Translate this page into:
Fine needle aspiration biopsy of metastatic malignant mesothelioma with myxoid change and signet ring cells: A case report and review of the literature
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Malignant mesothelioma (MM) is a rare neoplasm, which is most commonly encountered in cytology through effusion specimens. Fine needle aspiration biopsy of MM, particularly the epithelioid subtype, can be a source of diagnostic difficulty and may mimic sampling of an adenocarcinoma. This is the first case report to demonstrate abundant extracellular myxoid material and numerous intracellular vacuoles, including signet ring cells, in a fine needle aspirate of metastatic MM. A review of the literature for myxoid change and vacuoles in fine needle aspiration biopsies of MM discloses that vacuoles are found in up to 35% of aspirates of MM, but myxoid change is very rare, reported in <5% of the cases. Cytologists should be aware of this rare morphologic pattern of metastatic epithelioid MM.
Keywords
Cytology
fine needle aspiration
malignant mesothelioma
metastatic
myxoid
signet ring
vacuoles
INTRODUCTION
Malignant mesotheliomas (MMs) of the pleura are rare, comprising <1% of all cancer deaths worldwide.[1] Most MMs are caused by exposure to asbestos, so as asbestos has been gradually removed from the environment, the incidence of MMs has begun to decrease.[2] Epithelioid MMs are the most common subtype and cause diagnostic difficulties for pathologists in surgical biopsy and cytopathology specimens.
In Cytology, MM is most commonly encountered on effusion cytology specimens, including pleural and peritoneal effusions. The cytologic features of MM in effusion cytology have been previously well described.[34] It is much rarer to encounter MM on fine needle aspiration (FNA) biopsy. Several small series have been published on the cytologic appearance of MM on aspiration biopsy, of either the primary tumor[5678] or a metastatic deposit.[910]
Differentiating mesothelioma from adenocarcinoma can be challenging, particularly in FNAs. While uncommon, vacuoles, myxoid/mucinous material, and even signet ring cells have been described in cytologic preparations of mesotheliomas, leading to potential misdiagnosis as an adenocarcinoma. In this article, we highlight a case of a 69-year-old man who underwent FNA of a mediastinal lymph node involved by metastatic MM, and review the literature on myxoid change and vacuoles in FNAs of MM.
CASE REPORT
Clinical history
A 69-year-old man, former smoker, with a history of multiple medical problems and a recent 15 lb. weight loss presented with a large, right loculated pleural effusion, extensive right lung atelectasis, and mediastinal adenopathy. Two pleural fluid cytology specimens obtained were negative for malignancy. A pleural decortication was performed, and frozen section examination showed “atypical epithelial cells present, cannot exclude malignancy, defer to permanent evaluation.”
Meanwhile, the patient developed hemoptysis and underwent bronchoscopy to determine the site of bleeding. An endobronchial ultrasound (EBUS)-guided FNA of an enlarged station 7 lymph node was performed.
Cytologic findings
Both air-dried and alcohol-fixed smears were prepared, and on-site evaluation of adequacy was performed using Diff-Quik stained, air-dried smears. The Diff-Quik stained aspirate smears were hypercellular with cells arranged in loose clusters and singly dispersed in a background of metachromatic myxoid/granular material [Figure 1]. The cells had a low nuclear to cytoplasmic ratio, moderate to abundant dense cytoplasm, and distinct cell borders. Some cell clusters showed intercellular windows [Figure 2]. Nuclei were centrally to eccentrically placed, with moderate anisonucleosis and frequent binucleation. Cytoplasmic vacuoles were readily appreciated, most commonly as multiple small vacuoles located near the nucleus [Figure 3]. Occasional cells had single large vacuoles filled with granular metachromatic material similar to that seen in the background, imparting a signet ring appearance [Figure 4]. An on-site adequacy interpretation of “positive for malignancy, nonsmall cell carcinoma” was rendered.
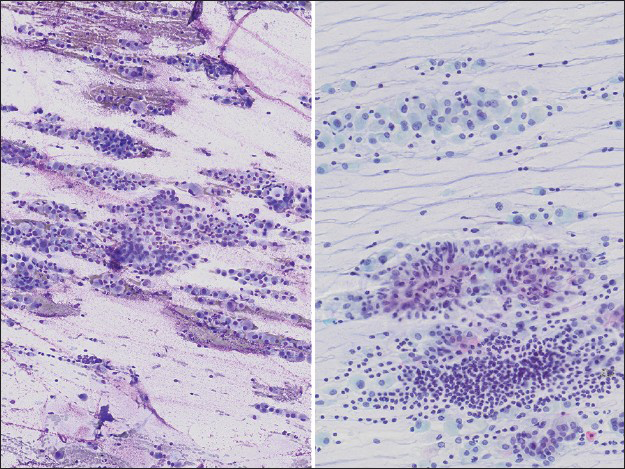
- Low power image of aspirate smear (left, Diff-Quik, ×40; right, Papanicolaou ×100) showing high cellularity with abundant extracellular myxoid material imparting a streaming appearance to the slide, admixed with single cells and occasional larger clusters
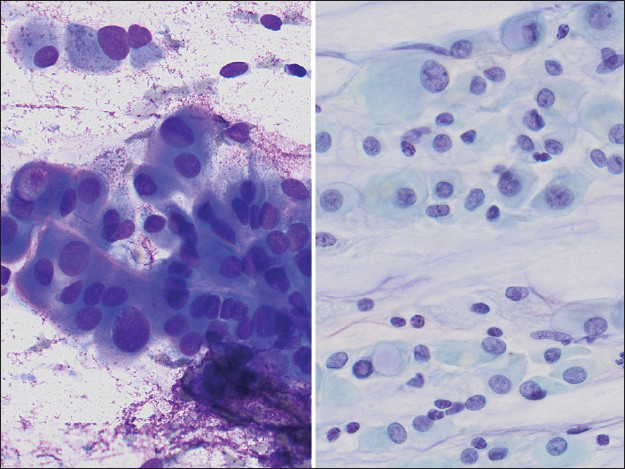
- High power image of the aspirate smear slides (left Diff-Quik, ×400; right Papanicolaou, ×400) showing cells with abundant, dense cytoplasm, well-defined cytoplasmic borders, cytoplasmic windows, occasional vacuoles, and round to oval nuclei, pale chromatin, and moderate variation in nuclear size
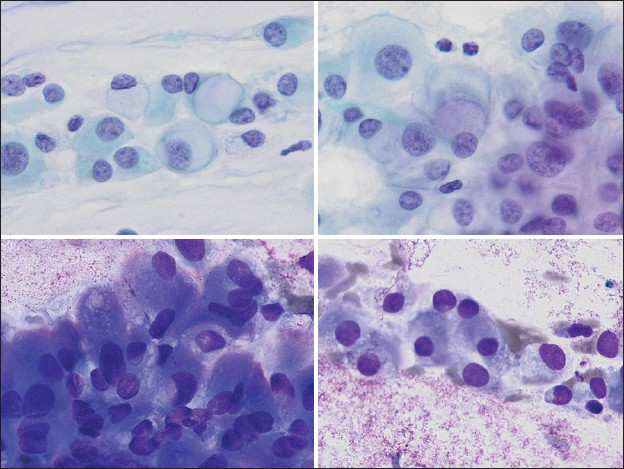
- Various vacuoles appreciated in the aspirate smears, varying from large, solitary vacuoles displacing the nucleus into an eccentric position (upper left, Papanicolaou, ×400), large solitary perinuclear vacuoles with grey-light blue material suggestive of mucin (upper right, Papanicolaou, ×400), or multiple small vacuoles, often overlying the nucleus (lower left and lower right, Diff-Quik, ×400)
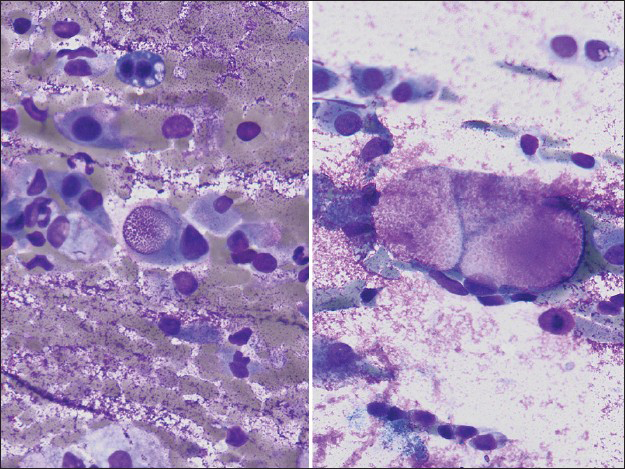
- Some tumor cells had intracytoplasmic vacuoles filled with material which stained magenta on Diff-Quik stain (×400, right and left)
The Papanicolaou-stained aspirate smears were similar, composed predominantly of single cells and loose clusters in a background of myxoid material [Figure 1]. The nuclei had evenly distributed finely granular chromatin and smooth nuclear contours with small, centrally located nucleoli [Figure 2]. Cytoplasmic vacuoles were less obvious on the Papanicolaou-stained slides, but occasional signet ring-like cells were seen [Figure 3]. Acinar formation, mitoses, and necrosis were not prominent.
Histologic findings
The pleural decortication specimen demonstrated sheets of epithelioid cells with abundant eosinophilic cytoplasm within a myxoid stroma. The cells demonstrated large cytoplasmic vacuoles, which were negative for mucin by mucicarmine and alcian blue stains. Invasion into the parietal pleural, chest wall soft tissue, and lung parenchyma was focally present. Immunohistochemical studies revealed that the cells were diffusely, strongly positive for calretinin, WT-1, and D2-40 and were negative for TTF-1 and Napsin A [Figure 5].
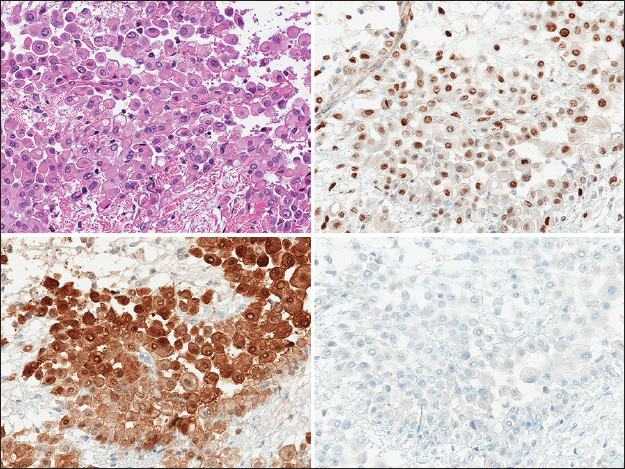
- The pleural decortication specimen demonstrated infiltrative sheets of cells with abundant eosinophilic cytoplasm and a discohesive arrangement within a myxoid stroma (upper left, H and E, ×400), which were positive for WT1 (upper right, ×400), calretinin (lower left, ×400) and negative for TTF-1 (lower right, ×400), consistent with a malignant mesothelioma
DISCUSSION
The presence of vacuoles in MM cells, though rare, has been previously reported in signet ring cell variants,[11] lipid-rich variants,[12] and adenomatoid variants of MM.[13] In signet ring cell variants, the vacuoles usually contain an acidic mucin such as hyaluronic acid or proteoglycans.[14]
In the cytology literature, one of the earliest papers to describe vacuoles in mesothelioma cells was by Boon et al.,[32] who examined pleural effusions involved by MM and adenocarcinoma. The vacuoles in MM were typically smaller, multiple, more uniform in size, and more centrally located compared to vacuoles in adenocarcinoma. They noted that the vacuoles were much easier to identify on air-dried May-Grunwald-Giemsa (MGG)-stained smears than in alcohol-fixed Papanicolaou-stained smears.
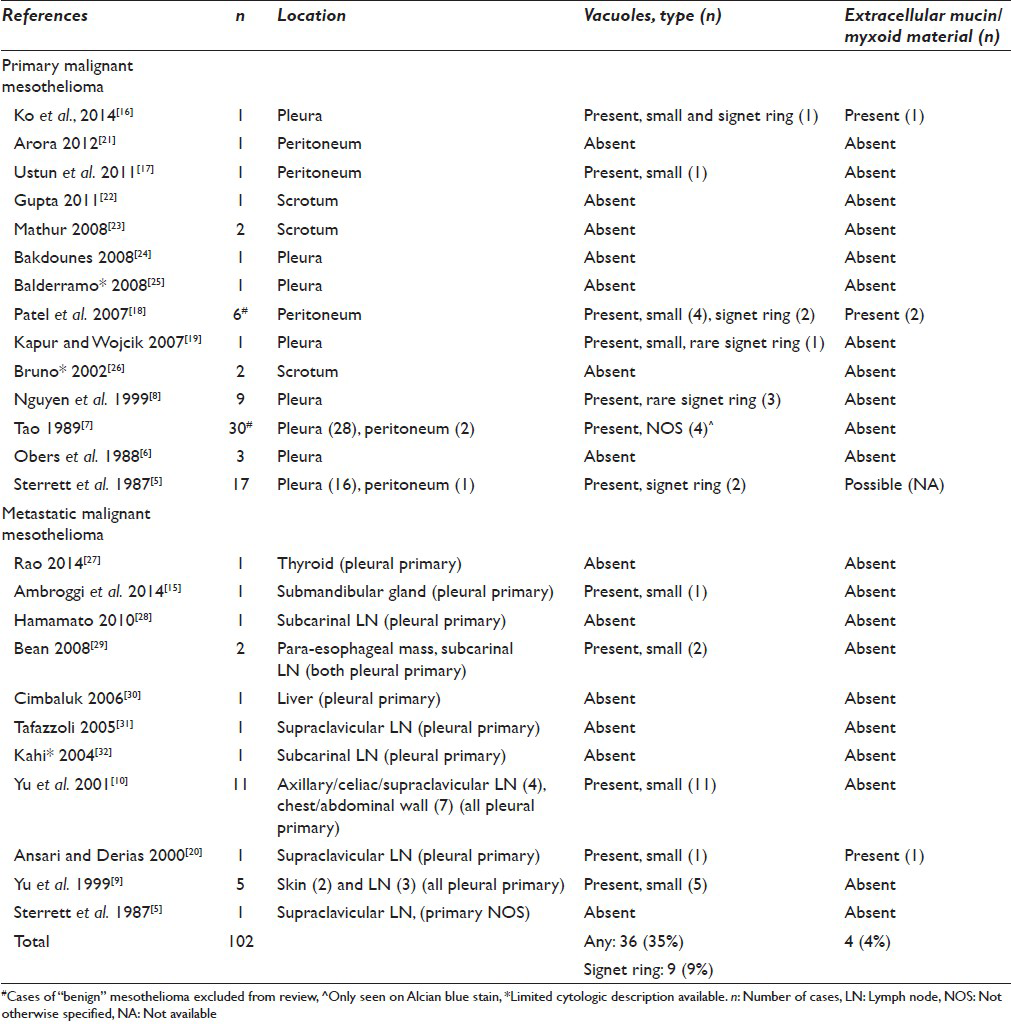
The first report of vacuoles in an FNA of MM was by Sterrett et al.[5] Of the 18 MM FNAs reviewed, 2 cases contained numerous signet ring cells. Both of these cases were epithelioid MMs, and the vacuoles were visible on both Papanicolaou and MGG stains. Reviewing the literature finds vacuoles in approximately 35% of aspirates of MMs [Table 1], most commonly multiple small vacuoles,[7910151617181920] although large solitary vacuoles causing displacement of the nucleus (so-called signet ring cells) were much less common, found in <10% of aspirates.[58161819]
Extracellular myxoid material in MM has been rarely reported in surgical pathology specimens. Hammar et al.[14] reported that up to 20% of epithelioid MMs produce hyaluronic acid or proteoglycan material that can be identified by acid mucin stains such as alcian blue. However, only rare MMs may produce large amounts of hyaluronic acid/proteoglycans visible without the aid of special stains.
Extracellular myxoid material is present in <5% of all reported aspirates of MMs in the literature [Table 1],[161820] and in most cases, it was only focally present. Ansari and Derias[20] reported “some extracellular globules” that stained magenta on Diff-Quik stained aspirate smears in one case of metastatic pleural MM. The subsequent surgical resection was grossly “mucoid,” and histology showed abundant extracellular material that was alcian blue positive, and hyaluronidase sensitive. Patel et al.[18] noted similar rare foci of extracellular myxoid material in two aspirates of peritoneal MM. The magenta, homogenous myxoid material appeared to be enveloping cells and was best seen on the Romanowsky-stained smears. Sterrett et al.[5] noted that “several” FNAs of MM contained cohesive clumps of myxoid material; however, they attributed this finding to reactive desmoplastic stroma.
Only one case of abundant myxoid material on an MM FNA has been reported similar to the current case report.[16] Computed tomography-guided FNA of a paraspinal mass demonstrated abundant extracellular myxoid material, along with dyshesive epithelioid cells with multiple vacuoles and signet ring cells, raising concern for a chordoma. The subsequent surgical resection showed a localized MM, microcystic variant.
In our case, the numerous intracellular vacuoles, abundant extracellular mucinous material, and signet ring cells were highly suggestive of adenocarcinoma on preliminary evaluation. This is a particular pitfall in situations where clinical and radiologic is either not available, or suggestive of a primary lung carcinoma. This is often the situation in a setting of rapid on-site evaluation for EBUS-guided FNAs.
CONCLUSION
This is the first case report highlighting abundant myxoid material and numerous intracellular vacuoles, including signet ring cells, in an FNA of metastatic MM. Clues suggestive of MM include numerous single bland dyshesive cells, intercellular windows in cohesive groups, and cells with multiple small vacuoles. Correlation with the clinical history and imaging, particularly in a patient with a pleural-based mass, may be helpful. Cytologists should be aware of this rare morphologic pattern of metastatic epithelioid MM.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent).
LIST OF ABBREVIATIONS (In alphabetic order)
EBUS - Endobronchial Ultrasound
FNA - Fine Needle Aspiration
IRB - Institutional Review Board
MGG - May-Grunwald-Giemsa
MM - Malignant Mesothelioma
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Malignant pleural mesothelioma: An epidemiological perspective. Ann Cardiothorac Surg. 2012;1:491-6.
- [Google Scholar]
- The diagnosis of malignancy in effusion cytology: A pattern recognition approach. Adv Anat Pathol. 2006;13:174-84.
- [Google Scholar]
- Fine needle aspiration cytology of malignant mesothelioma. Acta Cytol. 1987;31:185-93.
- [Google Scholar]
- Primary malignant pleural tumors (mesotheliomas) presenting as localized masses. Fine needle aspiration cytologic findings, clinical and radiologic features and review of the literature. Acta Cytol. 1988;32:567-75.
- [Google Scholar]
- Cytopathology of malignant mesothelioma of the pleura in fine-needle aspiration biopsy. Diagn Cytopathol. 1999;21:253-9.
- [Google Scholar]
- Cytomorphology of metastatic mesothelioma in fine-needle aspiration specimens. Diagn Cytopathol. 1999;20:328-32.
- [Google Scholar]
- Changing clinical course of patients with malignant mesothelioma: Implications for FNA cytology and utility of immunocytochemical staining. Diagn Cytopathol. 2001;24:322-7.
- [Google Scholar]
- Clinicopathologic characteristics of malignant mesotheliomas arising in patients with a history of radiation for Hodgkin and non-Hodgkin lymphoma. J Clin Oncol. 2013;31:4544-9.
- [Google Scholar]
- Lipid-rich diffuse malignant mesothelioma: A case report. Hum Pathol. 2000;31:876-9.
- [Google Scholar]
- Mucin-positive epithelial mesotheliomas: A histochemical, immunohistochemical, and ultrastructural comparison with mucin-producing pulmonary adenocarcinomas. Ultrastruct Pathol. 1996;20:293-325.
- [Google Scholar]
- Malignant pleural mesothelioma metastatic to the submandibular salivary gland, simulating glandular hypertrophy, diagnosed by fine-needle aspiration biopsy: A case report and literature review. World J Surg Oncol. 2014;12:129.
- [Google Scholar]
- Microcystic variant malignant mesothelioma presenting as a localized paraspinal mass. Cytojournal. 2014;11:16.
- [Google Scholar]
- Primary malignant deciduoid peritoneal mesothelioma: A report of the cytohistological and immunohistochemical appearances. Diagn Cytopathol. 2011;39:402-8.
- [Google Scholar]
- Cytomorphologic features of primary peritoneal mesothelioma in effusion, washing, and fine-needle aspiration biopsy specimens: Examination of 49 cases at one institution, including post-intraperitoneal hyperthermic chemotherapy findings. Am J Clin Pathol. 2007;128:414-22.
- [Google Scholar]
- Fine-needle aspiration of malignant mesothelioma with unusual morphologic features: A case report. Diagn Cytopathol. 2007;35:174-8.
- [Google Scholar]
- Diagnosis of malignant mesothelioma by fine needle aspiration of a cervical lymph node. A case report. Acta Cytol. 2000;44:70-4.
- [Google Scholar]
- Malignant biphasic peritoneal mesothelioma in a child: Fine-needle aspiration cytology, histopathology, and immunohistochemical features along with review of literature. Diagn Cytopathol. 2012;40:1112-1115.
- [Google Scholar]
- Fine needle aspiration cytology in malignant mesothelioma of the tunica vaginalis testis. Cytopathology. 2011;22:66-68.
- [Google Scholar]
- Malignant mesothelioma of tunica vaginalis: A report of 2 cases with preoperative cytologic diagnosis. Acta Cytol. 2008;52:740-743.
- [Google Scholar]
- Diagnostic usefulness and challenges in the diagnosis of mesothelioma by endoscopic ultrasound guided fine needle aspiration. Diagn Cytopathol. 2008;36:503-507.
- [Google Scholar]
- Diagnosis of pleural malignant mesothelioma by EUS-guided FNA (with video) Gastrointest Endosc. 2008;68:1191-1192.
- [Google Scholar]
- "Diagnosis of malignant mesothelioma of the tunica vaginalis testis by ultrasound-guided fine-needle aspiration. J Clin Ultrasound. 2002;30:181-183. Rao 2014
- [Google Scholar]
- Diagnostic usefulness of endobronchial ultrasound-guided transbronchial needle aspiration in a case with malignant pleural mesothelioma. Intern Med. 2010;49:423-426.
- [Google Scholar]
- Endoscopic ultrasound-guided fine needle aspiration is useful for nodal staging in patients with pleural mesothelioma. Diagn Cytopathol. 2008;36:32-37.
- [Google Scholar]
- Malignant biphasic pleural mesothelioma metastatic to the liver diagnosed by fine-needle aspiration. Diagn Cytopathol. 2006;34:33-36.
- [Google Scholar]
- Primary diagnosis of malignant mesothelioma by fine-needle aspiration of a supraclavicular lymph node. Diagn Cytopathol. 2005;33:122-125.
- [Google Scholar]
- Diagnosis of a malignant mesothelioma by EUS-guided FNA of a mediastinal lymph node. Gastrointest Endosc. 2004;60:859-861.
- [Google Scholar]
- Qualitative distinctive differences between the vacuoles of mesothelial cells and of cells from metastatic carcinoma exfoliated in pleural fluid. Acta Cytol. 1984;28:443-449.
- [Google Scholar]








