Translate this page into:
Fine-needle aspiration of metastatic renal cell carcinoma to a male breast: A rare initial presentation
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Sir,
Breast metastasis from extramammary tumors is extremely uncommon and accounts for only 3% of all breast masses.[12] Reported primary malignant tumors that spread to the breast include melanoma, ovarian, thyroid, pulmonary, hepatic and rarely renal cell carcinoma.[3456] Clinical symptoms at presentation are unreliable to distinguish between a primary breast carcinoma and a metastatic tumor to the breast. Prompt diagnosis of a metastatic tumor is imperative to prevent an unnecessary lumpectomy or mastectomy as well as to render appropriate management for what may be an occult primary tumor. We herewith report an interesting case of a de novo male breast mass that demonstrated atypical cytological features suspicious for malignancy on fine-needle aspiration (FNA), which initiated an excisional biopsy, revealing a metastasis from what was determined to be an occult renal cell carcinoma. On literature search, all the reported cases of metastatic renal cell carcinoma to the breast were in females, except one in which metastasis was noted in the skin of the male breast.[7] The present case describe the first report of clear cell renal cell metastasis in the male breast parenchyma with no skin involvement.
A 57-year-old male presented for a 1.3 cm palpable right breast mass, which was detected by self-examination. There were no associated overlying skin lesions. He denied having nipple discharge, pain, or any other masses. Ultrasonography of the right breast revealed a 1.3 cm solid non-specific mass at the 12:00 position of intermediate suspicion (ACR BIRADS Category: 4). There were no associated microcalcifications. FNA of the mass (two direct smears stained with the Papanicolaou stain) demonstrated moderately cellular smears of epithelioid to spindled cells arranged in three-dimensional clusters as well as two-dimensional sheets of cells with vague papillary configurations. A few singly dispersed cells were also noted. The cells demonstrated a low nuclear to cytoplasmic ratio, clear/vacuolated cytoplasm and regular to convoluted nuclear membranes, inconspicuous nucleoli, and rare intra-nuclear pseudo-inclusion. Some of the cellular groups demonstrated a fine capillary meshwork. The background was clean with no evidence of necrosis [Figure 1a–c]. The FNA was performed in a physician's office, so no on-site pathological evaluation was done; neither additional material for a cellblock preparation was provided. The differential diagnosis included a primary breast carcinoma or metastasis from an unknown primary such as clear cell renal cell carcinoma, pulmonary adenocarcinoma and clear cell “sugar” tumor of the lung, epithelioid hemagioendothelioma, adrenal cortical carcinoma or melanoma. Due to limited available material, further work-up by immunohistochemical stains could not be performed. A diagnosis of atypical cytological features suspicious for malignancy was rendered with a recommendation for a repeat FNA with a cellblock preparation or a biopsy of the mass.
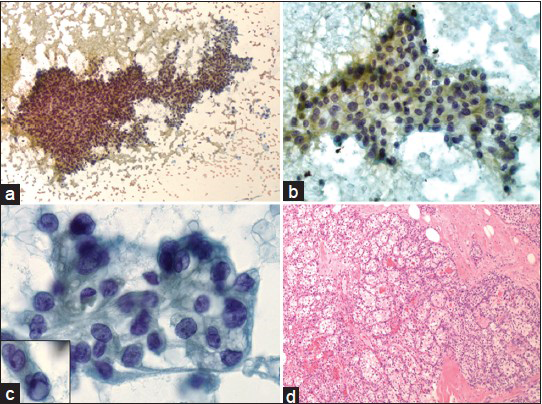
- (a) Moderately cellular smear demonstrates three-dimensional clusters of epithelioid to spindled cells in vague papillary configuration with occasional singly dispersed cells (Papanicolaou, ×100). (b) Two-dimensional sheets of epithelioid cells with low nuclear to cytoplasmic ratio (Papanicolaou, ×200). (c) The tumor cells show distinct cytoplasmic borders, clear/vacuolated cytoplasm, regular to convoluted nuclear membranes and inconspicuous nucleoli. Inset shows rare intra-nuclear pseudoinclusion (Papanicolaou, ×400). (d) Histopathologic section of the right breast lumpectomy reveals metastatic low-grade clear cell renal cell carcinoma (H and E, ×100)
At 1 week later, the patient underwent a right breast lumpectomy, which revealed fatty breast parenchyma with a circumscribed mass. Histological sections of the mass demonstrated a low-grade clear cell carcinoma [Figure 1d]. There were no mitoses or areas of necrosis. Immunohistochemical stains revealed that the tumor cells were positive for PAN-cytokeratin (CK), CK7, CD10 and PAX-8, and negative for CK20, TTF-1, ER and PR. A diagnosis of metastatic renal cell carcinoma, clear cell type was rendered. Subsequently, an abdomen computed tomography scan demonstrated an 8.6 cm peripherally enhancing mass involving the upper pole of the right kidney. At 1 month later, a right nephrectomy demonstrated a clear cell renal cell carcinoma (10.1 cm) with tumor extending into the perinephric fat and in the segmental branch of the renal vein (pT3aNxM1). No other sites of metastasis from the renal carcinoma were found radiologically. The patient has been on regular follow-up for the past 3 years with no recurrence or evidence of other metastases.
Renal cell carcinoma is also known as “the internist's tumor” because of bizarre and unpredictable initial presentations. Breast metastasis from renal cell carcinoma is rare with 16 cases reported in the literature including 7 cases presenting as the initial sign of metastatic disease.[8] In the majority, the breast masses were noted 1-18 years status post-nephrectomy of renal cell carcinoma of clear cell type.[9] The metachronous metastases have been found to carry a better prognosis than the synchronous metastasis.[10] Of the 16 cases reported, 15 have been described in women and in one man a palpable metastatic lesion was noted in the skin of the breast.[7] The present report is the first case of clear cell renal cell carcinoma metastasis appearing initially as a breast mass in a male breast without skin involvement.
Differentiation of primary breast cancer from secondary metastasis is very important because of differences in treatment and prognosis. Breast involvement may be a signal of rapid widespread dissemination of the neoplasm with a crude survival rate of only 10.9 months reported before, but occasionally long-term survival may also occur.[11] Breast radiology does not seem to always helpful but features such as microcalcifications and spiculations are not usually seen in metastatic extramammary breast tumors when compared to the primary breast carcinoma. Extramammary tumors do not involve the ducts and thus nipple retraction, skin dimpling, and nipple discharge are rare as is the regional lymph node involvement. No microcalcifications or skin changes were seen in our case, but in the absence of a cell-block preparation and the unusual cytomorphology prompted us to consider the other differential diagnosis besides primary breast carcinoma and to recommend a biopsy for a definitive diagnosis.
In summary, this case highlights that rare metastasis from an extramammary site such as kidney can occur and can be the initial presenting sign. This report is the first case of clear cell renal metastasis presenting initially as a breast mass in a male breast without skin involvement. A cell-block preparation for performing immunohistochemical studies in addition to both Diff Quik and Papanicolaou stains on FNA specimens can aid in the differentiation of primary breast carcinoma from metastasis.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors declare no conflict of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. No funding was provided, and no support in the form of grants, gifts, equipment, and/or drugs was received.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of Cyto Journal publications, the review process of this manuscript was conducted under a double-blind mode (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Fine-needle aspiration cytology of extra mammary metastatic lesions in the breast: A retrospective study of 36 cases diagnosed during 18 years. Cytojournal. 2010;7:10.
- [Google Scholar]
- Breast metastasis from renal cell carcinoma: Rare initial presentation of disease recurrence after 5 years. J Breast Cancer. 2012;15:244-7.
- [Google Scholar]
- Metastatic disease to the breast: The Washington University experience. World J Surg Oncol. 2007;5:74.
- [Google Scholar]
- Metastatic hepatocellular carcinoma presenting as gynecomastia in male: A diagnostic dilema in fine needle aspiration cytology. Cytojournal. 2012;9:21.
- [Google Scholar]
- Fine-needle aspiration of metastatic renal-cell carcinoma masquerading as primary breast carcinoma. Diagn Cytopathol. 1998;18:343-5.
- [Google Scholar]
- Fine needle aspiration biopsy diagnosis of metastatic neoplasms of the breast. A three-case report. Cytojournal. 2005;2:17.
- [Google Scholar]
- Bilateral breast metastases of a renal carcinoma: A case report and review of the literature. BMJ Case Rep 2008 2008 bcr0620080239
- [Google Scholar]
- Solitary breast mass as initial presentation of clinically silent metastatic renal cell carcinoma. Breast. 2006;15:427-9.
- [Google Scholar]
- Metastatic renal adenocarcinoma presenting in a breast screening programme. Eur J Surg Oncol. 1996;22:641-3.
- [Google Scholar]
- Solitary breast metastases from a renal cell carcinoma. Breast Cancer Res Treat. 2001;68:29-31.
- [Google Scholar]







