Translate this page into:
Evaluation and significance of hyperchromatic crowded groups (HCG) in liquid-based paps
-
Received: ,
Accepted: ,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objective
Hyperchromatic crowded groups (HCG), a term first introduced into the cytology literature by DeMay in 1995, are commonly observed in Pap tests and may rarely be associated with serious but difficult to interpret lesions. In this study, we specifically defined HCG as dark crowded cell groups with more than 15 cells which can be identified at 10× screening magnification.
Methods
We evaluated consecutive liquid-based (Surepath) Pap tests from 601 women (age 17–74 years, mean age 29.4 yrs) and observed HCG in 477 cases. In all 477 HCG cases, Pap tests were found to be satisfactory and to contain an endocervical sample. HCG were easily detectible at 10× screening magnification (size up to 400 um, mean 239.5 um) and ranged from 1 to 50 (mean 19.5) per Pap slide.
Results
HCG predominantly represented 3-Dimensional groups of endocervical cells with some nuclear overlap (379/477 – 79%), reactive endocervical cells with relatively prominent nucleoli and some nuclear crowding (29/477 – 6%), clusters of inflammatory cells (25/477 – 5.2%), parabasal cells (22/477 – 4.6%), endometrial cells (1/477 – 0.2%). Epithelial cell abnormalities (ECA) were present in only 21 of 477 cases (4.6%). 18 of 21 women with HCG-associated ECA were less than 40 years old; only 3 were =/> 40 years. HCG-associated final abnormal Pap test interpretations were as follows: ASCUS (6/21 – 28%), LSIL (12/21 – 57%), ASC-H (2/21 – 9.5%), and HSIL/CIN2-3 (3/21 – 14%). The association of HCG with ECA was statistically significant (p = 0.0174. chi-square test). In patients with ECA, biopsy results were available in 10 cases, and 4 cases of biopsy-proven CIN2/3 were detected. Among these four cases, HCG in the Pap tests, in retrospect represented the lesional high grade cells in three cases (one HSIL case and two ASC-H cases). Interestingly, none of the 124 cases without HCG were found to have an epithelial cell abnormality.
Conclusion
We conclude: a. HCG are observed in a high proportion of cervical smears. b. In the vast majority of cases, HCG are benign. c. ECA were only observed in cases with HCG. This observation is consistent with the hypothesis that the presence of HCG in Pap tests most often represents adequate sampling of the transformation zone, thus increasing the chances of detecting an epithelial cell abnormality. d. Only a few cases with HCG were associated with a serious ECA, but careful scrutiny of all HCG appears warranted to avoid the potential diagnostic pitfall of a significant false negative interpretation.
Background
The term hyperchromatic crowded groups (HCG) was introduced into the cytology literature in DeMay's 1995 first edition of The Art and Science of Cytopathology [1]. DeMay noted that “although hyperchromatic crowded groups are usually benign and include such entities as endometrial cells and severe atrophy; they can also be the general cytologic appearance of a variety of serious lesions.” He first noticed the possible significance of these groups when examining a large series of Pap tests interpreted as “negative” from a single patient who ultimately developed cervical cancer and initiated litigation [2]. Glandular cells tend to present much more frequently as HCG than squamous cells. Endocervical cells, endometrial cells, tubal metaplasia, and cone artifact are just some of the many benign glandular cell groups which may present as HCG, whereas atrophy is the most common benign cause of HCG associated with squamous epithelium [13]. Although relatively uncommon, epithelial cell abnormalities (ECA) may also present as HCG. Significant lesions such as carcinoma-in-situ (CIS), squamous cell carcinoma, adenocarcinoma in situ (AIS), endocervical adenocarcinoma, small cell carcinoma, endometrial adenocarcinoma, and even metastatic carcinoma may present in part or entirely as HCG [134].
There is currently limited literature as how to precisely differentiate benign HCG from precancerous or malignant HCG [134]. The difficulty of cytologic interpretation may on occasion represent a highly clinically significant diagnostic pitfall. Aside from seminal publications by DeMay, reports specifically analyzing the cytologic evaluation of HCG are still uncommon [5–7]. We undertook this study to further evaluate this challenge and to characterize some of the cytologic patterns associated with HCG.
Materials and methods
We define HCG in this study as groups of darkly staining cells which measured 100 μm or more and which were easily detectable at 10× screening magnification. Consecutive Pap tests from 601 women from 17 to 74 years old (mean 29) were selected for the study. All specimens were collected with the Cervex-Brush® in SurePath collection medium™. Of the 601 consecutive Pap tests, 38 had no endocervical cells or T zone metaplastic cells and 10 were unsatisfactory. The Pap slides were evaluated for the presence of HCG. Cytomorphologic features such as the size of the cellular groups, background, cohesiveness of the cell clusters, shape of the cell groups, presence of feathering or other cell group border features were noted. The individual size and shape of the cells, N/C ratio, cytoplasm, chromatin, nuclear contour, and presence of nucleoli were also noted.
Results
HCG were present in 477 of 601 cases (79.4%) and absent in 124 cases (20.6%). HCG were benign in over 95% of cases (Table 1). Benign HCG included 3-Dimensional groups of endocervical cells with some nuclear overlap (379/477 – 79%) (Figure 1), reactive endocervical cells with relatively prominent nucleoli and some nuclear crowding (29/477 – 6%) (Figure 2), groups of inflammatory cells (25/477 – 5.2%) (Figure 3), endometrial cells (1/477 – 0.2%) (Figure 4) and parabasal cells (15 atrophic and 7 postpartum) (22/477 – 4.6%) (Figure 5).
| Groups identified | #/out of | % |
|---|---|---|
| 3-Dimensional groups of endocervical cells with some nuclear overlap | 379/477 | 79.4 |
| Reactive endocervical cells with relatively prominent nucleoli and some nuclear crowding | 29/477 | 6.0 |
| Endometrial cells | 1/477 | 0.2 |
| Parabasal cells (15 atrophic and 7 postpartum) | 22/477 | 4.6 |
| Clusters of inflammatory cells | 25/477 | 5.2 |
| Total | 456/477 | 95.4 |

- 3-Dimensional groups of endocervical cells with some nuclear overlap, (40×).

- Reactive endocervical cells with relatively prominent nucleoli and some nuclear crowding (100×).

- Groups of inflammatory cells, (100×).

- Endometrial cells, (100×).

- Parabasal cells, (100×).
Twenty one of 477 HCG cases (4.6%) were associated with epithelial cell abnormalities (ECA). 18 of 21 women with HCG-associated ECA were below 40 years of age; only 3 were =/> 40 years (Figure 7). The final abnormal cytological interpretations/results in these abnormal cases were as follows: ASCUS (6/21 – 28%), ASC-H (2/21 – 9.5%), LSIL (12/21 – 57%), and HSIL (3/21 – 14%). The association of HCG with ECA was statistically significant (p = 0.0174, chi-square test). In the group with ECA, follow-up cervical biopsy results were available in 10 cases. One LSIL case with non-lesional benign HCG was found subsequently on follow-up cervical biopsy to harbor a high grade squamous intraepithelial lesion (CIN II) (Table 2). A total of four biopsy proven CIN2/3 cases were associated with HCG (Figure 6). Among these four cases, HCG in the smear actually represented the atypical lesional cells in three cases (one HSIL cytology and two ASC-H cytology cases). Although the total number of HCG-associated abnormal cases in this series is small, an interesting and unexpected finding was that in none of the 124 cases with satisfactory sampling of transformation zone but without HCG showed any epithelial cell abnormality.
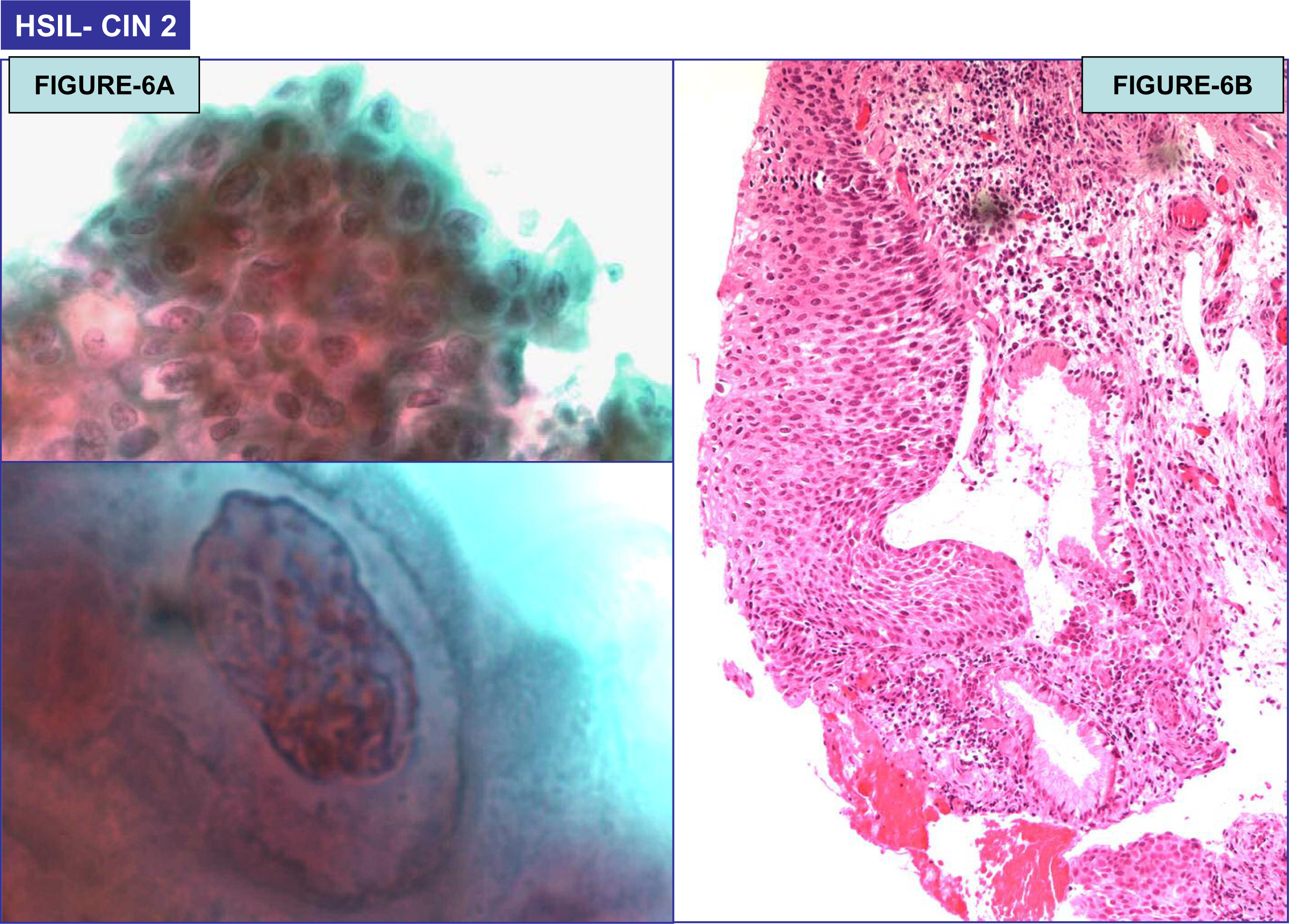
- (A): HSIL (Pap Test) (100×); (B): Cervical intraepithelial neoplasia 2 (CIN2) on biopsy (40×).
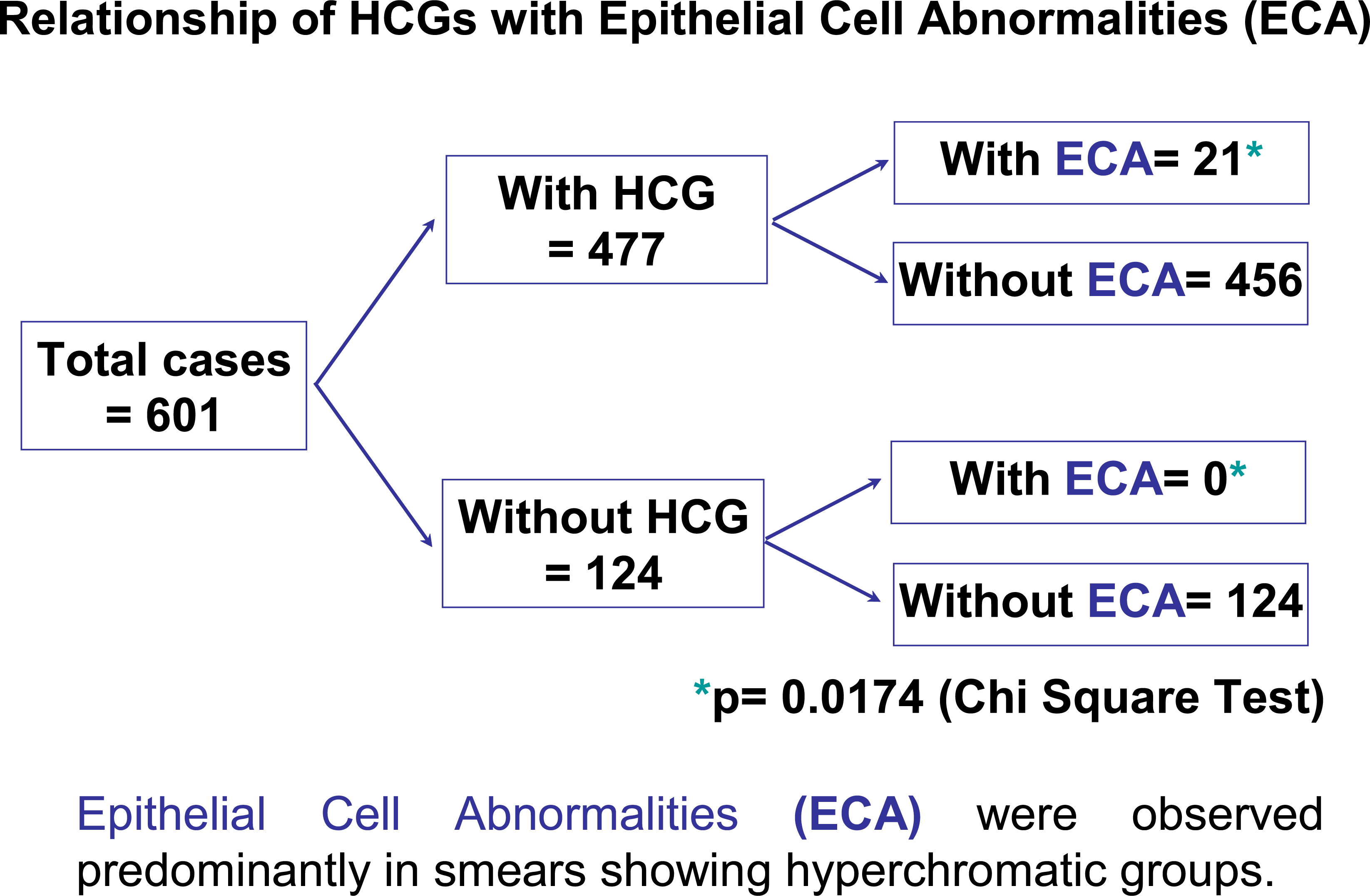
- Relationship of HCGs with Epithelial abnormalities (ECA).
| Total # cases | Cytologic interpretation | Biopsy interpretation |
|---|---|---|
| 1 | LSIL | CIN-2 |
| 1 | HSIL/CIN-2 | CIN-2 |
| 2 | ASC-H | CIN-2 |
Discussion
Hyperchromatic crowded groups, termed HCG by DeMay [1], are a frequent occurrence in Pap Tests. Benign glandular cells HCG are seen far more frequently than either abnormal glandular cell HCG or squamous cell HCG, normal or abnormal in routine Pap Tests. In this study, endocervical cells were by far the most common cause of benign HCG cell groups. We report here for the first time the significant association of HCG with endocervical sampling. All cases with HCG were consistent with optimum sampling of the transformation zone. We also report for the first time a significant increase in detection of epithelial cell abnormalities (ECA) in Pap tests with HCG as opposed to Pap tests without HCG. We conclude that these observations are best explained by the hypothesis that the presence of HCG in Pap tests most often represents adequate sampling of the transformation zone, thus increasing the chances of detecting an epithelial cell abnormality.
Endocervical sampling has been significantly enhanced with the advent of the endocervical brush [8], even as coincidentally increased sampling of the lower uterine segment (LUS) [910], endocervical tubal metaplasia (TME) [1112], brush-induced atypia (brush effect) [13], reparative endocervical changes [14], cone artifact [15], cervical endometriosis [16], and microglandular hyperplasia [1718] have added to the complex list of benign endocervical glandular HCG that must be distinguished from neoplastic lesions. The detection of TME, approximately 31% in cone or hysterectomy specimens [19], has undoubtedly risen in recent decades as a result of the improved ability of the cytobrush to sample the upper endocervix. TME may present as flat sheets or 3D HCG with a finely granular chromatin, dark nuclei, absent nucleoli. The presence of cilia and peg cells are very helpful. In the absence of ciliated cells, the reporting of atypical glandular cells on the Pap tests is a common outcome [1112].
Benign endometrial cells often present in the Pap tests as HCG. The cells are small, and crowded; the nuclei may be degenerate and hyperchromatic. Mitotic figures are not typically seen in exfoliated cells but may be seen in directly sampled cells [3]. Benign endometrial cell HCG spontaneously shed in menstrual smears may especially show concerning degenerative chromatin changes, but the cell groups typically lack features commonly described in neoplasia [20], especially the more pronounced nuclear atypia seen at least in higher grade endometrial cancers [21]. Tubal metaplasia of the endometrium and other forms of endometrial metaplasia, also termed epithelial cytoplasmic change [22], have been much more frequently recognized in endometrial surgical pathology specimens than in cytology specimens. The relatively few cytologic descriptions suggest that they may present as HCG and that their accurate recognition can be a significant diagnostic challenge [2324].
One of the most important abnormal glandular causes of HCG is endocervical neoplasia, including both adenocarcinoma in situ (AIS) and invasive adenocarcinoma [13]. Detection rates of AIS on Pap tests have risen with the widespread use of endocervical brush sampling [25]; however, there are many benign conditions which can mimic AIS [26]. Additional features such as marked hyperchromasia, altered nuclear polarity, increased N/C ratio, and associated single cells have been described as helpful diagnostic features [2627]. An increasingly common endometrioid variant of AIS has a distinct small cell pattern and may be an especially common cause of false negative interpretations as it can be difficult to distinguish from cells sampled from LUS [28]. Presence of endometrial glands and stroma in the cells derived from LUS are helpful cytologic features to distinguish LUS from AIS. Endometrial adenocarcinoma can also present as HCG. Nuclear enlargement and presence of nucleoli are helpful cytomorphologic features (28). Some studies have reported enhanced accurate recognition with liquid-based cytology [30–33].
Parabasal cells associated with an atrophic cellular pattern in postmenopausal or postpartum specimens are often seen as syncytial aggregates of cells with small closely packed parabasal cells with dark nuclei and scant cytoplasm. These may on occasion be difficult to distinguish from CIS [34]. Maturation of the cells at the periphery of the groups of parabasal cells is one clue which may help to recognize the atrophic nature of the smear. A study by Harris et al to evaluate the dense nuclei of HCG using digital measurements revealed that the atrophic groups had significantly lower nuclear area (mean 0.4 μm) than HSIL (mean 0.9 μm) [6]. In another recent study on the cytomorphologic spectrum of ASC-H from the authors’ aboratories, it was noted that parabasal cell HCG often present as small dark nuclei with variable cytoplasm and a moderate N/C ratio. The chromatin is typically dark but not clumped. These groups may be interpreted as ASC-H [35]. Recognition of this distinctive cytomorphologic pattern and HPV DNA testing can be very helpful in establishing the benign character of these cell groups [36].
The other most clinically significant differential diagnosis with HCG is that of a high grade squamous intraepithelial lesion (CIN2,3/CIS) with or without involvement of endocervical glands. The cytomorphologic features of HSIL include small dysplastic cells as compared to larger cells of LSIL. HCG in this study never represented lesional LSIL cells. Cells of HSIL usually occur as small single cells, but when present as syncytial-like aggregates, the HCG may be misinterpreted as other benign HCG. Close scrutiny under high-magnification is needed. Attention must be given to features such as increased nuclear size and hyperchromasia, fine-coarsely granular chromatin, and irregular nuclear contours. Nucleoli are usually absent but may be seen along the periphery of the groups, especially when the dysplasia involves endocervical glands [37]. In Liquid based preparations, it has been observed that dispersed single dysplastic cells are more often seen than sheets or syncytial groups [38]. The study of dense HCG by Harris et al found that HSIL groups contained the highest nuclear area (mean-0.9 μm) as compared to invasive squamous cell carcinoma (mean -0.8 μm) or endometrial adenocarcinoma (mean – 0.64 μm) [6]. Interpretation of HCG of HSIL may be challenging in conventional as well as liquid based preparations. In a recent study from the College of American Pathologists PAP Program, poorly performing conventional smear and Thin-Prep HSIL cases were compared. HCG were observed in both the preparations. The HSIL groups were often interpreted as glandular lesions when there was rounding up of cells with small nuclei and smooth contoured borders. On the other hand, these cell groups were interpreted as squamous lesions when elongated and large nuclei were present [3839]. The distinction of CIS involving the endocervical glands from AIS may impact management decisions. HCG may be seen in both the groups. The cells of AIS show spindling in the center with round to oval nuclei, peripheral palisading, and pseudostratification at the edge [40].
In summary, HCG are observed in a high proportion in cervical smears. Most of these cell groups turn out to be benign. However, there is a small proportion of HCG associated with serious abnormalities. The careful scrutiny of HCG is mandatory, as such cell groups are common in litigated cases alleged as interpretive false negatives [3]. A number of different cytomorphologic features, discussed above, can be utilized in distinguishing benign from high grade precancerous or malignant HCG. The judicious use of reflex HPV-DNA testing (34), p16INK4A or other immunocytochemical stains such as ProEx C [42], and computer-assisted imaging employing DNA sensitive stains [43] may aid interpretation of HCG-associated high grade squamous and glandular intraepithelial lesions or malignancy.
Abbreviations
AIS – adenocarcinoma in-situ; CIS-carcinoma-in situ; DNA, Deoxyribose nucleic acid; HCG – Hyperchromatic crowded groups; HPV, Human papilloma virus; LUS, Lower uterine segment; TME, Tubal Metaplasia of Endocervix;
Competing interests
The author(s) declare that they have no competing interests.
Authors’ contributions
(MC): Faculty collected all the data, participated in cytological evaluation, and drafting of manuscript, presentation of the data at EUROGIN conference.
(RMA): Faculty, drafting of manuscript, manuscript writing.
(VS) Mentor, conceptual organization, cytological-histological evaluation, and manuscript writing.
1: Presented in part as a platform presentation at European Congress of Research for genital tract infections and neoplasia (EUROGIN), Paris 2003.
2. CytoJournal editorial team thank the academic editor: Dr. Richard DeMay, MD, Professor of Clinical Pathology, University of Chicago, Cytopathology, MARP 212, MC2050, 5841 S. Maryland Ave. Chicago, IL 60637, USA, for organizing and completing the peer-review process for this manuscript.”
2: The authors thank Jon Allen, CT (ASCP) to organize some images in the manuscript.
References
- The Art & Science of Cytopathology. Chicago, Illinois: ASCP Press; 1996. p. :62-3.:93-3.:145-3.:173-3.:181-3.
- [Google Scholar]
- Hyperchromatic Crowded groups Pitfall in Pap Smear Diagnosis. Am J Clin Pathol. 2000;114((Suppl)):S36-S43.
- [Google Scholar]
- Lessons from Litigations. In: Chapter X in The Pap Test by RM DeMay. Chicago, Illinois: ASCP Press; 2005.
- [Google Scholar]
- Cytomorphological assessment of benign and malignant dense hyperchromatic groups in cervicovaginal smears. BioMed Sci Instrum. 2000;36:349-54.
- [Google Scholar]
- Cytopathology Resource Committee College of American Pathologists: Hyperchromatic crowded groups in cervical cytology – differing appearances and interpretations in conventional and ThinPrep preparations: a study from the College of American Pathologists Inter laboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2006;130(3):332-6.
- [Google Scholar]
- Analysis of five sampling methods for the preparation of cervical smears. Acta Ctyologica. 1898;33:843-848.
- [Google Scholar]
- Endometrial cells of the "Lower Uterine Segment" (LUS) in Cervical Smear Obtained by Endocervical Brushings: A Source of Potential Diagnostic Pitfall. Diagn Cytopathol. 1995;12:263-271.
- [Google Scholar]
- Tubal Metaplasia: A Frequent Potential Pitfall in the Cytologic Diagnosis of Endocervical Dysplasia on Cervical Smears. Acta Cytologica. 1992;17:383-387.
- [Google Scholar]
- Tubal metaplasia: A cytologic study with comparison to other neoplastic and non-neoplastic conditions of the endocervix. Diagn Cytopath. 1993;9:98-105.
- [Google Scholar]
- Artifactual endocervical atypia induced by endocervical brush collection. Diagn Cytopathol. 1994;11:79-83.
- [Google Scholar]
- Atypical repair on Pap smears: clinicopathologic correlates in 647 cases. Diagn Cytopathol. 2005;33:214-7.
- [Google Scholar]
- Atypical glandular cells of undetermined significance in cervical smears after conization. Cytologic features differentiating them from adenocarcinoma in situ. Acta cytol. 2001;45(2):163-8.
- [Google Scholar]
- Superficial Endometriosis of the Cervix: A Source of Abnormal Glandular Cells on Cervicovaginal Smears. Diagn Cytopath. 2004;30:88-91.
- [Google Scholar]
- Cytological Atypia Associated with Microglandular Hyperplasia. Diagn Cytopathol. 1994;10:326-331.
- [Google Scholar]
- Microglandular Hyperplasia has a cytomorphological spectrum overlappping with Atypical Squamous Cells – cannot exclude High-grade Squamous Intraepithelial Lesion (ASC-H) Diagnostic Cytopathology. 2004;30:57-61.
- [Google Scholar]
- The effects of endocervical canal typography, tubal metaplasia, and high canal sampling on the cytologic presentation of nonneoplastic endocervical cells. Am J Clin Pathol. 1996;105:403-410.
- [Google Scholar]
- Clinical significance of atypical glandular cells favor endometrial origin, in Pap Smears. Acta Cytologica 2006:48-54.
- [Google Scholar]
- Diagnosis of Endometrial Biopsies and Curettings. New York: Springer Publishers; 2005.
- [Google Scholar]
- Endometrial metaplasia as a source of atypical glandular cells in cervicovaginal smears. Diagn Cytopathol. 1996;14:25-31.
- [Google Scholar]
- Incidence Trends of Adenocarcinoma of the Cervix in 13 European Countries. Cancer Epidemiol Biomarkers Prev. 2005;14:2191-2199.
- [Google Scholar]
- Adenocarcinoma in situ with small cell (endometrioid) pattern in cervical smears: a test of the distinction from benign mimics using specific criteria. Cancer cytopathol. 1999;87:254-258.
- [Google Scholar]
- Glandular lesions of the cervix on thin-layer Pap test. Validity of cytologic criteria used in identifying significant lesions. Acta Cytologica. 2004;48:372-379.
- [Google Scholar]
- Adenocarcinoma in situ in cervical smears with a small cell (endometrioid) pattern: distinction from cells directly sampled from the upper endocervical canal or lower uterine segment. Am J Clin Pathol. 1998;109:746-748.
- [Google Scholar]
- Background features of endometrial carcinoma on ThinPrep cytology. Diagn Cytopathol. 2005;33:162-5.
- [Google Scholar]
- ThinPrep detection of cervical and endometrial adenocarcinoma: a retrospective cohort study. Cancer. 2002;96:338-43.
- [Google Scholar]
- Detection of Endometrial Adenocarcinoma with the ThinPrep Pap Test. Diagn Cytopathol. 2000;23:260-265.
- [Google Scholar]
- Diagnosis of Endometrial Lesions Utilizing Liquid-based Cytology. Cancer Cytopathology. 2005;105:56-64.
- [Google Scholar]
- Observer variability in endometrial cytology ustilizing k statistics. J Clin Pathol. 1992;45:292-294.
- [Google Scholar]
- Postmenopausal Squamous Cell Atypias: A Diagnostic Challenge. Diagn Cytopathol. 1994;11:226-230.
- [Google Scholar]
- ASC-H in Pap test-definitive categorization of cytomorphological spectrum. Cytojournal. 2006;3:14.
- [Google Scholar]
- Human Papillomavirus Testing and Liquid-Based Cytology: Results at Recruitment From the New Technologies for Cervical Cancer Randomized Controlled Trial. J Natl Cancer Inst. 2006;98:765-774.
- [Google Scholar]
- Cytologic features of squamous cell carcinoma in-situ involving endocervical glands in endocervical cytobrush specimens. Acta Cytol. 1997;38:687-692.
- [Google Scholar]
- Cytologic features of high-grade squamous intraepithelial lesion in Thin-prep Papanicolaou test slides: Comparison of cases that performed well in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med. 2006;128:746-748.
- [Google Scholar]
- Cytologic features of high-grade squamous intraepithelial lesion in conventional slides: what is the difference between cases that perform well and those that perform poorly. Arch Pathol Med. 2005;129:733-735.
- [Google Scholar]
- Endocervical adenocarcinoma In Situ: An analysis of cellular features. Diagn Cytopath. ;17(5):326-332.
- [Google Scholar]
- The Predictive value of p16 and Hybrid Capture 2 Human Papillomavirus Testing for High Grade Cervical Intraepithelial Neoplasia. Am J Clin Pathol. 2004;122:894-901.
- [Google Scholar]
- ProEx C: A New Immunohistochemical Test to Enhance High-Grade Squamous Intraepithelial Detection in Cervical cytology. ASC Bulletin. 2006;63:71-82.
- [Google Scholar]
- Performance of an imaging system vs. manual screening in the detection of squamous intraepithelial lesions of the uterinecervix. Acta Cytol. 2006;50:309-11.
- [Google Scholar]







