle without suction. An attending cytopathologist was present during the procedure. The aspirate revealed 0.9 cc's of sanguinous material.
Cytologic findings
The cytological features of the FNA biopsy in this patient are presented in Figures 1 and 2. The cytologic examination revealed paucicellular smears consisting of few clusters of large cells with abundant dense granular cytoplasm and round nuclei with prominent nucleoli. At a first glance, this was very alarming because they resembled malignant cells metastatic to the celiac lymph nodes. However, no lymphoid cells were identified and there was no evidence of inflammation, edema, or fibrosis. Some of these cells were seen in association with spindled stroma. These findings are representative of a benign celiac ganglion rather than a malignant metastatic lymph node.
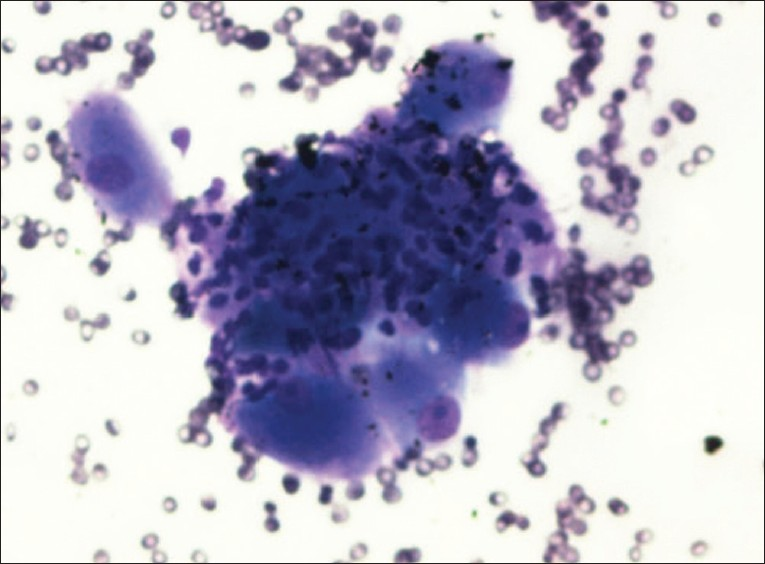
- EUS-Guided FNA of celiac ganglion, diff-quik (40×): Shows many ganglion cells with intervening stroma
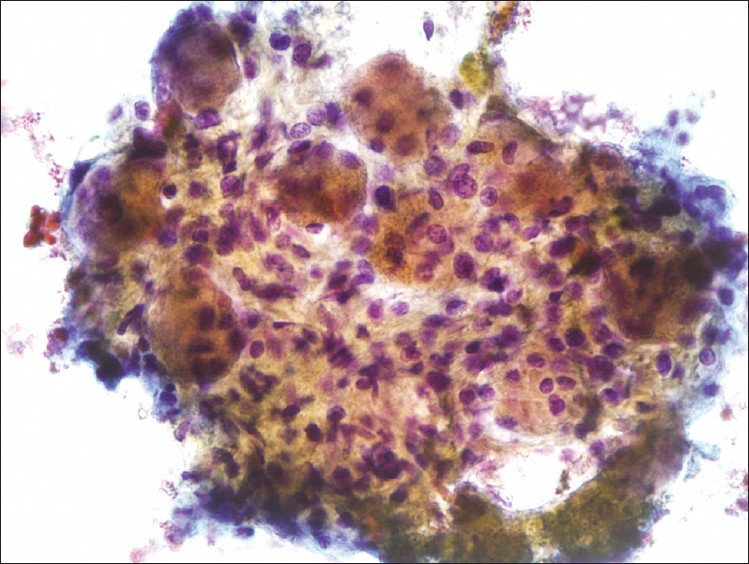
- EUS-Guided FNA of celiac ganglion, pap. (40×): Shows numerous large ganglion cells occurring in association with a spindled stroma
DISCUSSION
The innervations of many abdominal structures such as the pancreas, liver, and bile ducts are via sympathetic fibers originating from T7 through T10. The sympathetic fibers pass through celiac ganglia before giving off postganglionic fibers that innervates abdominal structures.[12] Clinically, the celiac ganglia play a major role in pain management for patients with chronic pancreatitis and pancreatic cancer.[13] EUS visualization of the celiac ganglia has been reported in literature mainly for the purpose of neurolysis as therapeutic pain control in these cases.[14–17]
Studies have shown that the celiac ganglia can be differentiated from the celiac lymph nodes via endoscopy in a majority of patients.[811] The celiac ganglia characteristically appear as almond-shaped structures with irregular margins, containing echo-rich foci.[8] They are usually located lateral to the celiac artery between the celiac artery and the left adrenal and posterior to the celiac lymph nodes.[8] However, it has been reported that there are many variations in the location and size of the celiac ganglia that can lead to confusions when obtaining a EUS-FNA sample.[9] These same studies conclude that in some cases, the differentiation of ganglia from celiac nodes can be difficult when based on sonographic appearances alone.[811]
One of the key clinical feature that helps when determining if the sample is from a node or a ganglion is the presence of transient pain, which begins immediately when suction is applied to the ganglion. The pain often will completely resolve after the FNA.[10] Therefore, it is important for the endoscopist to note any increases in discomfort with the introduction of the needle. In addition, when sampling a ganglion, the endoscopist will generally obtain scant aspirates that contain little to no grossly visible blood.[10] However, even with these features, cytologic examination is often required as a confirmatory test to assess the source of the sample.
Cytologically, the FNA findings are striking. The presence of large cells with prominent nucleoli can be mistaken for malignant cells representing metastasis to the celiac lymph node. This is more so when the clinical scenario requires to rule out a metastatic lesion, as in our patient. However, it must be noted that the sample had no lymphoid tissue, no evidence of inflammation, or fibrosis. There were no features that would point toward a malignant process including necrosis, mitotic figures, and nuclear pleomorphism. The typical cytologic features seen in a celiac ganglion FNA are large ganglion cells that are composed of abundant granular cytoplasm with regular nuclei and prominent nucleoli occurring within and in close association with a spindled stroma.[10] Lipofuscin pigment may be seen in the cytoplasm. If material is present in a cell block, a positive immunohistochemical stain for S-100 is useful. The FNA sample from our patient exhibited all of these cytologic features and is shown in Figures 1 and 2.
Ganglioneuromas and ganglioncytic paragangliomas are rare tumors that morphologically may resemble a celiac ganglion. However, the clinical presentation is different. Ganglioneuromas present as large mediastinal or retropertoneal masses in older children or young adults.[18] Ganglioncytic paragangliomas arises almost exclusively in the second portion of the duodenum presenting as a sub mucosal mass.[19]
In conclusion, in patients with suspected metastatic disease involving the abdominal lymph nodes, an EUS-FNA may be performed for diagnostic and staging purposes. In a majority of patients, the procedure will lead to a sampling of lymphoid tissue. We report a rare case of inadvertent sampling, of the celiac ganglion instead of the celiac lymph node. It is critical for the cytopathologist to recognize the sampled tissue is from a normal anatomical structure and to distinguish the cytological features of a benign sympathetic ganglion from a malignant process to avoid a diagnostic pitfall.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE. All authors are responsible for the conception of this study, participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a single case report without patient identifiers, approval from the Institutional Review Board (IRB) is not required at our institution.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/24/103025
REFERENCES
- Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73:283-90.
- [Google Scholar]
- Pancreatic cancer: Diagnosis and endoscopic staging. Eur Rev Med Pharmacol Sci. 2010;14:375-85.
- [Google Scholar]
- Staging and restaging of advanced esophageal cancer. Curr Opin Gastroenterol. 2008;24:530-4.
- [Google Scholar]
- Endoscopic ultrasound and staging of non-small cell lung cancer. Minerva Med. 2007;98:323-30.
- [Google Scholar]
- Utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of colorectal carcinoma. Diagn Cytopathol 2011 Sep 19 doi: 10.1002/dc.21804. [PMID: 21932358]
- [Google Scholar]
- Endoscopic ultrasound-guided fine-needle aspiration biopsy in the evaluation of bile duct strictures and gallbladder masses: A systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2011;23:113-20.
- [Google Scholar]
- Autonomic Nerves and Ganglia of Abdomen. In: Netter FH, ed. Atlas of Human Anatomy (4th edition). Philadelphia: Saunders Elsevier; 2006. p. :318-325.
- [Google Scholar]
- Frequency of visualization of presumed celiac ganglia by endoscopic ultrasound. Endoscopy. 2007;39:620-4.
- [Google Scholar]
- The celiac ganglia in man: Normal anatomic variations. Anesth Analg. 1979;58:461-5.
- [Google Scholar]
- Neural ganglia visualized by endoscopic ultrasound. Am J Gastroenterol. 2006;101:1787-91.
- [Google Scholar]
- EUS characteristics of celiac ganglia with cytologic and histologic confirmation. Gastrointest Endosc. 2006;64:35-9.
- [Google Scholar]
- Townsend. In: Sabiston Textbook of Surgery: The Biological Basis of Modern Surgical Practice. Philadelphia, PA: Elsevier Saunders; 2012. Internet Resource
- [Google Scholar]
- A review of the thoracic splanchnic nerves and celiac ganglia. Clin Anat. 2010;23:512-22.
- [Google Scholar]
- Endoscopic ultrasound-guided neurolysis in pancreatic cancer. Pancreatology. 2011;11(Suppl 2):52-8.
- [Google Scholar]
- Therapeutic endoscopic ultrasound for biliary and pancreatic disorders. Curr Gastroenterol Rep. 2010;12:141-9.
- [Google Scholar]
- EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: A meta-analysis and systematic review. In: Dig Dis Sci. Vol 54. 2009. p. :2330-7.
- [Google Scholar]
- EUS visualization and direct celiac ganglia neurolysis predicts better pain relief in patients with pancreatic malignancy. Gastrointest Endosc. 2011;73:267-74.
- [Google Scholar]
- The cytologic diagnosis of gangliocytic paraganglioma: A case report. Diagn Cytopathol 2011 Nov 18 doi: 10.1002/dc.21858
- [Google Scholar]








