Translate this page into:
Etiologic factors related to unsatisfactory ThinPrep® cervical cytology: Evaluation and potential solutions to improve
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution NonCommercial ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
In cervical cytology, the unsatisfactory rates for ThinPrep (TP) are slightly higher compared to SurePath. We examined various causes and explored potential for resolution of this discrepancy.
Materials and Methods:
Totally, 19,422 cases were reviewed and 1000 unsatisfactory specimens were selected and analyzed. 531 specimens were available for wash protocol. Out of 114 unsatisfactory specimens associated with atrophic cellular changes (ACC), 48 were resubmitted by provider and reevaluated.
Results:
Lubricant and lubricant-like debris/contamination (LUBE) was the most common cause of unsatisfactory specimens (68%; 681/1000) followed by blood (7.5%); ACC only (without other interfering factors) (2.4%); inflammation (3.0%); and combinations thereof (1.9%). 11.5% showed scant cellularity without an identifiable cause. 3.3% were virtually acellular. Wash protocol improved cellularity in 48% (256/531) of cases. However, only 29% (73/256) of those were satisfactory (with more than 5000 cells). Quantitative reduction in LUBE after wash protocol varied with different morphological subtypes. Interpretation patterns on satisfactory specimens after wash protocol were comparable to the results on selected cohort of specimens during the same study period. Out of 114 ACC, wash protocol was performed on 68 ACC specimens leading to satisfactory TP in 24% (16/68). Totally, 48 cases reported as unsatisfactory with ACC, were resubmitted by the providers between 2 weeks and 2 years. 44 (92%) showed increased cellularity, out of which 52% (23/44) did not show ACC.
Conclusion:
LUBE was the most common cause of unsatisfactory TP in addition to interference by blood and association with atrophic changes. Knowing the morphological spectrum of LUBE would help to identify it as the cause of unsatisfactory TP. Communicating the cause of unsatisfactory TP such as LUBE, ACC, and blood would hint the provider to take appropriate precaution during submission of the repeat specimen, leading to improved patient care.
Keywords
Cervical cancer
cytology
liquid based cytology
lubricant
Papanicolaou smear
ThinPrep
wash protocol
unsatisfactory
INTRODUCTION
ThinPrep® (Hologic Inc., Bedford, MA) is one of the liquid based cytology (LBC) methods of Papanicolaou (Pap) testing for early detection of cervical cancer and its precursor lesions. For a conventional Pap test, the collected cervical cytology specimen is directly smeared on a glass slide, wet-fixed in 95% ethanol, and then stained with Pap stain for cytomorphologic evaluation. In contrast, the ThinPrep® Pap (TP) test sample is collected and placed in a weak fixative medium in a collection vial and sent to a cytopathology laboratory for further processing. A few clinical advantages of LBC include prevention of air-drying artifact (which is not uncommon with conventional smears) and preservation of the residual specimen (for ancillary reflex tests such as human papillomavirus [HPV] testing) without the inconvenience (for the patient and the clinician) of collecting a new specimen.
Unsatisfactory rates for TP are lower than conventional Pap smears (1–5.9%); however, rates are relatively higher (1.1–3.4%) compared to other LBC method such as SurePath (0.3–1.3%).[123456] In this prospective study, we examined various causes of unsatisfactory TP specimens. In our tertiary care university hospital, we have a significant community outreach component and experience periodic surges in the unsatisfactory rates. We also evaluated possible approaches to overcome these factors and improve the unsatisfactory rate.[123]
MATERIALS AND METHODS
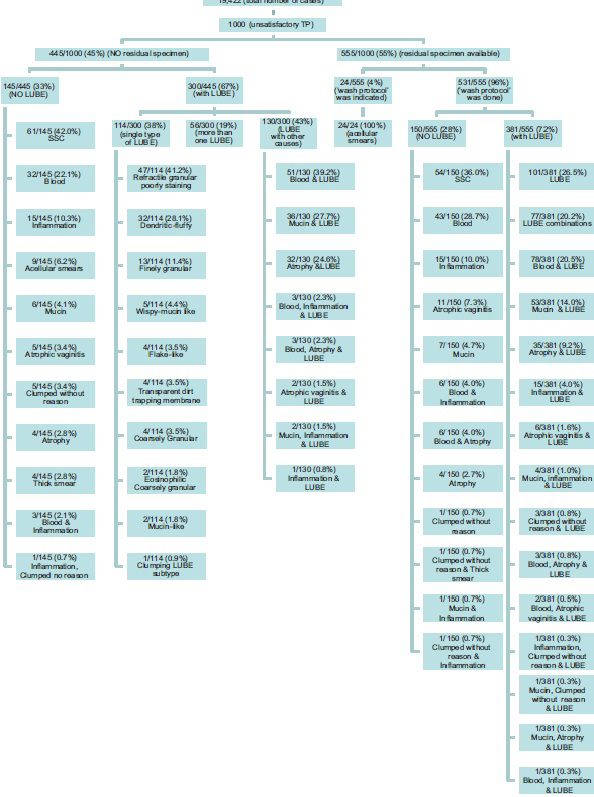
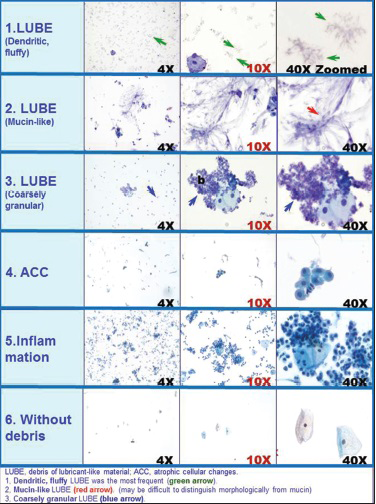
We analyzed 19,422 TP specimens from women 12 through 86 years of age (with the mean age of 51 years) over a 5½ months period after IRB approval. By strict adherence to the “2001 Bethesda System” criteria, 1000 TP were deemed inadequate for interpretation. This yields relatively higher unsatisfactory rate of 5.1%.[7] The cases were analyzed to identify the potential causes of the unsatisfactory interpretations [Figure 1] and assessed for the amount of residual specimen. Of these 1000, there were enough residual TP specimens in 531 cases. A variety of factors such as utilization of specimen for reflex HPV testing, processing for repeat preparation, or other logistics were responsible for the nonavailability of residual TP specimen in 469 cases. These 531 specimens (which included 381 with debris such as lubricant and lubricant-like debris/contamination (LUBE) [Figure 2] and 150 others) were reprocessed with wash protocol mentioned below.[8]
- ThinPrep® specimen distribution
- Common etiologic factors for unsatisfactory ThinPrep
All specimens were anonymized and after wash protocol (see below) [Figure 3], all repeat TP were examined by two cytotechnologists with final review by two or more cytopathologists. The cellularity and amount of residual LUBE were evaluated.
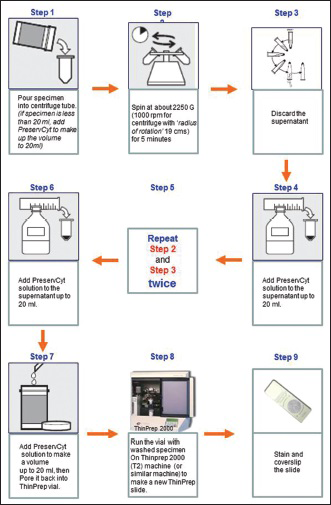
- Wash protocol for preparation of repeat ThinPrep slide to improve cellularity (see also video at: https://www.youtube.com/watch?v = nFvqz9BGfXc)
Out of 114 specimens initially reported as unsatisfactory with scant cellularity in association with atrophic cellular changes (ACC) including atrophic vaginitis (mostly reported with a note to repeat after topical or systemic estrogen therapy to reverse ACC), 48 were repeated by resubmitting new specimens within a time period ranging from 2 weeks to 2 years (average of 5 months). These resubmitted TP specimens were also analyzed.
Multiple wash protocols were evaluated to test efficiency in overcoming interference related to LUBE. The simplest protocol achieving the best outcome (increased cellularity of the repeated TP) was finalized.[8] This simple protocol [Figure 3] is as follows:
Protocol [Figure 3]
-
Pour specimen in centrifuge tube and spin at about 2250 G force/relative centrifugal force (G) (1000 revolutions per minute on centrifuge with a radius of rotation 19 cm) for 5 min.[9] (If the amount of specimen is <20 ml, add PreservCyt® to make it to 20 ml, mix, and centrifuge)
-
Discard the supernatant
-
Add PreservCyt® to make it to 20 ml, mix, and centrifuge at about 2250 G for 5 min
-
Discard the supernatant
-
Repeat the previous step (add 20 ml PreservCyt®, mix and centrifuge at about 2250 G for 5 min) one more time
-
Discard the supernatant and reconstitute specimen with PreservCyt® to make it to 20 ml
-
Run it on TP machine to prepare the smear and stain with Pap stain
-
Save the residual in the TP vial for elective tests including reflex HPV testing as indicated.
(For a brief video demonstration see https://www.youtube.com/watch?v = nFvqz9BGfXc).[8]
RESULTS
LUBE related scant cellularity was the most common cause (68%; 681 out of 1000) of unsatisfactory interpretations. The remaining 32% of causes were: Blood (7.5%), ACC (2.4%), inflammation (3.0%), mucin (1.3%), clumping without other cause (0.6%), thick smear (0.4%), and combination of any of these causes (1.9%). Thirty-three (3.3%) specimens were virtually acellular. 11.4% of specimens showed scant cellularity without any identifiable associated factors (suggesting insufficient sampling at the time of collection) [Figure 1 and Table 1].
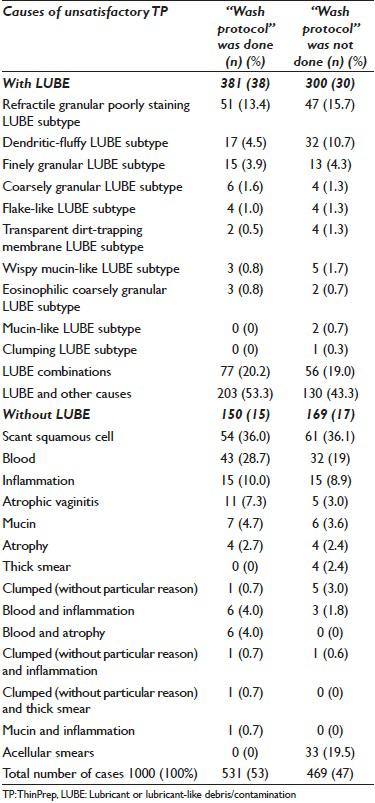
LUBE demonstrated various morphological patterns in Pap stained TP [Figure 2 and Table 2]. Poorly staining refractile granular LUBE was the most common variant with or without other associated causes noted in 39% (264/681), but this variant was relatively difficult to identify due to the morphological overlap with erythrocyte stroma (lysed red blood cells). Mucin-like and wispy mucin-like LUBE were difficult to distinguish from regular mucin. Some LUBEs were also associated with a tendency to cell clumping with thick cell groups, thus interfering with cytomorphological evaluation.
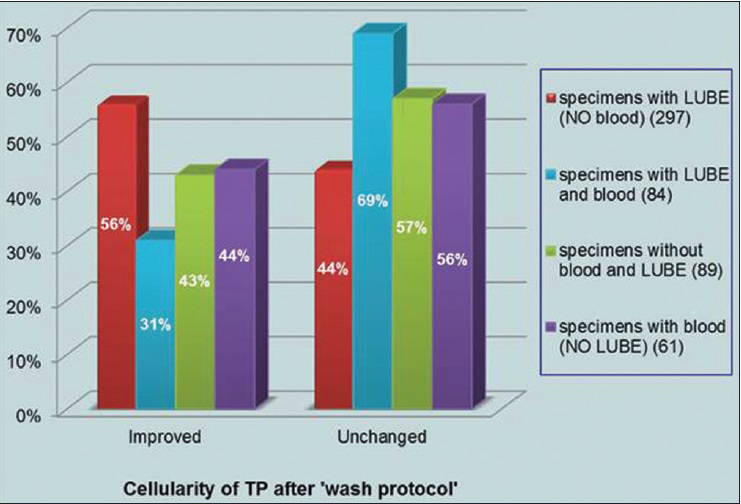
Repeat TP and staining after wash protocol showed increased cellularity in 48% (256 out of 531) of the specimens [Figure 4]. In specimens with blood and LUBE together, the cellularity improved in relatively fewer cases [31%, 26 out of 84; Figure 5]. Out of 256 with improved cellularity, 29% (73 out of 256) were satisfactory (over 5000 cells per TP) with good cellularity in 22% (56 out of 256) and satisfactory but with borderline cellularity (just over 5000 cells per TP) in 7% [17 out of 256; Figure 6]. The overall satisfactory rate achieved in these specimens with LUBE after the wash protocol was 13.7% (73 out of 531). Thus, for effective reduction in the unsatisfactory rate, the preferred approach should be to avoid contamination with LUBE at specimen collection stage itself.
The proportion of LUBE in the background decreased variably with different morphological subtypes of LUBE (78% [62/80] for dendritic-fluffy type [Figure 4], 80% [16/20] for flake-like, 70% [14/20] for wispy mucin-like, 100% [14/14] for transparent dirt-trapping membranes).
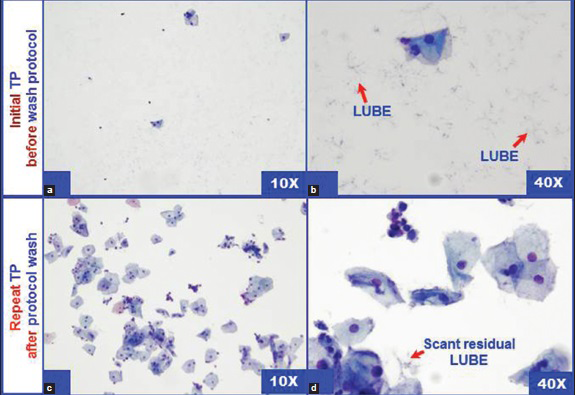
- Improved cellularity with reduction in LUBE (dendritic-fluffy) in ThinPrep after (c and d) “wash protocol” as compared to the cellularity before “wash protocol” (a and b)
- Cellularity results on repeat ThinPrep after “wash protocol”
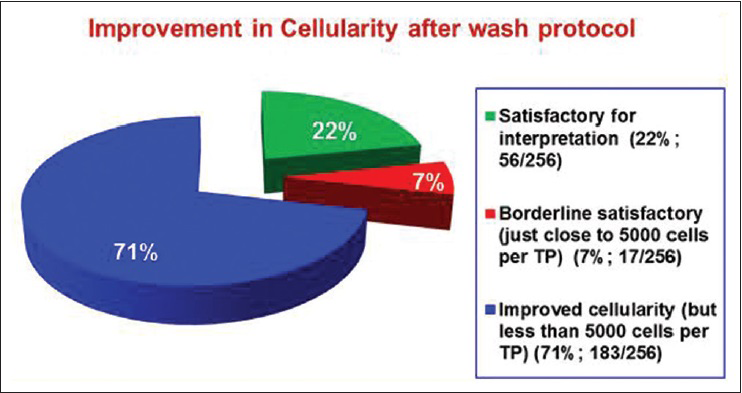
- Pattern of improvement in the cellularity of repeat ThinPrep for 256 specimens (out of 531) after wash protocol with reference to the final satisfactory status per Bethesda 2001 criteria
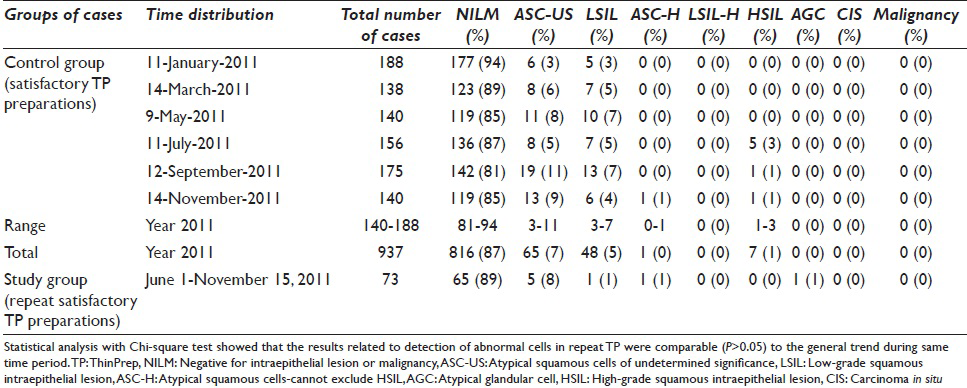
Cytologic interpretation on repeat TP (after wash protocol in the specimens which became satisfactory) showed 89% negative for intraepithelial lesion or malignancy, 8% atypical squamous cells of undetermined significance, 1% low-grade squamous intraepithelial lesion (LSIL), 1% atypical squamous cells – cannot exclude a high-grade squamous intraepithelial lesion (ASC-H), and 1% atypical glandular cells (AGC) [Table 3]. Although relatively small in number, the interpretation pattern was compared with routine results on satisfactory TP in the same population (selected randomly from three random days corresponding with the study period). Although AGC and HSIL together were comparable, LSIL rate was relatively lower for specimens undergoing wash protocol [1% as compared to 5% in the general pool; Table 3].
ACC with scant squamous cellularity was noted in 11.4% (114 out of 1000) specimens. This category showed higher frequency (73.7%; 84/114) of association with LUBE contamination (these cases were signed-out with recommendation to repeat after local or systemic estrogen therapy without contamination with LUBE) as compared to (26.3%; 30/114) ACC specimens without LUBE (these cases were signed-out with recommendation to repeat after local or systemic estrogen therapy). Out of 114 ACC specimens, the wash protocol could be applied to 68 specimens. Nineteen (27.9%) specimens showed improved cellularity (19/68). Out of these 19 cases, a total of 16 became satisfactory: 11% (2/19) were borderline satisfactory and 74% (14/19) were satisfactory with good cellularity.
Forty-eight (with and without LUBE contamination) specimens previously with ACC and reported as unsatisfactory were repeated (within 2 weeks - 2 years) and showed increased cellularity in 44 (ACC was still observed in 48%, 21/44). The proportion of specimens in repeated cases with previous atrophic changes, LUBE decreased to 30% in the resubmitted cohort.
Sixteen out of 30 initially unsatisfactory specimens with ACC but without LUBE were repeated as a new specimen collected at later date and showed a reduction in the unsatisfactory rate to 6.3% (1/16), the remaining 14 cases could not be followed as repeated specimen.
Thirty-two out of 84 unsatisfactory ACC specimens with LUBE contamination were repeated as a new collection at a later date with relatively reduced unsatisfactory rate to 9% (3/32). Fifty two cases could not be confirmed whether TP was repeated without LUBE.
DISCUSSION
The Pap test has been highly effective in decreasing the prevalence of advanced cervical cancer and related morbidity/mortality in the western world.[1011] Recently, there have been a variety of enhancements made to the Pap test as regards the reproducible quality of cytology preparations for final cytologic interpretation. There have been many other ongoing enhancements including standardized Bethesda nomenclature,[12 13] publications communicating further refinements such as morphological spectrum of ASC-H,[14] LSIL-H as new category,[15] ancillary role of immunomarkers such as HPV testing and p16.[1617] All of these factors have taken the humble test induced initially by Georgios Nikolaou Papanicolau to the highly evolved test currently in use today. Further refinements in processing by methodologies such as by LBC: TP® (Hologic, Inc., Bedford, MA)[18] and SurePrep® (Becton, Dickinson and Company, Franklin Lakes, NJ)[19] have added more benefits to this test. However, one of the limiting factors with TP is scant cellularity due to a variety of technical causes leading to relatively higher unsatisfactory rate.[14520212223]
In brief, the TP sample processing begins with a collection of the cervical specimen in PreservCyt® vials. An adequate number of squamous epithelial cells including the cells from transformation zone must be collected and transferred in adequate numbers to the glass slide for satisfactory cytomorphologic evaluation. The fundamental measure of adequacy being number of squamous cells (more than 5000 nucleated squamous cells). The TP machine collects the cells on the surface of a filter membrane with small pores sufficient to aspirate PreservCyt® in the vial while trapping the diagnostic cells on its surface.
The TP machine effects this collection by suction through the pores in the filter membrane. The suction stops when the holes are blocked by material collected on the filter membrane irrespective of the nature of the material collected including the diagnostic cells. Any debris such as blood, inflammatory cells, and contaminants capable of blocking the holes including LUBE can and will prevent the collection of enough epithelial cells onto the filter membrane.
As reported previously, LUBE was the most frequent factor resulting in decreased cellularity in the final TP slides.[1] The possibility of compromising of additional testing modalities such as HPV testing due to the paucity of cells (including cases with atrophic change) and abundance of LUBE in TP vial also exist. Other studies also have reported similar observations.[62425] Additional significant etiologic factors associated with high unsatisfactory rate were association with atrophic changes and blood.
In this study over a 5½ months period, a total of 19,422 TP specimens were processed with an unsatisfactory rate of 5.1%. The higher unsatisfactory rate may be related to variety of etiologic factors including variation in collection methodologies (preference for the use or avoidance of LUBE), the nature of the patient population such as preponderance of elderly postmenopausal women (prone to atrophic smear), sampling skills, etc. The most common cause in this study was an association with LUBE (68%, 681 out of 1000). Only a few cases (3.3%, 33 out of 1000) were virtually acellular. Remaining 28.6% unsatisfactory specimens were associated with blood, atrophic changes, inflammation, and their combinations. A few showed scant cellularity without any associated identifiable factor (suggestive of insufficient sampling during collection) [Table 1]. Other possible reasons include keeping cervical brush with collected cytology material in TP fixative (PreservCyt®) for a while, and then swirling the brush to loosen and disperse the cells in PreservCyt®. This may fix the specimen on brush as a thin film which would be dislodged as fragments/clumps while swirling to disperse the specimen. These fragments may block the pores in TP membrane and interfere with adequate cellularity in final TP.
The practical aspect of identifying various microscopic morphological patterns of LUBE in Pap stained TPs [Figure 2 and Table 2] would be the recognition of LUBE as a possible reason for inadequate Pap test result as well as an appropriate communication in final report to prevent trend of repeated unsatisfactory results on future sample submissions. As a general observation, LUBE was better detected at the periphery (rim of) of the TP circle. The most common morphological variety was poorly staining refractile granular LUBE [Tables 1 and 2]. However, this variant was relatively difficult to interpret due to morphological overlap with erythrocyte stroma. Mucin-like and wispy mucin-like LUBE were difficult to distinguish from regular mucin [Figure 2]. Rare LUBE was associated with a tendency to cell clumping with increased thickness of cell groups. This group improved with repeat wash protocol but with suboptimal morphological details. Acid wash leads to increased tendency for cell clumping when performed on the specimen contaminated with LUBE. Some studies have reported improved cellularity when a repeat TP was prepared on residual specimens (without any wash or processing) but with lower success rate, especially if the specimens had LUBE contaminant.[6]
The optimal approach to prevent LUBE interference challenge would be to avoid contamination with any debris (including those associated with lubricant used clinically for vaginal examination and for negotiating the speculum, personal use of lubricant, use of topical medication, postcoital seminal fluid contamination, etc.,) which may potentially block the pores in the TP filter membrane. Proper collection of cervical cytology specimens by avoiding contamination with LUBE and blood should be the preferred recommendation. Additional insurance is instructions to the patients seeking cervical cytology specimens for Pap test, to avoid topical use of any LUBE like products prior to the collection of sample and other possible interfering factors including postcoital seminal fluid debris. This approach would avoid contamination by interfering debris potentially blocking the TP filter membrane pores and thereby improving cellularity of final TP and consequently lower unsatisfactory rate [Table 4].

Other important factor is to identify LUBE with its garden variety of morphologic patterns in TP [Figure 2] and to communicate the interference due to LUBE in the report as a feedback with a note “to repeat the adequately cellular sample without LUBE contamination as clinically indicated.”
The wash protocol used in this study was standardized after evaluating a few combinations of a variety of steps and reagents including potential incorporation of weak surfactants. The final protocol reported in this study is the simplest one with minimal steps achieving the best results to improve the cellularity [Figure 3].[18] The improvement in the cellularity was noted not only in specimens with lubricant [Figure 4], but also in some specimens with other debris or without an identifiable cause. However, this improvement only achieved a satisfactory rate of 11% (56 out of 531) in processed specimen.
Although relatively small in number, the interpretation pattern was comparable to that with routine results on satisfactory TP (except for LSIL) in same population during the study period [Table 3]. LSIL rate was relatively lower for specimens undergoing wash protocol (1% as compared to 5% in general pool). The significance of this observation should be explored further.
The improved cellularity after wash protocol varied with morphological type of LUBE noted in the initial hypocellular TP [Figure 4] (78% [62/80] for dendritic-fluffy type, 80% [16/20] for flake-like, 70% [14/20] for wispy mucin-like, 100% [14/14] for transparent dirt-trapping membranes).
As compared to the specimens only with LUBE (297 specimens), the wash protocol was less effective for the specimens with presence of blood with (84 specimens) or without LUBE (89 specimens) [Figure 5]. Cellularity of the specimens with blood and LUBE improved in relatively fewer cases (31%; 26 out of 84) as compared to 56% in specimens only with LUBE without blood [Figure 5]. Specimens with LUBE processed after acid wash protocol showed a higher tendency for cell clumping with increased thickness.
Association of LUBE in 114 specimens with ACC was slightly higher (73.7%; 84/114) as compared to general population (68%; 681/1000). This may be due to the technical benefit of LUBE for negotiating the speculum in these cases. Most of these cases were signed-out initially with a note to repeat after topical or systemic estrogen therapy to reverse ACC and avoiding LUBE contamination.
The wash protocol could be applied to 68 ACC specimens (out of 114 cases) with improved cellularity in 19 (28%). Out of these 19 cases, total of 16 showed improved cellularity: Satisfactory with good cellularity in 21% (14/68) and borderline satisfactory in 3% (2/68).
Sixteen out of 30 previously unsatisfactory specimens with ACC (without LUBE contamination) repeated as a new collection at later date showed relatively reduced unsatisfactory rate of 6.3% (1/16). Thirty-two out of 84 previously unsatisfactory specimens with ACC (with LUBE contamination) repeated as a new collection at later date showed relatively reduced unsatisfactory rate to 9% (3/32). This improvement may be due to the feedback related to ACC and LUBE in the report on previously unsatisfactory specimen.
The repeat TP after wash protocol with adequate cellularity showed cytologic interpretation pattern comparable with the routine results on satisfactory TP in same population during same time period. However, LSIL rate observed after wash protocol on this small cohort was relatively lower [Table 3].
In summary, LUBE was one of the most common identifiable factors for unsatisfactory TP, with blood contamination and association with atrophic changes as additional causes [Figure 2; Tables 1 and 2]. Although the best option is to avoid contamination with LUBE during specimen collection, wash protocol may overcome LUBE interference [Table 4]. The interpretation results of TP after wash protocol showed patterns comparable to the interpretation patterns on specimens processed as routine TP [Table 3]. In addition, knowing morphologic patterns of LUBE helps to recognize it in the TP and instead of just reporting the specimen as “unsatisfactory,” it is recommended to add an educational note/recommendation to the provider about LUBE interference in the final report with a note “to repeat the adequately cellular cervical specimen without LUBE contamination as clinically indicated.” This is also applicable to blood contamination. Similarly for “Unsatisfactory” ACC specimens, a recommendation to “repeat after topical or systemic estrogen therapy as clinically indicated” may decrease the unsatisfactory rate in the repeated specimens in this subset with ACC. Possible solutions to overcome these three common factors leading to unsatisfactory TP are summarized in Table 5.

COMPETING INTERESTS
No competing interest to declare by any of the authors.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study. Authors take responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
ACC - Atrophic cellular Changes
ACC - Atrophic Cellular Changes
AGC - Atypical Glandular Cells
HPV - Human Papillomavirus
IRB - Institutional Review Board
LBC - Liquid Based Cytology
LSIL - Low-Grade Squamous Intraepithelial Lesion
LUBE - Lubricant and Lubricant-Like debris/Contamination
Pap - Papanicolaou
TP - ThinPrep.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
We thank Michael Carroll CT (ASCP), Andrea Black CT (ASCP), Dongping Shi, MD, Erica Brandt, BS (Student); and Karen Jones (Lab Assistant) for their support during the course of study. Kathleen Rost (Executive Secretary) for the secretarial support. We appreciate Daniel Neill, MD for his editorial help and support. This study was supported by limited funding from Hologic, Inc. Bedford, MA.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2015/12/1/21/165955
REFERENCES
- Frequency and etiology of unsatisfactory cervical cytology by ThinPrep method in a tertiary care urban setting – A snapshot of brief duration. Mod Pathol. 2012;25(Suppl 1):93A. [Abstract 382]
- [Google Scholar]
- Lubricant-related high unsatisfactory rate with ThinPrep-can the cellularity be improved. Mod Pathol. 2013;26(Suppl 2):94A. [Abstract 387]
- [Google Scholar]
- Evaluation and resolution of etiologic factors for unsatisfactory cervical cytology ThinPrep preparations. J Am Soc Cytopathol. 2012;1(1 Suppl):S1-126. [Abstract #225]
- [Google Scholar]
- Unsatisfactory reporting rates: 2006 practices of participants in the college of American pathologists interlaboratory comparison program in gynecologic cytology. Arch Pathol Lab Med. 2009;133:1912-6.
- [Google Scholar]
- Unsatisfactory rates vary between cervical cytology samples prepared using ThinPrep and SurePath platforms: A review and meta-analysis. BMJ Open. 2012;2:e000847.
- [Google Scholar]
- The unsatisfactory ThinPrep® Pap Test™: Analysis of technical aspects, most common causes, and recommendations for improvement. Diagn Cytopathol. 2013;41:588-94.
- [Google Scholar]
- NCI Bethesda System. Unsatisfactory for Evaluation. Available from: http://nih.techriver.net/listing.php?tableOutline=2-2
- [Google Scholar]
- Protocol to Overcome Lubricant-Like Debris (Shidham) – Higher Resolution FREE on YouTube. Available from: https://www.youtube.com/watch?v=nFvqz9BGfXc
- [Google Scholar]
- How to Convert Centrifuge RPM to RCF or G-force? G-force=0.00001118xRxRPM2. Clinfield Limited. Available from: http://clinfield.com/2012/07/how-to-convert-centrifuge-rpm-to-rcf-or-g-force/
- [Google Scholar]
- Cervical Cancer Screening (PDQ®); Description of the Evidence – Background: Natural History, Incidence, and Mortality. National Cancer Institute at National Institute of Health. Available from: http://www.cancer.gov/cancertopics/pdq/screening/cervical/HealthProfessional/page2#Section_207
- [Google Scholar]
- ASC Statement on New Technologies in Cervical Cytology Screening. American Society of Cytopathology. Available from: http://www.cytopathology.org/new-technologies/
- [Google Scholar]
- Second edition of ‘The Bethesda System for reporting cervical cytology’ – Atlas, website, and Bethesda interobserver reproducibility project. Cytojournal. 2004;1:4.
- [Google Scholar]
- NCI Bethesda System-2001 Bethesda System Terminology. Available from: http://nih.techriver.net/bethesdaTable.php
- [Google Scholar]
- ASC-H in Pap test – Definitive categorization of cytomorphological spectrum. Cytojournal. 2006;3:14.
- [Google Scholar]
- Should LSIL with ASC-H (LSIL-H) in cervical smears be an independent category?. A study on SurePath specimens with review of literature. Cytojournal. 2007;4:7.
- [Google Scholar]
- Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: Realistic estimates from the ASCUS-LSIL Triage Study. JAMA. 2001;285:1500-5.
- [Google Scholar]
- p16 immunocytochemistry on cell blocks as an adjunct to cervical cytology: Potential reflex testing on specially prepared cell blocks from residual liquid-based cytology specimens. Cytojournal. 2011;8:1.
- [Google Scholar]
- ThinPrep, Hologic, In. Available from: http://www.hologic.com/en/product-support/diagnostic-cytology/thinprep-2000/
- [Google Scholar]
- The BD SurePath™ Liquid-based Pap test. Available from: http://www.bd.com/tripath/physicians/surepath.asp
- [Google Scholar]
- The effect of lubricant contamination on ThinPrep (Cytyc) cervical cytology liquid-based preparations. Cytopathology. 2008;19:236-43.
- [Google Scholar]
- The effect of vaginal speculum lubrication on the rate of unsatisfactory cervical cytology diagnosis. Obstet Gynecol. 2002;100:889-92.
- [Google Scholar]
- Use of water-soluble gel in obtaining the cervical cytologic smear. Acta Cytol. 1997;41:1861-2.
- [Google Scholar]
- Does speculum lubricant affect liquid-based Papanicolaou test adequacy? Cancer Cytopathol. 2014;122:221-6.
- [Google Scholar]
- Interference potential of personal lubricants and vaginal medications on ThinPrep pap tests. J Am Board Fam Med. 2011;24:181-6.
- [Google Scholar]
- Protocol for the processing of bloody cervical specimens: Glacial acetic acid and the ThinPrep Pap Test. Diagn Cytopathol. 2006;34:210-3.
- [Google Scholar]








