Translate this page into:
Fine needle aspiration biopsy of the liver : Algorithmic approach and current issues in the diagnosis of hepatocellular carcinoma
*Corresponding author
-
Received: ,
Accepted: ,
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The role of fine needle aspiration biopsy (FNAB) in the evaluation of focal liver lesions has evolved. Guided FNAB is still useful to procure a tissue diagnosis if clinical, biochemical and radiologic findings are inconclusive. Major diagnostic issues include: (i) Distinction of benign hepatocellular nodular lesions from reactive hepatocytes, (ii) Distinction of well-differentiated hepatocellular carcinoma (WD-HCC) from benign hepatocellular nodular lesions, (iii) Distinction of poorly differentiated HCC from cholangiocarcinoma and metastatic carcinomas, (iv) Determination of histogenesis of malignant tumor, and (v) Determination of primary site of origin of malignant tumor. This review gives a general overview of hepatic FNAB; outlines an algorithmic approach to cytodiagnosis with emphasis on HCC, its variants and their mimics; and addresses current diagnostic issues. Close radiologic surveillance of high-risk cirrhotic patients has resulted in the increasing detection of smaller lesions with many subjected to biopsy for tissue characterization. The need for tissue confirmation in clinically obvious HCC is questioned due to risk of malignant seeding. When a biopsy is indicated, core needle biopsy is favored over FNAB. The inherent difficulty of distinguishing small/early HCC from benign hepatocellular nodular lesions has resulted in indeterminate reports. Changing concepts in the understanding of the biological behavior and morphologic evolution of HCC and its precursors; and the current lack of agreement on the morphologic criteria for distinguishing high-grade dysplastic lesions (with small cell change) from WD-HCC, have profound impact on nomenclature, cytohistologic interpretation and management. Optimization of hepatic FNAB to enhance the yield and accuracy of diagnoses requires close clinicopathologic correlation; combined cytohistologic approach; judicious use of ancillary tests; and skilled healthcare teams.
Review
Until recently, guided fine needle aspiration biopsy (FNAB) was accepted as a safe procedure to procure tissue diagnosis in the management of patients with focal liver lesions. However, the role of FNAB in the evaluation of such lesions, especially hepatocellular carcinoma (HCC), and the diagnostic challenges it poses have evolved [1-4]. Much diagnostic information can be gleaned nowadays from an ever-expanding array of tumor markers and sophisticated imaging modalities. This has obviated the need, in some practices, for tissue confirmation in clinically obvious HCC. Surveillance of high-risk cirrhotic patients, has contributed to an increasing detection of focal liver lesions that are < 2 cm in diameter 56]. Study of autopsy material and cirrhotic explants, coupled with immunohistochemical and/other ancillary techniques, have led to better understanding of the morphologic evolution and biological behavior of certain hepatocellular nodular lesions [7-10]. This changing concept in the evolution of HCC and its precursor lesions has great impact on nomenclature 11, cytohistologic interpretation, management strategies and treatment outcomes 512]. Critical refinement of current histopathologic criteria for the diagnosis of small ("early") HCC is necessary with increasing demands for tissue characterization of small "suspicious" nodules [13-17]. The window of opportunity in the treatment of HCC is limited as patients tend to present with advanced cancers.
The review gives an algorithmic approach to the FNAB diagnosis of focal liver lesions; an overview of current diagnostic issues concerning hepatic FNAB; problems and pitfalls in the cytodiagnosis of well-differentiated hepatocellular nodular lesions; and diagnostic utility of immunohistochemistry.
General approach to focal liver lesions
Focal lesions in the liver range from cysts and inflammatory processes to neoplasms, be they benign or malignant, primary or metastatic 1118]. This realm of pathology comes with its share of diagnostic challenges and pitfalls. Although imaging and tumor markers can provide a diagnosis in many instances, tissue confirmation may be warranted under circumstances where the clinical, biochemical and imaging profiles are not conclusive 19. A combined smearing and microhistology approach is strongly recommended [20-24]. Smears are air-dried and stained with Diff-Quik/May-Grunwald-Giemsa as well as fixed in 95% alcohol and stained by the Papanicolaou method. Particulate material is formalin-fixed for preparation of cell blocks for histologic study and for special and immunostains. Use of FNAB needles (21 gauge) with cutting mechanism may provide microbiopsy tissue cores.
Apart from cystic/inflammatory conditions, the major diagnostic issues are: (i) Distinction of benign hepatocellular nodular lesions, namely, macroregenerative nodule (MRN), dysplastic nodule (DN), focal nodular hyperplasia (FNH) and liver cell adenoma (LCA), from reactive hepatocytes; (ii) Distinction of well-differentiated HCC (WD-HCC) from benign hepatocellular nodular lesions; (iii) Distinction of poorly differentiated HCC (PD-HCC) from cholangiocarcinoma (CC) and metastatic carcinomas; (iv) Determination of histogenesis of malignant tumor; and (v) Determination of primary site of origin of malignant tumor.
Algorithmic approach
A diagnostic algorithm for FNAB diagnosis of focal liver lesions is given in Table 1. The emphasis is on HCC, its variants, and their differentiation from benign and malignant mimics 20.
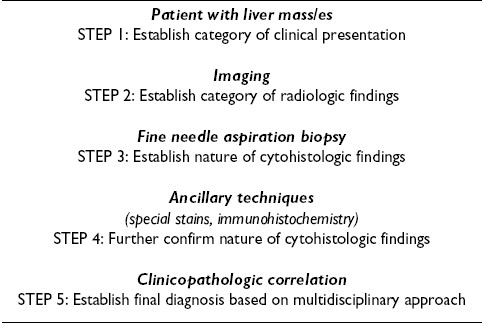
Step 1: Establish category of clinical presentation
A patient may present with a liver mass/es under the following clinical scenarios: (i) Routine medical check-up, (ii) Chronic liver disease due to, for example, hepatitis B or C virus infection, and alcoholism, (iii) Known cancer cases, (iv) Symptomatic patients, and (v) In childhood. Important relevant data include serum alpha-fetoprotein (AFP) and carcinoembryonic antigen (CEA) levels, hepatitis virus markers, results of liver function tests, presence of cirrhosis, biliary tract disease, calculi, liver fluke infestation, and history of hormone usage. Radiologic correlation is mandatory.
Step 2: Establish category of radiologic findings
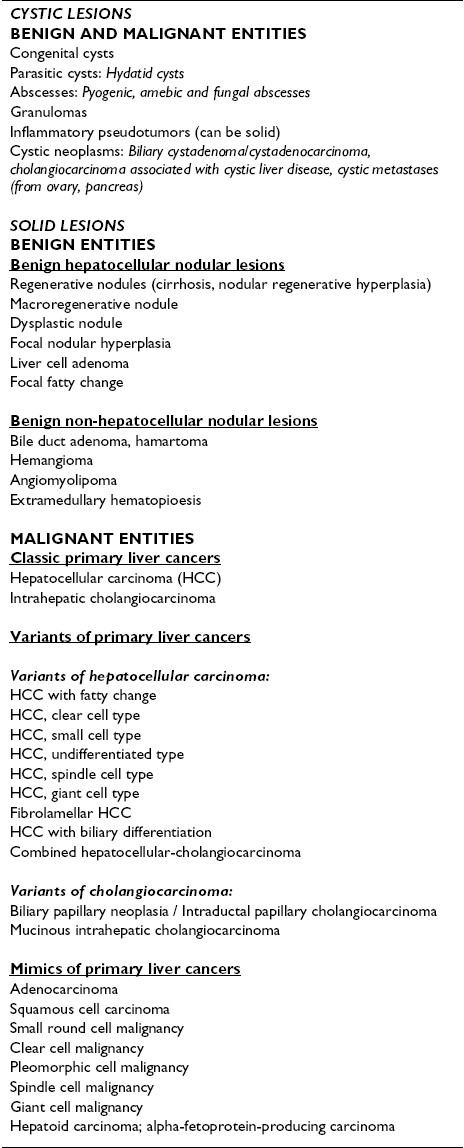
In many instances, a preoperative diagnosis can be achieved with a high degree of accuracy based on non-invasive imaging techniques and close clinical correlation. FNAB is useful in defining those lesions without characteristic imaging appearance. The solid or cystic nature of the lesion; number, size and location of the lesion/s; absence or presence of hepatomegaly, cirrhosis, steatosis, regional lymphadenopathy and calculi; and status of the biliary tract are important clues to the final diagnosis. Imaging of liver masses can be divided into two categories, namely, cystic and solid lesions. The focus is on a rational and pragmatic approach to hepatic FNAB. A list of entities is given in Table 2as a working guide.
Step 3: Establish nature of cytohistologic findings
FNAB of normal/reactive liver
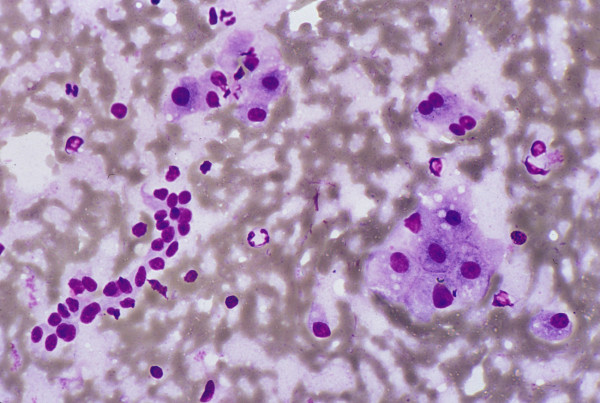
The liver parenchyma comprises a heterogeneous population of hepatobiliary and related cells, namely, hepatocytes, bile duct and ductular epithelia Figure 1; and Kupffer, endothelial, mesothelial and inflammatory cells. Hepatocytes often contain intracytoplasmic inclusions, such as, fat vacuoles, Mallory bodies and to a lesser extent, hyaline bodies; as well as intranuclear cytoplasmic inclusions. Pigments, such as lipofuscin Figure 2, bile and iron, may be present.
-
Normal and reactive hepatocytes: FNAB. Monolayered
clusters of loosely-cohesive, well-defined, polygonal cells
with ample dense, granular cytoplasm, round central nuclei
and low nuclear-cytoplasmic ratio. Note the double-layered
row of ductular epithelial cells with scanty cytoplasm (May-
Grunwald-Giemsa).
-
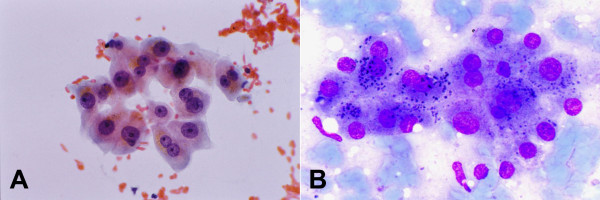
Normal and reactive hepatocytes: FNAB. (A) Hepatocytes show dense granular cytoplasm, round central nuclei, well-delineated
nuclear membrane, distinct nucleoli, granular chromatin and binucleation. Note polymorphism displayed by nonneoplastic
hepatocytes. The cells contain brown granules of lipofuscin pigment in the cytoplasm (Papanicolaou). (B) Lipofuscin appears
as black granules. The two elongated nuclei are likely to be Kupffer cells (May-Grunwald-Giemsa).
FNAB of liver with large and small cell change
The terms "large cell change" and "small cell change" have replaced large and small cell dysplasia 18. Hepatocytes with large cell change, considered a low-grade lesion, exhibit both cell and nuclear enlargement with nuclear atypia but retaining the normal nuclear-cytoplasmic ratio (N/C) of ≤ 1/3, by eyeballing the diameters of the nucleus and the cell Figure 3. On the other hand, in small cell change, considered a high-grade lesion and putative precancerous link with HCC, the hepatocytes are small and monotonous with subtle increase in N/C ratio; they impart an impression of nuclear crowding. Both types of changes are common in cirrhosis. The atypical cells can form dysplastic foci (< 1 mm diameter) or nodules (>1 mm diameter) in both cirrhotic and non-cirrhotic livers 18.
-
Hepatocytes with large cell change: FNAB. There is
simultaneous nuclear and cell enlargement of the hepatocytes,
thus maintaining the nuclear-cytoplasmic ratio of 1/3.
Note mild nuclear atypia (Papanicolaou).
FNAB of a liver mass: Stepwise approach
Liver aspirates can come from malignant or benign conditions of hepatocellular or non-hepatocellular origin [25-27]. Certain entities can give characteristic gross appearances when smeared 20. Naked eye inspection coupled with low power scanning view of the smears is a helpful adjunctive step to determine: (i) Patterns of spread of smears, (ii) Malignant or benign cell picture, (iii) Hepatocytic nature or otherwise, and (iv) Monotonous hepatocytic population or heterogeneous liver parenchymal components.
Patterns of spread of smears
A. Uniformly granular pattern. Hypercellular. Regularly irregular tissue fragments; evenly distributed in rows with tendency to be equidistant. Most likely HCC Figure 4.
-
Naked eye inspection of hepatic aspirate. Uniformly granular
pattern of spread of classic hepatocellular carcinoma.
Note the regularly irregular tumor fragments which tend to
be equidistant (Papanicolaou).
B. Non-uniformly granular pattern . Hypercellular. A mixed picture of irregular tissue fragments of variable size/shape; may include uniform granules. Variable cell picture -malignant to benign lesions of any origin.
C. Scanty pattern. Hypocellular. Streaks or fine clumps of cells. Variable cell picture -desmoplastic malignancies or benign conditions, such as cyst contents and hepatocellular nodular lesions. Pitfalls: Necrotic or cystic neoplasm; non-lesional sample.
D. Fluid pattern. Amorphous look. Thin fluid or thick pus. Usually benign and non-hepatocellular, such as abscess or infected cyst contents. Pitfalls: Dissociated tumor cells, such as lymphomas.
E. Microbiopsy pattern. Short and narrow, difficult-to-focus tissue cores, usually composed of nonneoplastic liver parenchyma with intact architecture. Admixed with other patterns, if the specimen is representative and includes lesional tissue. The clue is from the other patterns. Rarely, tumor tissue.
FNAB of hepatocellular carcinoma
HCC can be small/focal, solitary/large, and multifocal/diffuse; with satellite nodules and large vein involvement. Classic HCC is usually graded into well, moderately or poorly differentiated lesions. Histologic patterns comprise trabecular-sinusoidal, pseudoacinar and solid types; combinations are frequent 28. Close attention should be paid to architectural details in cell blocks/microbiopsies and smears. Accurate distinction from metastases, especially unresectable lesions, is necessary for appropriate therapy. One should be aware that there are limitations to the cytodiagnosis of HCC 2329-31].
Smears
-
Hypercellular smears with uniformly granular pattern of spread of the cells.
-
Cohesive clusters of malignant hepatocytes with arborizing, tongue-like projections of broad cords (>2 cells thick) that may be wrapped by peripheral endothelium Figure 5, Figure 6.
-
Rows of transgressing endothelium in larger aggregates; basement membrane material ("sinusoidal capillarization") best seen in Giemsa preparations.
-
Cohesion is the rule; however, tendency to dissociation noted in highly WD-HCC and PD-HCC.
-
Pseudoacini containing bile or pale secretions are not uncommon. The spaces are surrounded by polygonal cells with central nuclei similar to adjacent cells Figure 7.
-
Hepatocytic characteristics include polygonal cells with well-defined borders, ample granular cytoplasm, central round nucleus, well-delineated nuclear membrane, prominent nucleolus and fine, irregularly granular chromatin. Mitoses increase with nuclear grade. Cytologic features of malignancy are wanting at the highly WD-HCC end whereas resemblance to hepatocyes is lacking at the PD-HCC end.
-
Tumor cells may be smaller, larger or of the same size as normal hepatocytes. WD-HCC cells tend to be conspicuous by their small size, monotony, subtle increase in N/C ratio and nuclear crowding. PD-HCC cells tend to be pleomorphic Figure 8.
-
Atypical naked hepatocytic nuclei may abound Figure 9.
-
Multinucleated tumor giant cells may be of "osteoclastic" or pleomorphic type. The former shows nuclear features akin to adjacent HCC cells. Tumor giant cells may be found in all grades of HCC. Their presence does not necessarily upgrade the tumor.
-
Bile may be present within tumor cells or in canaliculi or pseudoacini.
-
Intracytoplasmic fat and glycogen vacuoles are common. Intracytoplasmic inclusions include hyaline, pale and Mallory bodies. Intranuclear cytoplasmic inclusions are not specific.
-
Bile duct epithelial cells, if present, are few and far apart. Kupffer cells may be seen.
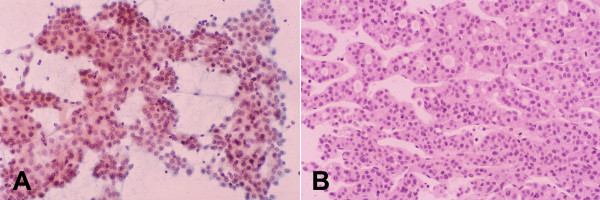
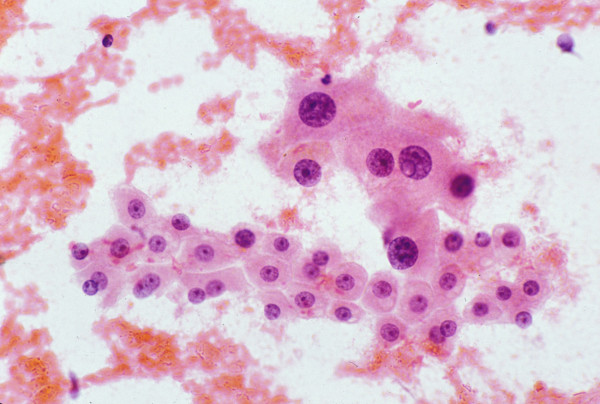
-
Well-differentiated hepatocellular carcinoma with trabecular and pseudoacinar patterns: FNAB. (A) Thick arborizing
cords of malignant hepatocytes showing cellular monotony, increased nuclear-cytoplasmic ratio, and impression of nuclear
crowding. The circular spaces among the cords represent pseudoacini (Papanicolaou). (B) Corresponding histologic section of
the tumor shows trabecular-sinusoidal arrangement with pseudoacini. Note the uniformity of the tumor cells and cords 2 to 3
cells thick (H&E).
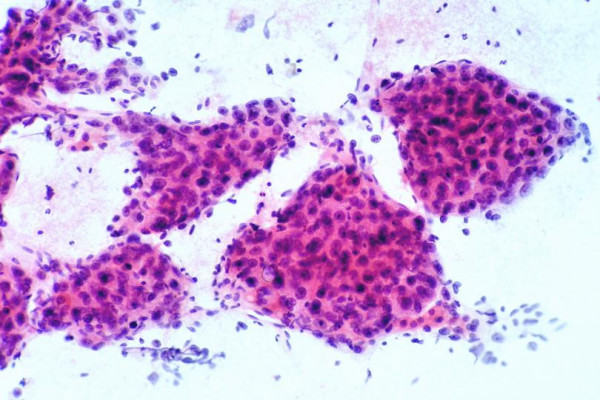
-
Moderately differentiated hepatocellular carcinoma:
FNAB. Thick cords of malignant hepatocytes are wrapped by
peripheral endothelium. They appear to be floating on transverse
section view (Papanicolaou).
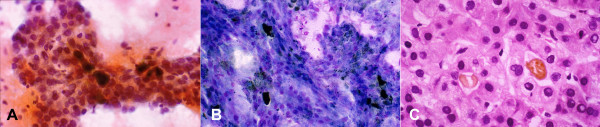
- Moderately differentiated hepatocellular carcinoma with pseudoacinar pattern: FNAB. (A) Pseudoacini filled with bile
which appears as dark brown blobs (Papanicolaou) (B) Bile appears black (May-Grunwald-Giemsa). (C) Corresponding histologic
section of the tumor shows cystically dilated canaliculi filled with golden-brown bile and surrounded by polygonal cells
with central nuclei (H&E).
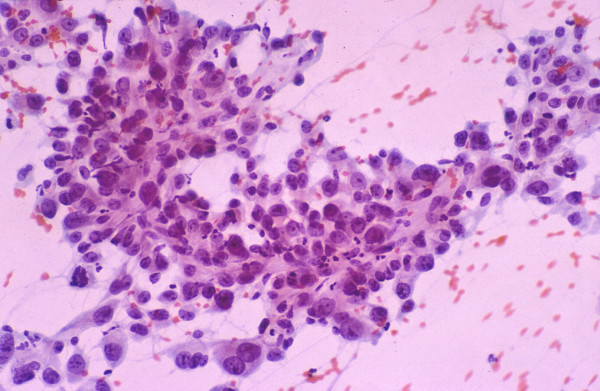
-
Poorly differentiated hepatocellular carcinoma: FNAB.
High-grade tumor shows marked pleomorphism but still
retaining some hepatocytic characteristics (Papanicolaou).
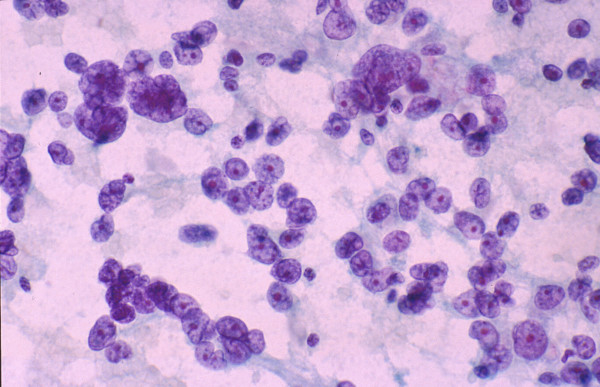
-
Poorly differentiated hepatocellular carcinoma: FNAB.
Atypical naked hepatocytic nuclei exhibit pleomorphism, thin
nuclear membrane, nuclear contour irregularities, prominent
nucleoli and multinucleation (Papanicolaou).
Cell blocks/microbiopsies
-
Microhistology provides additional invaluable architectural details such as trabecular-sinusoidal pattern formed by broad trabeculae (>2 cells thick); pseudoacini; unpaired arteries; and deficient/virtually absent reticulin framework.
FNAB of well-differentiated hepatocellular nodular lesions
The accuracy of cytodiagnosis at this end of the spectrum is often an issue with indeterminate reports being rendered 1432]. The diagnostic dilemmas are: (i) Is the sample representative? (ii) Are the hepatocytes malignant ( highly WD-HCC ) or benign? (iii) If benign, are they neoplastic ( LCA ) or nonneoplastic hepatocytes? (iv) If nonneoplastic, are they intralesional ( FNH, DN or MRN ) or extralesional hepatocytes ( cirrhosis or normal liver ), with/without fatty change ? Cytologic features predictive of HCC include increased N/C ratio, cellular monomorphism, nuclear crowding, trabeculae >2 cells thick, atypical naked hepatocytic nuclei and lack of bile duct cells 2333]. Cytologic parameters distinguishing highly WD-HCC from cirrhosis include well-defined cytoplasmic borders, scant cytoplasm, monotonous cytoplasm, thick cytoplasm, eccentric nuclei and increased N/C ratio 34.
FNAB of variants of hepatocellular carcinoma
HCC is well known for its histologic variations and subtypes. A large tumor can harbor areas that are more easily recognizable than others. This has significant practical implications on the number of passes and sampling in hepatic FNAB.
The variants of HCC include:
-
HCC with fatty change : Fatty change can occur in HCC without associated steatosis. Small (early) lesions are prone to fatty change due to inadequate vascularization. WD-HCC cells with cytoplasmic fat vacuoles can be mistaken for hepatocytes from fatty liver or focal fatty change Figure 10 3536]. PD-HCC cells with fat vacuoles may mimic malignant lipoblasts or signet-ring adenocarcinoma cells.
-
HCC, clear cell type: Clear cell change in hepatocytes is due to abundant cytoplasmic glycogen or lipid content 37. Tumor cells with empty-looking vacuoles after removal of glycogen during processing may mimic fatty change. Focal clear cell change is frequent. Diffuse clear cell change occurs in < 10% of cases of HCC 38.
-
HCC, small cell type: This type is reminiscent of neuroendocrine tumors with tendency to dissociation and microacinar formation but no obvious trabecular pattern 3940]. Scrutiny of the nuclear/chromatin features should reveal the true histogenesis of the small round cells.
-
HCC, undifferentiated type : The tumor cells are larger than the small cell category with no obvious hepatocytic characteristics.
-
HCC, spindle cell type: This is rare and is more likely to be seen with tumor giant cells as part of a larger tumor 41.
-
HCC, giant cell type: This pure variant is rare.
-
Fibrolamellar HCC: This occurs in non-cirrhotic livers of young patients and has a good prognosis. It comprises large, discohesive polygonal hepatocytes with abundant oncocytic cytoplasm and lamellar fibrosis. Pale bodies are common 4243].
-
HCC with biliary differentiation : Some HCC are positive for biliary markers (AE1/3, CK19) 44. The stem cell theory with bipotential progenitor cells capable of developing into either hepatocytes or biliary epithelial cells provides a satisfactory explanation for primary liver cancers arising from different stages of the cell lineage 45.
-
Combined hepatocellular-cholangiocarcinoma (CHCC-CC): This is a rare tumor containing unequivocal elements of HCC and CC that are intimately admixed with a transitional component 4647]. The HCC cells are expected to be AFP and Hep Par 1-positive and show polyclonal CEA ( p CEA) canalicular staining. The CC cells are AE1/3-positive and show brush border/diffuse cytoplasmic p CEA reactivity. The intermediate cells exhibit hybrid features with equivocal immunoprofiles.
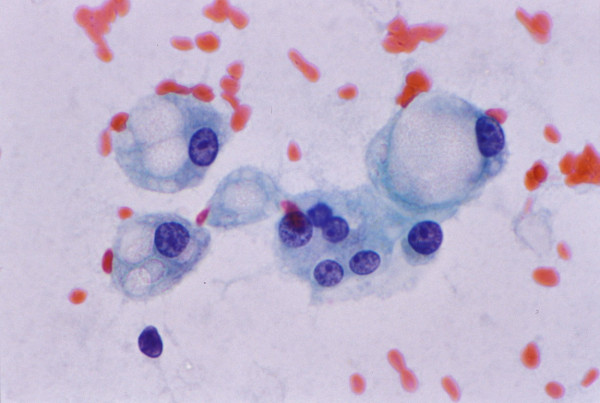
-
Fatty change: FNAB. Hepatocytes exhibit polymorphism
and contain cytoplasmic fat vacuoles of varying sizes pushing
nucleus to one side. Cytologic picture can mimic signet-ring
cell adenocarcinoma (Papanicolaou).
FNAB of cholangiocarcinoma and its variants
Intrahepatic CC are rare and usually occur in non-cirrhotic livers in close proximity with sizeable bile ducts. Predisposing diseases include clonorchiasis, opisthorchiasis, hepatolithiasis and primary sclerosing cholangitis. CC are usually well to moderately differentiated adenocarcinomas with variable degree of desmoplasia. They can be categorized into papillary and/or tubular adenocarcinomas. Mucin production is minimal. A squamous component may be present. Adjacent biliary epithelial changes include carcinoma-in-situ and biliary ductular proliferation. There are three cytologic smear patterns, namely (i) Scanty cell smear pattern, (ii) Adenocarcinoma with proliferating ductular clusters, and (iii) Adenocarcinoma without prominent ductular clusters 20.
The variants of CC include:
FNAB of non-hepatocellular carcinoma malignancies
That the liver is a common target for metastases makes the separation between primary and secondary malignancies all the more difficult, especially when the particular histologic subtype can arise in the liver as well. Categorizing them based on cytologic patterns is a good start.
-
Adenocarcinoma: Most are metastases from stomach, colorectum, pancreas, breast and lungs. Colorectal metastases have much tumor diathesis. Signet-ring cell adenocarcinomas are likely to be gastric in origin. Pancreaticobiliary tract adenocarcinomas can have squamous components. For any adenocarcinoma in hepatic aspirates, CC, HCC with pseudoacini and CHCC-CC have to be considered.
-
Squamous cell carcinoma: Most are metastatic or arise in the pancreaticobiliary tract. Large, spindly, "tadpole-shaped" or bizarre cells with dense cytoplasm, keratinization and much necrosis may be seen. Adenosquamous variants are not uncommon.
-
Small/intermediate round cell malignancy: This includes neuroendocrine tumors (NET), small cell undifferentiated carcinomas (SCUC), undifferentiated nasopharnygeal carcinomas and lymphomas 4049]. Other possibilities include melanoma, Merkel cell tumor, metastatic adenocarcinomas from prostate, stomach and breast (lobular carcinoma), CC, certain sarcomas and HCC, small cell type. Most NET are from the gastrointestinal tract (GIT), pancreaticobiliary tract or lung; primary hepatic NET is unusual 50. SCUC usually originates in the lung. Hepatic metastases from nasopharnygeal carcinomas tend to be markedly necrotic mimicking abscesses radiologically. Lymphoma seldom presents as a primary neoplasm although hepatic involvement is common in advanced disease 51. It has been reported in hepatitis B virus infection, systemic lupus erythematosus, primary biliary cirrhosis and acquired immunodeficiency syndrome. A high index of suspicion is necessary. It can be mistaken for poorly differentiated carcinoma or HCC. Inflammatory processes, such as inflammatory pseudotumors [52-54], and sinusoidal hematopoietic cells have first to be excluded. Nodular extramedullary hematopoiesis can rarely mimic metastases.
-
Clear cell malignancy: This can also arise in the kidney, adrenal and ovary 55. Renal cell carcinoma contains transgressing endothelium and papillary endothelium in fibrovascular cores but lacks peripheral endothelium. Nuclear features also differ. However, renal cell carcinoma has not been called the great mimic for no good reason. Metastases can occur years later. Other pitfalls include hepatocytes with fatty change and metastases from liposarcoma and signet-ring cell adenocarcinoma.
-
Pleomorphic cell malignancy: This includes large cell undifferentiated carcinomas (LCUC), large cell lymphomas, germ cell tumors and various sarcomas. LCUC is not a pure or single entity; tumors may show glandular and neuroendocrine differentiation. Common sites are lung, GIT and female genital tract.
-
Spindle cell malignancy: Well-differentiated spindle cell tumors include leiomyosarcoma (LS), neurogenic tumors and fibroblastic/stromal tumors, including gastrointestinal stromal tumor (GIST) 56. At the poorly differentiated end, LS, malignant fibrous histiocytoma, undifferentiated sarcoma or even sarcomatoid HCC or CC with a spindle cell component, have to be considered. Apart from distinguishing LS from leiomyoma, other differential diagnoses include benign reactive processes 57. The epithelioid variant of LS occurs primarily in the GIT. Hepatic metastases can be mistaken for epithelial tumors, such as, HCC, CC, metastatic carcinoma and melanoma. GIST also has spindle and epithelioid cell types 58. Metastatic GIST may pose diagnostic problems due to their variable morphologic spectrum and cytologic atypia; c-kit staining is necessary. Hepatic angiosarcoma associated with Thorotrast and epithelioid hemangioendothelioma are rare. In the young, differential diagnoses of spindle cell lesions include inflammatory pseudotumor, infantile hemangioendothelioma, mesenchymal hamartoma and undifferentiated (embryonal) sarcoma.
-
Giant cell malignancy: This can be carcinomatous or sarcomatous. Giant cell carcinomas have been noted in lung, pancreas, thyroid, kidney and breast. Hepatic metastases have to be distinguished from HCC, giant cell type.
-
Hepatoid carcinoma; AFP-producing carcinoma: Primary hepatic tumors are not the only source of AFP. Neither is the liver the only site of origin for hepatocytic-looking carcinomas. Most of them arise in the lung and GIT. Those with hepatoid features mimic classic HCC in having a proclivity for vascular permeation and distant metastases, in this case to the liver. Others produce AFP but are non-hepatoid ; being usually adenocarcinoma, undifferentiated carcinoma and small or large cell neuroendocrine carcinoma 59.
Suffice it to say that when dealing with FNAB of liver masses, one must be fully aware of any past history of malignancy or rule out any hitherto undetected cancer. Some of the limitations in the categorization of tumors obtained by FNAB can be overcome by immunohistochemistry. The advantages of an exact cytodiagnosis are obvious - it may save the patient a diagnostic laparotomy, especially in inoperable cases, and allow for specific chemotherapy to be instituted without delay. However, at best, information gleaned from a precise cytodiagnosis can sometimes only favor a particular primary site.
Step 4: Further confirm nature of cytohistologic findings
The initial cytologic assessment is crucial as it forms the basis upon which ancillary tests are ordered; the results of which should be interpreted in the larger context of the case.
Special stains
· Reticulin
Study of the reticulin framework, stained by Gomori′s method, is important in the analysis of well-differentiated hepatocellular nodules 6061]. HCC have deficient or absent reticulin; the reticulin framework in LCA and FNH may not be well developed either.
· Periodic acid-Schiff with and without diastase; Mucicarmine
This is usually performed to distinguish glycogen from epithelial mucin. Hepatocytes are loaded with glycogen. Cells exhibiting glandular (biliary) differentiation may show intracytoplasmic or intraluminal mucin production.
· Fat
Fat can be demonstrated in nonneoplastic and neoplastic hepatocytes by staining fresh or formalin-fixed tissue with Oil Red O. It has no discriminant value in defining the biological status of hepatocytes but may be of help in deciphering the contents of cytoplasmic vacuoles of cells of unrecognizable histogenesis.
· Iron
Iron appears as black cytoplasmic granules in hepatocytes stained with Giemsa stains and dark brown with Papanicolaou stains. The pigment can be confirmed with Perl′s prussian blue method. Iron accumulation in hepatocytes favors a benign process. On the other hand, in populations where hepatic iron accumulation is common, the absence of iron in hepatocytes should alert one to the presence of a proliferative process, be it regenerative or neoplastic.
Immunohistochemistry
A whole battery of antibodies is available for the comparative immunohistochemical study of primary and metastatic liver tumors. The two major diagnostic issues are (i) whether the hepatocytes are malignant or benign, and (ii) what the histogenesis of the malignant cells is [See "Diagnostic utility of immunohistochemistry" ].
Step 5: Establish final diagnosis based on multidisciplinary approach
Close clinicopathologic correlation is mandatory for enhancing the yield of FNAB diagnoses and the reduction of indeterminate reports.
Current diagnostic issues
Reappraisal of role of hepatic fine needle aspiration biopsy
1. It is still to procure a tissue diagnosis as part of the evaluation of focal liver lesions but under circumstances where the clinical, biochemical and imaging profiles are not conclusive
A benign cytodiagnosis obviates unnecessary surgery. Surgical resection is indicated for any resectable malignant hepatic mass, be it primary or secondary. In unresectable malignant lesions, a precise cytohistologic typing is crucial for appropriate alternative therapy. The need for biopsy diagnosis in HCC is now a hotly debated topic 2412. The stand in some practices is that a needle biopsy may be indicated only if it is not possible to diagnose HCC by other means, namely, serum AFP concentration, spiral computed tomography (CT) and magnetic resonance imaging (MRI). In advanced HCC, the need for biopsy may be obviated due to the non-availability of effective therapy. In early/small HCC, even if a biopsy is performed, the diagnostic sensitivity of the procedure may be as low as 60% 3. If there is a high index of suspicion for HCC despite negative cytologic sampling, specific therapy may be instituted.
2. It is a safe and well-tolerated, minimally invasive procedure with low risk of complications in suitable candidates and in skilled hands
Needle track seeding by malignant cells is the main reason often cited by opponents of FNAB of the liver 262-64]. There is no reliable data to establish the risk; the figure of 0.006% is regarded as a gross underestimation by many authors 36566. Needle biopsies, in general, are usually not indicated in patients deemed suitable for liver transplantation due to possible seeding 5. The move to institute definitive treatment for classic HCC diagnosed solely on clinical, biochemical and radiologic grounds without tissue confirmation has crept slowly into some practices. The risk of false positive diagnosis of HCC with subsequent aggressive therapy, such as liver transplantation, by far outweighs the risk of seeding.
3. It is a technique with high sensitivity and specificity when practised in a multidisciplinary setting by skilled operators
Tissue procurement by FNAB under radiologic guidance and cytologic interpretation of the aspirated material are both highly operator-dependent. An experienced screener on-site can give a rapid assessment of adequacy.
4. Surveillance for early HCC in high-risk patients has resulted in the detection of "suspicious" nodules in cirrhotic livers that are often < 2 cm in diameter
The usual surveillance tools are serum AFP concentration and ultrasonography (US). AFP is not a very good screening test since it has a sensitivity of 39-64%, a specificity of 76-91% and a positive predictive value of between 9-32% 667]. On the other hand, US has, as a screening test in hepatitis B surface antigen (HBsAg) carriers, a sensitivity of 71% and specificity of 93%, but its positive predictive value is only 14% 6. Although a spectrum of hepatocellular nodular lesions can be encountered, especially in a background of cirrhosis, studies have revealed that about half of them are not HCC. Tissue characterization is, therefore, mandatory and FNAB may be the simplest and most practical means to reach small, deep-seated lesions 1.
The European Association for the Study of the Liver (Barcelona-2000 EASL Conference) 5has drawn up guidelines for the current clinical management of HCC. HCC can be diagnosed in a setting of liver cirrhosis if a focal liver lesion is >2 cm in diameter with arterial hypervascularization, and shows coincident features in at least two imaging techniques (US, spiral CT, MRI and angiography); or characteristic features in one imaging technique associated with serum AFP level of >400 ng/ml. It follows then that there is no necessity to obtain cytologic and/or histologic confirmation for all cases of suspected HCC. It is recommended that punctures be limited to patients with nodules < 2 cm diameter for the differentiation of HCC from regenerative nodules. In the future, tumor biopsy may assume a different role by becoming a useful tool for procuring tissue for the molecular profiling of the disease. It is important to note that the above recommendations are not meant for everyone. It should be carried out in highly selected patients by highly skilled healthcare teams in specialized tertiary centers.
Fine needle aspiration biopsy versus core needle biopsy
Fine needle aspiration biopsy is useful for (i) cirrhotic patients with poor liver function with risk of bleeding; (ii) liver masses with obstructive jaundice and risk of bile leakage, those near big vessels, or where there is need to go through bowel; (iii) small (< 2 cm diameter), deep-seated and difficult to approach nodules that require close patient co-operation during the procedure; (iv) representative sampling of sizeable lesions by re-direction of the needle and multiple passes; and (v) on-site rapid assessment of adequacy and rendering of provisional diagnosis, as well as for appropriate triage of tissue specimens for ancillary studies (e.g. microbiology, flow cytometry, genetic testing, molecular diagnostics, cell block preparation and electron microscopy) 22.
Core needle biopsy (CNB), with the availability of more material, provides tissue for histologic and immunohistochemical studies, especially in two major areas of diagnostic difficulties, namely, in the (i) differentiation of WD-HCC from benign hepatocellular nodules; and (ii) separation of HCC from CC and metastases. A critical comparative evaluation of the risks of seeding by CNB and FNAB is needed. For the proponents of CNB, the view is that a single pass with larger bore needles (< 20 gauge) may be preferable to multiple passes by finer needles needed to obtain sufficient material for cytohistologic examination. The incidence of seeding may increase as a result of the number of punctures of tumors detected at an early stage in patients with a longer life expectancy 66.
Consensus: The diagnostic accuracy in terms of sensitivity, specificity and positive predictive value of FNAB for HCC is almost similar to that of CNB. The accuracy rate is highly operator-dependent and increases with both techniques combined. The specificity and positive predictive value of FNAB in the diagnosis of malignant hepatic lesions has been shown to be close to 100% in most studies 192268 69. These results are comparable to the accuracy of CNB. In a comparative study, it was reported that both procedures, FNAB and CNB, had the same diagnostic accuracy of 78% when considered separately and of 88% when considered in combination 21. The conclusion was that the great advantage of combining the two techniques was the reduction in false negative results. Performing both procedures at the same sitting may not be feasible due to medical contraindications and may also not be acceptable clinical practice. Using larger caliber cutting needle biopsies can be associated with a greater number of complications 68. This quagmire can be overcome by using cutting FNAB needles. At our institution, the utilization of such (21 gauge) aspiration needles has provided us with ample material for smear preparations as well as tissue cores resembling microbiopsies rather than mere cell blocks 2034]. Our practice has been to retrieve sizeable particulate matter from the material on the glass slides prior to smearing and fixing them in formalin for cell block preparation. Many studies have attested to the improved diagnostic yield and accuracy of FNAB using the combined cytohistologic approach 2370]
FNAB can provide rapid on-site diagnosis when the smears are stained with Diff-Quik or Ultra-fast Papanicolaou stain 28. In the era of rising costs in medical practice and higher patient/practitioner/institution expectations of efficiency and faster turn-around time, FNAB can obviate the need to wait for tissue processing if accurate cytologic diagnoses can be rendered. Another cost-saving advantage, especially for less developed countries, is that smears are cheap, convenient and easy to prepare as long as there is an experienced person to interpret them.
Considering the overall advantages and cost-analysis, FNAB can be suggested as the initial method of choice for evaluation of focal liver lesions in most clinical settings. The final choice should be decided on the basis of the working clinical diagnosis and the institutional/personal experience.
Separation of well-differentiated hepatocellular carcinoma from benign hepatocellular nodular lesions
Factors that pose diagnostic problems and pitfalls
-
HCC can be small and focal, solitary and large, multifocal, or diffuse and infiltrating; thereby, mimicking small benign lesions on the one hand and metastases on the other, especially in imaging studies.
-
Serum AFP, though fairly specific, has poor sensitivity for the diagnosis of HCC, regardless of tumor size or degree of differentiation. There is significant elevation in about 50-60% of HCC 2433]. Small WD-HCC are usually not associated with serum AFP elevation. On the other hand, transient increases may be seen with inflammatory flares in chronic viral hepatitis. Published data at the current moment suggest using values of >400 ng/ml for diagnostic confirmation of HCC 67.
-
The diagnostic dilemma at the highly WD-HCC end is that the hepatocytic histogenesis is obvious but proof of malignancy may be lacking 243233. The cell cords tend not to be >2 cells thick and the cellular pleomorphism and subtle increase in the N/C ratios of the hepatocytes may not be appreciated under light microscopy. In fact, highly WD-HCC tend to be composed of small, uniform, strikingly monotonous neoplastic hepatocytes with slightly increased N/C ratios, imparting an impression of nuclear crowding 34. The recognition of polymorphism with variation in cell and nuclear sizes and a normal N/C ratio of 1/3 should alert one to the likelihood of benignity of the hepatocytes 20.
-
Routine surveillance by more than one imaging technique in high-risk patients tends to detect nodule/s of various natures in the cirrhotic livers 1071]. HCC tend to occur in a cirrhotic background together with MRN and low- and high-grade DN. Pure light microscopic interpretation with immunohistochemical input may no longer suffice in the diagnostic workup towards an accurate tissue diagnosis.
-
Smaller and smaller lesions (< 2 cm diameter) are being increasingly detected by imaging and subjected to FNAB for tissue diagnosis.
-
Early HCC tend to be small and highly well-differentiated, making differentiation from MRN and DN difficult. Increasing numbers of equivocal nodular lesions are being detected in cirrhotic livers by various imaging modalities. The histopathologic interpretation of these nodules is highly controversial, let alone cytologic assessment. Eastern pathologists tend to call them early HCC while the Western fraternity tends to go for a diagnosis of high-grade DN 1417].
-
Small HCC are prone to fatty change due to inadequate neoangiogenesis; poor vascularity makes them difficult to visualize and characterize by imaging methods 814]. Increasing degree of heterogeneity is exhibited as the lesion enlarges 15.
-
Well-differentiated hepatocellular nodules of any nature can occur in non-cirrhotic livers and have to be distinguished from each other and from the surrounding liver.
-
Fatty change can occur in WD-HCC, LCA, FNH and DN without associated steatosis in the surrounding liver parenchyma. An entity called focal fatty alteration/change can also mimic these nodules 2036].
-
Current histologic criteria for the diagnosis of highly WD-HCC include loss of reticulin, thickening of trabeculae (>2 cells thick), sinusoidal capillarization, unpaired arterioles, cellular pleomorphism/heterogeneity, "clonal" growth patterns, pseudoacini, mitotic activity 1416] and stromal invasion of portal tracts. Some of these criteria are lacking in cytologic material.
-
In high-risk cases, some "non-malignant" nodules with large cell change (low-grade DN) or small cell change (high-grade DN) may be precursor lesions 111872-74]. Morphometric studies suggest that small cell change may be the more sinister lesion biologically. HCC occurs in 5-40% of cirrhotic patients and foci of cancer are found in a third of DN 75. Some of these precursor lesions may be indistinguishable from malignant hepatocellular nodules by light microscopy alone.
-
Current concepts on how premalignant lesions develop and how HCC may arise within them impact on the accuracy of pathologic diagnosis of hepatocellular nodules 7910. Inadequacies of FNAB evaluation of hepatocellular nodules cannot be ignored given the focality of proliferative clones of atypical cells within the nodules and the shortcomings of pure morphologic interpretation.
Diagnostic utility of immunohistochemistry
There are two major applications for immunohistochemical markers in the diagnostic workup of focal liver lesions 2076]. One is to decipher the exact histogenetic origin of obvious tumor nodules - that is, the histologic typing and the primary site 77. It may not always be possible to distinguish between the poorly differentiated entities of HCC, CC and metastatic carcinomas 2578]. By the same token, adenocarcinomas occurring in the liver may be metastatic or primary in origin. Of interest lately is the increasing documentation of AFP-producing extrahepatic hepatoid/non-hepatoid carcinomas that have a propensity for vascular invasion and liver metastases 5979]. The immunoprofile of these tumors, originating mostly in the GIT and lungs, is almost identical to that of HCC. Serum AFP levels tend to be very high. The other application concerns the distinction of the various lesions within the realm of well-differentiated hepatocellular nodular lesions. [See " FNAB of well-differentiated hepatocellular nodular lesions "]. For ascertainment of malignancy in hepatocellular nodules, the antibody panel should comprise at least AFP, p CEA or CD10, and CD34 [80-82]. Additional markers, such as Hep Par 1 and cytokeratins, should be included if the histogenesis of the tumor is to be studied. Markers of cell proliferation, proliferating cell nuclear antigen (PCNA) and Ki67; and p53 are not routinely used.
-
AFP is fairly specific but not sensitive for HCC. Tissue AFP immunoreactivity is expected in HCC Figure 11but it may be patchy and minimal. Sensitivity is about 50% (range, 20-75%) and is low at both ends of the histologic spectrum of HCC 4483-86]. A study of 56 patients with small HCC (< 2 cm diameter) showed AFP-positivity in 44.6% of the tumors 87. A variable staining pattern may be encountered with CHCC-CC. MRN in cirrhosis are not associated with elevated serum or stainable tissue AFP 88; neither are DN or LCA. The specificity of AFP for HCC is being challenged by reports of AFP-producing extrahepatic hepatoid/non-hepatoid carcinomas 59. There is an increasing tendency to drop this immunomarker from the panel for HCC workup.
-
p CEA stains bile canaliculi and ductal epithelium but not hepatocytes. A characteristic "chicken-wire fence" canalicular staining pattern is seen in normal liver 89. Biliary epithelial cells show diffuse cytoplasmic and brush border staining. A normal canalicular pattern is expected for benign hepatocellular nodular lesions. However, some LCA and even, some FNH, may exhibit deficient canaliculi. There are two patterns of staining in HCC - canalicular and/or diffuse cytoplasmic staining. The canalicular pattern is abnormal and deficient in HCC with twisted and often dilated structures; it may not even be appreciable in high-grade HCC 78. Instead, diffuse cytoplasmic staining may be seen in less differentiated HCC, making distinction from a poorly differentiated adenocarcinoma difficult. In the context of a carcinoma, a canalicular pattern is specific for HCC.
-
CD10 is expressed in normal and neoplastic liver, exhibiting a similar canalicular staining pattern to p CEA 9091]. Although it does not differentiate between benign and malignant hepatocellular nodular lesions, CD10 is very useful in distinguishing HCC from non-HCC malignancies. The sensitivity of CD10 (68.3%) is far better than immuno-staining for AFP (23.8%) but less sensitive than p CEA (95.2%) in the diagnosis of HCC 92.
-
Hep Par 1 (Hepatocyte antigen) is a sensitive marker for hepatocytic differentiation and is part of the antibody panel for distinguishing HCC from CC and metastases. However, not all HCC stain uniformly Figure 12and not all Hep Par 1-positive tumors are of hepatocellular origin or arise in the liver 8693-96]. Its variable and heterogeneous staining pattern, which can range from 100% -positive cells in WD-HCC to < 5% in some PD-HCC cases, may lead to false negative results in small samples 97. MRN, DN, FNH and LCA tend to exhibit 100% positivity. Hence, this antibody has no discriminant value in the evaluation of the biological status of well-differentiated hepatocellular nodular lesions.
-
Cytokeratins (CK 7, 8, 18, 19, 20; CAM 5.2; AE1/AE3). Mature hepatocytes stain with CK 8 and 18 and CAM 5.2 but not with CK 7, 19 or 20 or AE1/AE3. CAM 5.2 is the most reliable cytokeratin antibody for HCC. AE1/AE3 negativity is expected in hepatocellular lesions. Focal CK 7 and 19 positivity can be seen in high-grade HCC. HCC is generally CK 20 negative 98.
-
CD34 is an intercellular adhesion protein found in normal endothelium but absent in normal sinusoids. CD34 highlights regions of sinusoidal capillarization where there is basement membrane material deposition. Diffuse sinusoidal CD34 reactivity is seen in HCC, even small WD-HCC 99. However, significant reactivity is also seen in LCA and some FNH. In cirrhotic and DN, staining is absent or minimal, and confined to the periportal/periseptal regions 100.
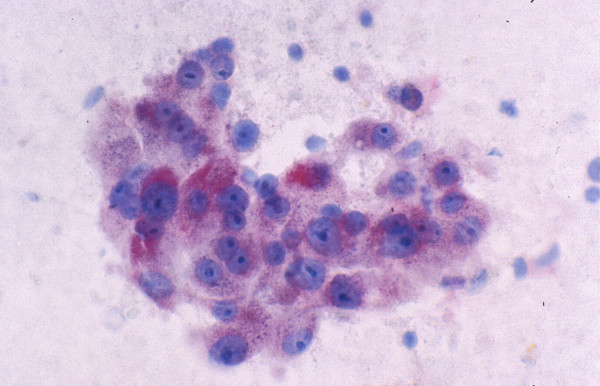
-
Hepatocellular carcinoma: FNAB. The tumor cells stain
positively for alpha-fetoprotein (Immunostain).
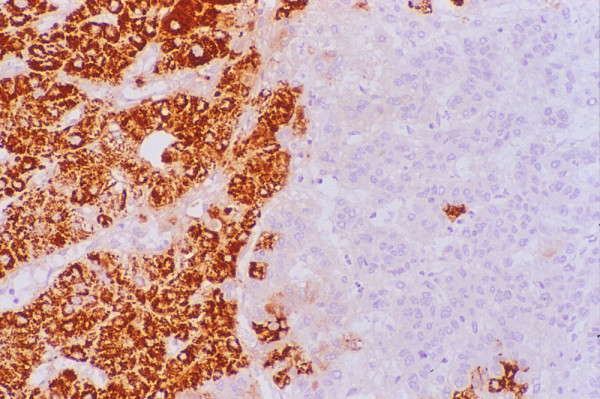
-
Hepatocellular carcinoma: Histology. Parts of the tumor
show intense granular cytoplasmic reactivity with HepPar1
(Immunostain).
Conclusion
Tissue confirmation is recommended in the diagnosis of focal liver lesions as the risk of aggressive therapy is greater than the risk of malignant seeding. FNAB has many advantages that CNB lacks. The lack of tissue can be overcome by using cutting aspiration needles that can provide material for smear cytology and microhistology. Guided FNAB is a highly operator-dependent procedure as is the preparation and interpretation of the cytologic material. Optimization of FNAB in the diagnosis of focal liver lesions, increase in the yield of true positive diagnoses, and rendering of fewer indeterminate reports require close clinicopathologic correlation; combination of smear cytology and microhistology, use of a discriminant panel of special and immunostains; and team work by skilled operators on all fronts.
List of abbreviations
FNAB : Fine needle aspiration biopsy
HCC : Hepatocellular carcinoma
MRN : Macroregenerative nodule
DN : Dysplastic nodule
FNH : Focal nodular hyperplasia
LCA : Liver cell adenoma
WD-HCC : Well-differentiated hepatocellular carcinoma
PD-HCC : Poorly differentiated hepatocellular carcinoma
CC : Cholangiocarcinoma
AFP : Alpha-fetoprotein
CEA : Carcinoembyronic antigen
N/C ratio : nuclear-cytoplasmic ratio
CHCC-CC : Combined hepatocellular-cholangiocarcinoma
p CEA : polyclonal carcinoembyronic antigen
NET : Neuroendocrine tumor
SCUC : Small cell undifferentiated carcinoma
GIT : Gastrointestinal tract
LCUC : Large cell undifferentiated carcinoma
LS : Leiomyosarcoma
GIST : Gastrointestinal stromal tumor
CT : Computed tomography
MRI : Magnetic resonance imaging
US : Ultrasonography
CNB : Core needle biopsy
PCNA : Proliferating cell nuclear antigen
Competing interests
The author(s) declare that they have no competing interests.
Authors′ contributions
This is solely my work.
The author would like to thank T. C. Tan for photographic assistance.
"Co-editors of Cytojournal, Vinod B. Shidham, MD, MRCPath, FIAC and Barbara F. Atkinson, MD thank:
The academic editor CAROLYN ERNST GROTKOWSKI, M.D., QUEST DIAGNOSTICS INCORPORATED, Mount Laural, NJ 08054, USA ( Carolyn.E.Grotkowski@questdiagnostics.com) for completing the peer-review process for this manuscript."
References
- Liver Transpl 2004, 10 (2 Suppl 1) : S26-S29
- Gut 2001, 48: 440 (Letter to editor)
- J Hepatol 2001, 35: 254-258
- J Hepatol 2001, 35: 874 (Letter to editor)
- Hepatology 1998, 27: 273-278
- Gastroenterology 1997, 112: 1617-1623
- J Hepatol 2000, 33: 282-289
- Gastroenterology 1998, 115: 1216-1222
- Semin Liver Dis 1995, 15: 360-371
- Hepatology 1995, 22: 983-993
- Hepatology 1999, 30: 889-893
- Liver 1998, 18: 14-19
- Liver Transpl 2004, 10 (2 Suppl 1) : S3-S8
- Liver 1999, 19: 12-18
- Histopathology 2001, 38: 167-174
- In Atlas of Tumor Pathology.3rd series. Fascicle In Atlas of Tumor Pathology 3rd series Fascicle 31 Armed Forces Institute of Pathology, Washington, DC; 2001
- [Google Scholar]
- Diagn Cytopathol 2000, 23: 326-328
- Diagnostic algorithms A Southeast Asian perspective [] Bangkok: Year Book Publisher Co Ltd; 2004
- Acta Cytol 2003, 47: 332-336
- Fine needle aspiration cytology or needle core biopsy. J Clin Gastroenterol :S101-S108.
- [Google Scholar]
- microbiopsy for the diagnosis of well-differentiated hepatocellular carcinoma and macroregenerative nodule.Definition of standardized criteria from a study of 100 cases. Acta Cytol :515-523.
- [Google Scholar]
- Acta Cytol 1994, 38: 347-354
- Diagn Cytopathol 1999, 21: 370-377
- Semin Diagn Pathol 1995, 12: 64-76
- Diagn Cytopathol 1995, 12: 364-371
- Cancer (Cancer Cytopathol) 2004, 102: 27-33
- Acta Cytol 1993, 37: 309-316
- Acta Cytol 2003, 47: 581-589
- Acta Cytol 1991, 35: 661-670
- Cancer 1999, 87: 270-277
- Acta Cytol 2003, 47: 16-26
- Acta Cytol 1999, 43: 610-616
- Diagn Cytopathol 1994, 11: 385-389
- Acta Cytol 2002, 46: 567-570
- Diagn Cytopathol 1986, 2: 290-294
- Diagn Cytopathol 1997, 17: 306-310
- Acta Cytol 1997, 41: 583-586
- Acta Cytol 1996, 40: 937-947
- Cancer 1996, 77: 51-57
- Diagn Cytopathol 1990, 6: 275-279
- Diagn Cytopathol 1999, 21: 180-187
- Am J Pathol 1996, 149: 1167-1175
- Pathology 2001, 33: 130-141
- Cancer 2002, 94: 2040-2046
- Acta Cytol 1999, 43: 131-138
- Diagn Cytopathol 2000, 22: 293-298
- Diagn Cytopathol 1998, 19: 29-32
- Diagn Cytopathol 1997, 16: 383-391
- Acta Cytol 1996, 40: 257-262
- Acta Cytol 1997, 41: 1325-1328
- J Gastroenterol 2000, 35: 641-645
- Acta Cytol 1995, 39: 453-462
- Cancer 1999, 87: 380-389
- Diagn Cytopathol 1994, 10: 232-241
- Acta Cytol 1993, 37: 300-308
- Diagn Cytopathol 2002, 27: 298-302
- Wee A, Thamboo TP, Thomas A: Alpha-fetoprotein-producing liver carcinomas of primary extrahepatic origin: Fine needle aspiration biopsy experience in 2 cases. Acta Cytol 2003, 47:799-808.
- Mod Pathol 1997, 10: 1258-1264
- Acta Cytol 1995, 39: 596-598
- Radiology 1992, 183: 787-788
- Radiology 1991, 178: 253-258
- Liver Transpl 2000, 6: 67-72
- J Hepatol 1996, 25: 334-338
- Gut 1999, 45: 626-627
- Sherman M: Alphafetoprotein: an orbituary. J Hepatol 2001, 34:603-605.
- Am J Gastroenterol 1990, 85: 1009-1013
- Dig Dis Sci 1996, 41: 2326-2331
- Hepatogastroenterology 2000, 47: 522-527
- Am J Surg Pathol 1993, 17: 1113-1123
- Semin Diagn Pathol 1998, 15: 285-299
- Am J Gastroenterol 1996, 91: 836-837
- Virchows Arch (A) Pathol Anat Histopathol 1993, 422: 381-388
- In Diagnostic Immunohistochemistry .Edited by: Dabbs DJ. New York In Diagnostic Immunohistochemistry Edited by: Dabbs DJ New York Churchill Livingstone; 2002:374-378
- [Google Scholar]
- Cytopathology 2000, 11: 262-267
- Hum Pathol 2002, 33: 1175-1181
- Histopathology 1997, 31: 47-54
- Diagn Cytopathol 2004, 30: 1-6
- Pathol Int 1999, 49: 310-317
- Histopathology 1989, 14: 503-513
- Am J Surg Pathol 1991, 15: 280-288
- Am J Clin Pathol 1993, 99: 551-557
- Mod Pathol 1997, 10: 686-692
- Oncol Rep 1998, 5: 355-358
- Liver 1995, 15: 30-34
- Acta Cytol 1997, 41: 1147-1155
- Am J Surg Pathol 2001, 25: 1297-1301
- Diagn Cytopathol 2004, 30: 92-97
- Mod Pathol 2002, 15: 1279-1287
- Am J Surg Pathol 2002, 26: 978-988
- Mod Pathol 2003, 16: 137-144
- Cancer (Cancer Cytopathol) 2001, 96: 49-52
- Cancer (Cancer Cytopathol) 2001, 93: 288-291
- Histopathology 1998, 33: 318-324
- Mod Pathol 1996, 9: 901-909
- Cancer (Cancer Cytopathol) 2000, 90: 273-278
- Acta Cytol 2000, 44: 218-222







