Translate this page into:
Flow cytometry, molecular analysis, and other special techniques (in Serous Fluid Cytopathology)

*Corresponding author: Ali Gabali, MD, PhD, Professor, Director of Hematopathology and Hematopathology Fellowship, Department of Pathology, Wayne State University School of Medicine, Karmanos Cancer Center, Detroit, Michigan, United States. agabali@med.wayne.edu
-
Received: ,
Accepted: ,
How to cite this article: Gabali A. Flow cytometry, molecular analysis, and other special techniques (in Serous Fluid Cytopathology). CytoJournal 2022;19:18.
HTML of this article is available FREE at: https://dx.doi.org/10.25259/CMAS_02_13_2021
Abstract
Morphological and architectural pattern evaluations play a major role in the rpretation of hematopoietic neoplasms. However, confirmation of diagnosis, classification, prognosis, and risk stratification are highly dependent on the utilization of multiple ancillary studies. The importance of these ancillary studies increases in evaluating serous fluid samples, as these samples lack architecture and patterns. Likewise, the morphology can be disturbed by sample preparation. The most common ancillary studies utilized are flow cytometry, immunohistochemistry for immunophenotyping, Fluorescent In Situ Hybridization (FISH), cytogenetics for structural and gene rearrangements, and molecular studies for mutational analysis. Among them, flow cytometry analysis is the handiest test to perform with high diagnostic yield on serous fluid specimens. In this article we will discuss the use, caveat, and role of the most common ancillary studies on serous fluid specimen evaluation. This review article will be incorporated finally as one of the chapters in CMAS (CytoJournal Monograph/Atlas Series) #2. It is modified slightly from the chapter by the initial authors (Choladda Vejabhuti, MD and Chung-Che (Jeff) Chang, MD, PhD) in the first edition of Diagnostic Cytopathology of Serous Fluids.
Keywords
Lymphoma
Leukemia
Serous Fluid
Flow cytometry
Immunohistochemistry
Fluorescent In Situ Hybridization (FISH)
Cytogenetics
Molecular analysis

Flow cytometry, molecular analysis and other special techniques, have become important adjunctive tools for the evaluation of effusion fluids. Although the majority of these techniques are applied to hematopoietic neoplasms, there has been an increased use of these methods for solid neoplastic and non-neoplastic processes, especially characterizing infectious diseases. Even though these ancillary techniques have limitations and may not be indicated in every case, their application may be helpful in rendering the diagnosis in many cases. • The purpose of this review is to summarize the application of these molecular, and other, special techniques to effusion fluids.
FLOW CYTOMETRY
Serous effusion is an appropriate specimen for flow cytometric analysis to confirm the diagnosis of malignant lymphoid neoplasm. However, unlike the role of flow cytometry in the diagnosis of lymphoid lesions by fine-needle aspiration or biopsies, which is well-accepted, its use in serous fluids is not largely utilized. However, recently its use in body fluid analysis has increased significantly. • It is usually difficult to differentiate between reactive lymphocytosis and malignant lymphoma in serous effusions, especially in low-grade malignant lymphomas, on morphology alone. Therefore, flow cytometric analysis can be useful in this situation.[1]
FLOW CYTOMETRY AND HEMATOPOIETIC NEOPLASMS
When the diagnosis of lymphoma/leukemia is suspected on cytopathologic examination and/or the patient has had a previous history of lymphoma/leukemia, flow cytometry can be helpful in the diagnosis and the classification of these tumors. • The algorithms in Figures 1 and 2 and Table 1 summarize the approaches of using flow cytometry data for aiding in the diagnosis of hematopoietic lesions.
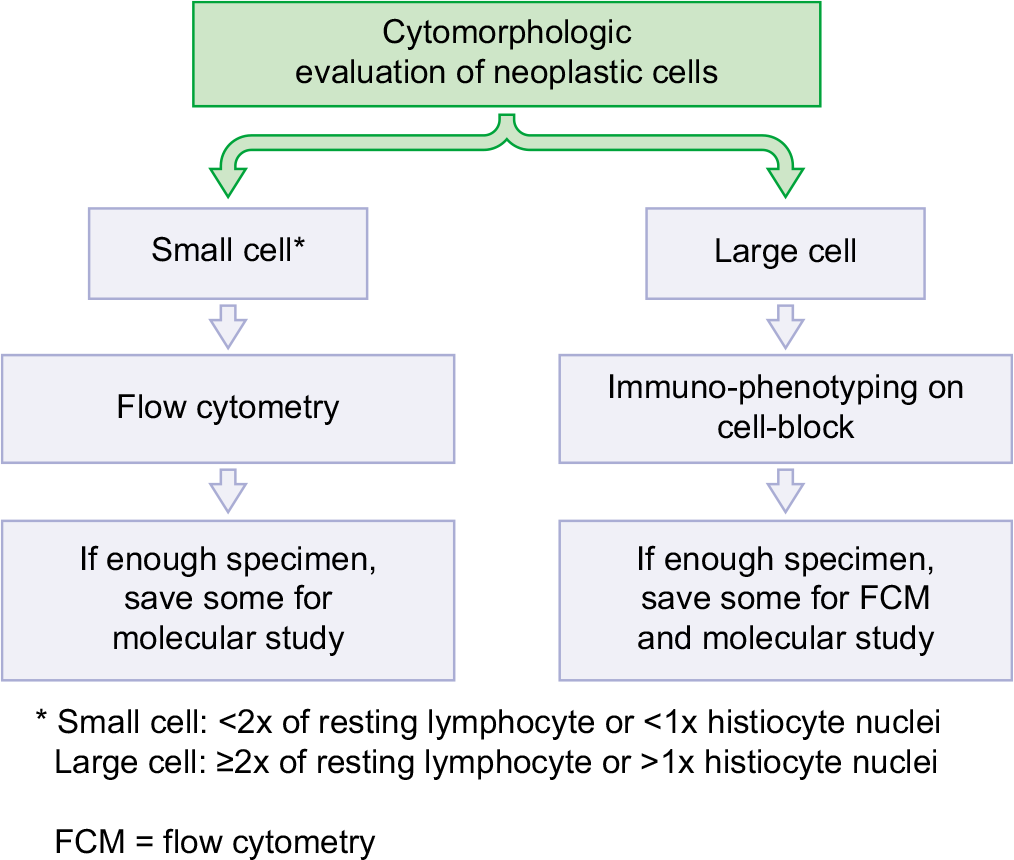
- Algorithm for evaluation of serous effusion suspicious for lymphoma.

- Flow cytometry immunophenotyping of lymphomas/lymphoid leukemias.
| ALL | AML | |
|---|---|---|
| B-cell | T-cell | CD13+ |
| CD19+ | CD2, 3, 5, 7 + | CD33+ |
| CD20+/− | CD4/CD8 + | CD34 (negative in acute promyelocytic leukemia and leukemia associated with NPM1 mutation or monocytic differentiation) |
| TdT+ | Either dual CD4/CD8 positive or negative | HLA-DR +/− |
| CD34+/− | Dual TdT+ | CD41 and CD61, Megakaryocytic differentiation |
| CD34+/− | CD71, Glycophorin A: Erythroid differentiation | |
| CD64, CD4, CD14: Monocytic differentiation | ||
The main differential diagnoses when one encounters small lymphocytes are usually between low-grade lymphomas and reactive effusion. Flow cytometry is usually the most helpful in this setting to distinguish the two. The encountering of large neoplastic cells usually brings up the diagnostic consideration of blue cell tumors and other large cell tumors such as poorly differentiated carcinomas, melanomas, and sarcomas.[2–4] Flow cytometric analysis is often less helpful in this circumstance (because the large lymphoma/blasts cells can be missed during sample processing). In such cases immunocytochemistry on cell-block sections is more useful [see Figures 1, 2 and Table 1].
UNIQUENESS OF FLOW CYTOMETRIC STUDIES OF HEMATOPOIETIC NEOPLASMS IN SEROUS EFFUSIONS
Use of flow cytometry as a screening tool
Questions usually arise if all serous effusion specimens with numerous small, mature-appearing lymphoid cells should be sent for flow cytometry. A few prospective studies investigating flow cytometry as a screening tool in serous effusion specimens are reported.[1,5,6] Finch et al[6] evaluated flow cytometric analysis in pleural lymphocytosis, which was defined as specimens containing a majority of small, mature-appearing, small lymphocytes or cytologically atypical lymphoid cells.
By flow cytometry, 73.9% of pleural fluids were categorized as reactive and 12.3% were positive for non-Hodgkin lymphoma. But when patients did not have a prior history of non-Hodgkin lymphomas, only 4% of pleural effusions were positive for lymphoma by flow cytometry. The above data did not suggest the use of flow cytometry as a screening tool to diagnose non-Hodgkin lymphomas in pleural lymphocytosis.[5] In contrast, Wells and Jorgensen[7] presented a study in an abstract form that included 309 pleural effusions from 281 patients. The majority of these cases were without a known history of a lymphoproliferative disease. The specimens were triaged for flow cytometric analysis based on cytologic examination. Eight percent (26/309) of all cases evaluated by the flow cytometric analysis demonstrated a new positive diagnosis. The authors concluded that 8% of all cases was a significant number and may be a valuable way to confirm the primary diagnosis of lymphoid malignancy. • In our opinion, the decision to send the specimen for flow cytometric analysis should be based primarily on cytomorphologic evaluation, clinical context, and patient clinical history.
The usefulness of flow cytometry in selected patient populations
• When flow cytometry is performed in a selected patient population that has a known history of or is suspected of malignant lymphoma either clinically or cytomorphologically, flow cytometry is proved to be a more valuable tool.[1,6] Czader and Ali performed a retrospective study on 115 serous effusions from pleural (n = 86), peritoneal (n = 20), and pericardial (n = 9) fluids. In these cases, the main indication for flow cytometric analysis was the presence of spontaneous serous effusions in patients with known histories of malignant lymphoma. The application of flow cytometry and morphology together were able to assign all but one case that was originally diagnosed as atypical/ suspicious (16% of specimens studies) to either benign or malignant categories resulting in appropriate clinical staging and management in the majority of the cases.[1] In the same study, 4 cases with benign morphology were reclassified to the atypical/suspicious category after flow cytometric studies demonstrated an aberrant population. Another study by Finch et al showed that, in 34.2% of patients with a previous history of malignant lymphoma and 33.3% of patients with cytologically atypical lymphoid cells in their pleural fluid, the diagnosis was confirmed by using flow cytometric studies.[6]
Significance of an aberrant or monoclonal lymphoid population by flow cytometry
The finding of an aberrant or monoclonal lymphoid population by flow cytometry in serous effusions is not equal to a diagnosis of malignant lymphoma. • The presence of monoclonality by flow cytometry without appropriate clinical context and morphologic findings should be interpreted cautiously and does not always equate with a diagnosis of malignant lymphoma. In other types of cytology specimens such as fine needle aspirations, studies of flow cytometric analysis of reactive lymph nodes with follicular hyperplasia and lymphoid follicles of Hashimoto’s thyroiditis, patients have shown that germinal center (GC) B-cells (CD10+/CD20+) can show upwardly skewed kappa-to-lambda light chain (K/L) ratios. This may give a false impression of monoclonality.[8] Likewise, a monoclonal lymphoid population in a serous effusion may be identified but does not always mean a diagnosis of malignant lymphoma. For example, a case in Czader’s study[1] showed a kappa restricted B-cell population in the setting of a chronic hepatitis C infection without evidence of lymphoma.
FLOW CYTOMETRY AND NON-HEMATOPOIETIC NEOPLASM
Unlike the flow cytometry role in hematologic neoplasm, the application of these techniques in non-hematopoietic neoplasm is not well accepted in clinical practice and is still under investigation. There have been studies using flow cytometry on non-hematopoietic neoplasms on bench fine needle aspiration specimens[9] and paraffin-embedded tissue.[10] However, such applications on serous effusions have not been reported in the literature.
The percentage of CD16+, CD56+, and CD3– natural killer lymphocytes by flow cytometry may be helpful in identifying metastatic adenocarcinoma in serous effusion.[11,12] An association of a higher percentage of natural killer (NK) cells (12–33%[12] and 29–68%[11]) with metastatic adenocarcinoma has been reported. In contrast to this, mesotheliomas, lymphomas, leukemias, malignant melanomas, and reactive mesothelial hyperplasia showed a lower percentage of NK cells (1–16%[12] and 2–20%[11]). • It was further suggested that the presence of ≥12% NK cells should make the cytopathologist suspect the presence of metastatic adenocarcinoma.[12]
ELECTRON MICROSCOPY
With an increasing use of immunohistochemistry, there has been a decreasing use of electron microscopy in the diagnosis of malignant neoplasm. The main usefulness of electron microscopy in serous effusion fluid is in the differential diagnosis between malignant mesothelioma and metastatic adenocarcinoma after cytopathologic confirmation of malignancy. The presence of slender and elongated microvilli, abundant intermediate filaments, and lack of secretory granules favored mesothelioma, whereas short stubby microvilli and secretory granules suggested a diagnosis of adenocarcinoma.[13,14] As a result, overlapping features may exist and cause difficulty in interpretation.[13] • The main challenge with serous fluid specimens as compared to tissue biopsy is to locate and identify the malignant cell unequivocally for ultrastructural evaluation. Spuriously, the reactive mesothelial cells in the background may be evaluated and misinterpreted as mesothelioma. Furthermore, in clinical practice, electron microscopic studies are considered expensive, laborious, lengthy, and, nowadays, the diagnosis is usually reached with the help of immunocytochemistry.
FLUORESCENCE IN-SITU HYBRIDIZATION (FISH) AND METASTATIC SEROUS EFFUSION
Application of fluorescence in-situ hybridization (FISH) technique has not become popular in serous effusion analysis. In hematopietic neoplasm, FISH analysis is standard practice to identify chromosomal structural and gene rearrangement abnormalities. A cell-block may be of more value for FISH testing than lose cells. In carcinoma, recent studies have demonstrated the possible role of this method for detecting malignant cells in effusions with higher sensitivity.[15–22] The potential uses of FISH using different centromere specific probes on chromosomes 1, 3, 7, 8, 9, 10, 11, 12, 17, and 18 (with different combinations) have been examined in serous effusions for detecting pleural malignant mesothelioma,[15] metastatic breast cancer,[16] and metastatic carcinoma.[16–21] The studies suggested that FISH can be helpful in cytologically negative or ambiguous effusions and yielded highly sensitive and specific results when used together with cytologic examination.[15–22] Diagnostic superiority was demonstrated in metastatic effusions from the breast, lung, pancreas, gynecologic, and gastrointestinal carcinomas.[18]
Furthermore, cases with known primary tumors associated with abnormal FISH patterns would facilitate the appropriate choice of centromeric probes for detection of metastasis.[20] However, the limitation of FISH has been observed in cases consisting of a small population of malignant cells hidden against a background of inflammatory or reactive cells.[21]
• It should be highlighted that cytomorphologic examination is still the gold standard for tumor staging and is the only method accepted in the classical AJCC cancer staging.[18,23] Prospective studies are needed to demonstrate the clinical benefit of FISH to detect disseminated tumor cells in correlation with clinical outcomes.[18]
MOLECULAR GENETICS
Molecular genetics and hematopoietic neoplasms
Determination of clonality of neoplastic lymphoid/leukemic cells using molecular genetics has been increasingly applied in a variety of specimens, including peripheral blood, bone marrow biopsy, bone marrow aspiration, paraffin-embedded tissue, and even fine-needle aspiration specimens such as cell-blocks and cytologic smears.[24–33] These techniques have also been successfully applied to effusion specimens.[27,34–36]
Mihaescu et al[36] compared the use of molecular genetics to the diagnosis of lymphoid-rich effusions in 95 consecutive patients with concomitant immunocytochemistry. The specimens included 74 pleural, 20 peritoneal, and one pericardial fluid. A proportion of patients (28 cases) had a previous diagnosis of non-Hodgkin lymphoma. Polymerase chain reaction (PCR) and Southern blot analysis (only done on PCR negative samples) to assess a clonal rearrangement of the IgH or TCR-g genes or a BCL2/IgH fusion gene were performed and were successful in 90 cases. Monoclonality was identified in 40 (42%) of the 95 effusions analyzed. Although flow cytometry was not studied, the authors found that molecular genetic analysis did provide additional information in some cases that were inconclusive by immunocytochemistry.[36]
Currently, applications of molecular diagnostic techniques are considered an important tools for clinical diagnosis of hematopoietic malignancies. • In addition, to morphology and immunophenotyping (both flow cytometry and immunocytochemistry) FISH, cytogenetics and molecular testing can be important ancillary methods for reaching an accurate diagnosis and classification.
Tables 2 and 3 summarize the major molecular genetic abnormalities in lymphomas and acute leukemias.
| Lymphomas | Cytogenetic abnormalities | Molecular genetic abnormalities | Prognostic significance |
|---|---|---|---|
| CLL/SLL | 13q14 deletions | Favorable | |
| Follicular lymphoma | t(14;18)(q32;q21) | BCL2-IgH fusion | None |
| Mantle cell lymphoma | t(11;14)(q13;q32) | BCL1-IgH fusion | None |
| MALT lymphoma | t(11;18)(q21;q21) | API2-MALT1 fusion | No responses to H. pylori eradication therapy |
| t(14;18)(q32;q21) | IGH-MALT1 fusion | N/A | |
| t(1;14)(p22;q32) | BCL10-IgH fusion | N/A | |
| Burkitt lymphoma | t(8;14)(q24;q32) | cMYC-IgH fusion | N/A |
| ALCL | t(2;5)(p23;q35) | ALK-NPM fusion | Favorable |
Abn, abnormality; ALCL, anaplastic large cell lymphoma; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; MALT, mucosa associated lymphoid tissue; N/A, not applicable or information not available.
| Leukemia | Cytogenetic Abn | Molecular Genetic Abn | Prognostic significance |
|---|---|---|---|
| AML | t(8;21)(q22;q22) | ETO-AML1 fusion | Favorable |
| inv(16)(p13;q22) | MYH11-CBFβ fusion | Favorable | |
| t(16;16)(p13;q22) | MYH11-CBFβ fusion | Favorable | |
| t(15;17)(q22;q21) | PML-RARα fusion | Favorable | |
| t(6;9)(p23;q34) | DEK-CAN fusion | Intermediate | |
| t(11;q23) | Fusions involving MLL | Unfavorable | |
| t(9;11)(p22;q23) | MLL-AF9 fusion | Unfavorable | |
| ALL | t(9;22)(q34;q11) | BCR-ABL fusion | Unfavorable |
| t(12;21)cryptic | TEL-AML1 fusion | Favorable | |
| t(1;19)(q23;p13) | E2A-PBX fusion | Unfavorable | |
| t(11;q23) | Fusions involving MLL | Unfavorable | |
| t(4;11)(q21;q23) | MLL-AF4 fusion | Unfavorable |
Abn: Abnormality, ALL: Acute lymphoblastic leukemia, AML: Acute myeloid leukemia.
MOLECULAR GENETICS AND SOFT TISSUE TUMORS
The knowledge of molecular pathogenesis of soft tissue tumors has grown tremendously over the last decade. Molecular diagnostic techniques, particularly reverse transcriptasePCR (RT-PCR) and FISH, have become important tools to detect the characteristic fusion genes associated with soft tissue tumors [Table 4]. Nonetheless, the application of these techniques to serous effusion specimens is rarely required in clinical usage for two reasons. First, sarcomas rarely cause serous effusions. Secondly, when sarcomas are present in serous effusions, the diagnosis of sarcoma has almost always already been established[2,37] Table 4 summarizes the major molecular genetic abnormalities in soft tissue tumors.
| Type of soft tissue tumor | Cytogenetic Abn | Molecular genetic Abn |
|---|---|---|
| 1. Rhabdomyosarcoma | ||
| Spindle cell | NA | NA |
| Embryonal /Botryoid | Gains of 2, 7, 8, 12, 13; losses of 1, 6, 9, 14, and 17 |
IGF2, GOK, PTCH TP53 |
| Alveolar | t(2;13)(q35;q14); t(1;13)(p36;q14) |
PAX3-FKHR PAX7-FKHR |
| 2A. Non-rhabdomyosarcoma-EWS family | ||
| EWS/PNET | t(11;22)(q24;q12) t(21;22)(q22;q12) t(7;22)(p22;q12) |
EWS-FLI-1 EWS-ERG EWS-ETV1 |
| DSRCT | t(11;22)(p13;q12) | EWS-WT1 |
| Clear cell sarcoma | t(12;22)(q13;q12) | EWS-ATF1 |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22-23;q11-12) t(9;15)(q22;q21) t(9;17)(q22;q11) |
EWS-TEC (CHN) TCF12-TEC TAF2N-TEC |
| Extraskeletal mesenchymal chondrosarcoma | der(13;21)(q10;q10) | NA |
| 2B. Non-rhabdomyosarcoma–others | ||
| Synovial sarcoma | t(X;18)(p11;q11) |
SYT-SSX1 SYT-SSX2 |
| Inflammatory myofibroblastic tumor | t(1;2)(q25;p23) t(2;19)(p23;p13) |
TPM3-ALK TPM4-ALK |
| Myxoid/round cell liposarcoma | t(12;16)(q13;p11) t(12;22)(q13;q12) |
TLS-CHOP EWS-CHOP |
| Alveolar soft part sarcoma | t(X;17)(p11;q25) | ASPL- TFE3 |
N/A, not applicable/not know
Modified from reference 55.
POLYMERASE CHAIN REACTION FOR DIAGNOSIS OF PLEURAL TUBERCULOSIS
Although the detection of mycobacterial DNA in pleural effusions by PCR appears to be a promising approach for the rapid diagnosis of pleural tuberculosis, there is no consensus regarding the usefulness of PCR testing for the diagnosis of pleural tuberculosis.[38] The reported sensitivity of PCR testing for pleural tuberculosis varies, ranging from 17.5 to 83%,[38–47] probably due to different PCR methods and diverse clinical diagnostic criteria. The reported specificity of the tests ranged from 78 to 100%, approaching 100% in most.[38–47] The above data suggest that PCR is not recommended in a routine clinical practice, although clinical utility of PCR testing for diagnosis of pleural tuberculosis may be improved in the future.
OTHER TECHNIQUES
In this section, we describe techniques that are applicable to serous effusions, but their role at present in routine clinical practice is controversial. For a detailed review of the emerging techniques for serous effusion application, please refer to other timely articles such as that by Ross.[48]
CYTOGENETICS
Although effusion fluid is suitable for cytogenetic evaluation, the use of this technique in the clinical specimen is limited. Several reports suggested the role of cytogenetics in distinguishing malignant and benign pleural effusions by using the presence of several chromosomal aberrations in neoplastic effusions.[49] It was initially believed that aneuploidy or polyploidy were specific for malignant cells, with the specificity approaching 100% in the diagnosis of malignant effusions. However, benign reactive mesothelial cells with chromosomal abnormalities have been described.[49] Moreover, the technique itself also limits its application to clinical specimens. It is laborious, time consuming, and requires fresh material, with a high number of malignant cells, specialized laboratory facilities, and trained technologists and cytotechnologists.
CHROMOGENIC IN-SITU HYBRIDIZATION (CISH)
CISH is a non-fluorescent alternative to FISH. The technique is feasible in the clinical laboratory and with routine specimens. The application to fine-needle aspirates of breast cancer has been described.[50] Nonetheless, such applications to serous effusions have not been reported extensively in the literature.
DIGITIZED IMAGING
A study describing the possible usefulness of digitized imaging in serous effusions has been reported.[51] However, more studies need to be published to evaluate the interobserver variability to confirm its clinical applications.
PROTEOMICS; TWO-DIMENSIONAL GEL ELECTROPHORESIS; MALDI AND SELDI
Since the potential use of high-throughput mass-spectroscopy based blood for the detection of localized ovarian cancer by Petricoin et al.[52] was published, a few studies using the same method for detection of metastatic carcinoma in serous effusions have been published.[53,54] The methods are expensive and at present are mostly applicable as research tools.
DNA PLOIDY ANALYSIS
Although the detection of DNA aneuploidy by using flow cytometric methods can be performed in the clinical laboratory and is economical, this method has not been implemented as a routine diagnostic screening tool in serous effusions. The literature data suggests a high false-negative rate.[5]
CASE STUDIES
Case 1
History
A 78-year-old female presented with dyspnea. A chest X-ray revealed pulmonary vascular congestion, pulmonary edema, and bilateral pleural effusions. The heart was markedly enlarged with globular configuration, suggestive of pericardial effusion. A pericardiocentesis was performed and submitted for cytologic examination and cell-surface flow cytometric analysis.
Cytomorphologic evaluation
The Giemsa and Papanicolaou (PAP)-stained Cytospin preparations [Figure 3a,b] showed a monomorphic population of medium to large lymphocytes admixed with a few acute inflammatory cells.
![Diffuse large B-cell lymphoma, pericardial fluid. (a,b) A predominantly monotonous population of medium-to-large lymphoid cells (arrows) admixed with a small number of acute and chronic inflammatory cells. [a,b, 40X (a, Giemsa-stained Cytospin preparation; b, PAP-stained Cytospin preparation)] (c–h) The two-dimensional histogram of four-color flow cytometric evaluation. The two-dimensional histograms show four-color flow cytometric analysis of B lymphocytes, which express CD19 and CD20 (d–f). These neoplastic B cells are medium to large, as illustrated by intermediate forward scatter (FSC) (c), correlating with the cytomorphologic correlation (a,b). They also co-express CD10 (e,f), are negative for CD5 (h), and demonstrate kappa light chain restriction (g).](/content/105/2022/19/1/img/Cytojournal-19-18-g003.png)
- Diffuse large B-cell lymphoma, pericardial fluid. (a,b) A predominantly monotonous population of medium-to-large lymphoid cells (arrows) admixed with a small number of acute and chronic inflammatory cells. [a,b, 40X (a, Giemsa-stained Cytospin preparation; b, PAP-stained Cytospin preparation)] (c–h) The two-dimensional histogram of four-color flow cytometric evaluation. The two-dimensional histograms show four-color flow cytometric analysis of B lymphocytes, which express CD19 and CD20 (d–f). These neoplastic B cells are medium to large, as illustrated by intermediate forward scatter (FSC) (c), correlating with the cytomorphologic correlation (a,b). They also co-express CD10 (e,f), are negative for CD5 (h), and demonstrate kappa light chain restriction (g).
Provisional cytomorphologic interpretation
Suspicious for lymphoma. Final interpretation pending immunophenotyping (flow cytometric analysis).
Flow cytometric findings
• Four-color flow cytometric analysis of B lymphocytes (depicted in red) showed expression of CD19 and CD20 [Figure 3d-f]. These neoplastic B cells are medium to large, as illustrated by intermediate forward scatter (FSC) [Figure 3c], correlating with the cytomorphologic findings [Figure 3a,b]. They also co-express CD10 [Figure 3e,f] with kappa restriction [Figure 3g] and with negativity for CD5 [Figure 3h].
Final diagnosis
Large B-cell lymphoma involving pericardial cavity.
Discussion
A monomorphic population of medium-to-large lymphocytes seen in Cytospin preparations exhibited intermediate forward and side scatter [Figure 3c,d] in flow cytometric analysis, demonstrating a clonal population of B cells [approximately 54% of total cellularity, shown in red color; Figure 3 a,b]. The cells co-expressed CD19, CD20, CD10, and surface kappa light chain [Figure3 e-g]. They were negative for CD5 [Figure 3h]. The cytologic examination and flow cytometric analysis supported the diagnosis of large B-cell lymphoma, most likely of pericardial cavity origin (see follow-up below).
Follow-up examinations
Radiologic examinations for lymphoma staging, which included CT scan of chest, abdomen, pelvis, PET/CT scan of base to mid skull, and MRI scan of the neck, did not reveal evidence of lymphoma. Bone marrow examination (including cell-surface flow cytometric analysis) was also negative for lymphoma.
Case 2
An 84-year-old male with a previous diagnosis of follicular lymphoma with bone marrow involvement 2 years ago in remission presented with nausea and abdominal pain. Subsequent investigation revealed abdominal ascites. Paracentesis was performed and the peritoneal fluid was submitted for cytologic examination and cell-surface flow cytometric analysis.
Cytomorphologic evaluation
Giemsa and PAP-stained Cytospin smears [Figure 4a,b] were hypocellular and showed small mature lymphocytes resembling chronic inflammatory cells admixed with polymorphonuclear cells and reactive mesothelial cells.
![Follicular lymphoma, ascitic fluid. (a,b) Mixed hypocellular population of small benign-appearing lymphocytes (L), occasional polymorphonuclear cells (N) and reactive mesothelial cells (RM). [a,b 40X (a, Giemsa-stained Cytospin preparation; b, PAP-stained Cytospin preparation)] (c–h) The two-dimensional histogram of four-color flow cytometric evaluation. The two-dimensional histograms show four-color flow cytometric analysis of B lymphocytes, which express CD19 and CD20 (d–f). These neoplastic B cells are small to medium, as illustrated by intermediate forward scatter (FSC) (c), correlating with the cytomorphologic correlation (a,b). They also co-express CD10 (f), are negative for CD5 (h), and demonstrate kappa light chain restriction (g).](/content/105/2022/19/1/img/Cytojournal-19-18-g004.png)
- Follicular lymphoma, ascitic fluid. (a,b) Mixed hypocellular population of small benign-appearing lymphocytes (L), occasional polymorphonuclear cells (N) and reactive mesothelial cells (RM). [a,b 40X (a, Giemsa-stained Cytospin preparation; b, PAP-stained Cytospin preparation)] (c–h) The two-dimensional histogram of four-color flow cytometric evaluation. The two-dimensional histograms show four-color flow cytometric analysis of B lymphocytes, which express CD19 and CD20 (d–f). These neoplastic B cells are small to medium, as illustrated by intermediate forward scatter (FSC) (c), correlating with the cytomorphologic correlation (a,b). They also co-express CD10 (f), are negative for CD5 (h), and demonstrate kappa light chain restriction (g).
Provisional cytomorphologic interpretation
Negative for malignancy. Reactive mesothelial cells and lymphocytes present. With reference to history of follicular lymphoma, final interpretation pending immunophenotyping (flow cytometric analysis).
Flow cytometric findings
Four-color flow cytometric analysis of B lymphocytes (depicted in yellow) showed expression of CD19 and CD20 [Figures 4d-f]. These B cells are small, as illustrated by low forward scatter and low side scatter (SCC) [Figure 4c], and constitute only approximately 5% of the total cellularity. They also co-express CD10 [Figure 4f], are negative for CD5 [Figure 4h], and demonstrate kappa restriction [Figure 4g]. The above findings are best interpreted as clonal B-cell population identified, consistent with involvement by follicular lymphoma.
Final diagnosis
Clonal B-cell population identified, consistent with involvement by follicular lymphoma.
Discussion
Cytospin shows a mixed hypocellular population of small reactive-appearing lymphocytes, occasional polymorphonuclear cells, and reactive mesothelial cells [Figure 4a,b]. Flow cytometric analysis demonstrates a population of small clonal B cells (shown in yellow color), approximately 5% of total cellularity, with low forward and side scatter [Figure 4c,d]. The cells co-express CD19, CD20, CD10, and surface kappa light chain [Figure 4e,g]. They are negative for CD 5 [Figure 4h]. The clonal population identified by flow cytometric analysis supported the involvement of the effusion by follicular lymphoma. • Low-grade follicular lymphoma cells cannot be distinguished objectively from a reactive lymphoid population by morphology alone. Flow cytometry is strongly recommended in cases with a clinical suspicion of lymphoma as a recurrent disease or for initial primary diagnosis. A similar approach is applicable to specimens that show a significant number of lymphocytes.
Follow-up examinations
CT scan and MRI of abdomen and pelvis revealed extensive ascites, massive retroperitoneal, mesenteric, and pelvic lymphadenopathy. The spleen was normal in size. MRI of the chest showed massive mediastinal and hilar lymphadenopathy as well as moderate axillary lymphadenopathy.
Acknowledgment
Author of this review article and editors of CMAS #2 series thank the initial authors (Choladda Vejabhuti, MD and Chung-Che (Jeff) Chang, MD, PhD) for all their efforts for the first edition material on which the current review (as chapter #13 in the final CMAS #2) is based.
Author thanks Andrew Kumar, MD (Resident, Wayne State University School of Medicine, Detroit, MI, USA) and Janavi Kolpekwar for copy-editing support.
ABBREVIATIONS (IN ALPHABETIC ORDER)
AJCC = American Joint Committee on Cancer
ALCL - anaplastic large cell lymphoma
ALL - Acute lymphoblastic leukemia
ALL/LBL - precursor T- or B- lymphoblastic leukemia/ lymphoma
AML - Acute myelogenous leukemia
B-ALL/LBL = precursor B lymphoblastic leukemia/ lymphoblastic lymphoma
CISH = chromogenic in-situ hybridization
CLL/SLL = chronic lymphocytic leukemia/small lymphocytic lymphoma
CT = Computed Tomography
DSRCT = Desmoplastic small round cell tumors
DNA = deoxyribonucleic acid
FCM = flow cytometry
FISH = fluorescence in-situ hybridization
FL = follicular lymphoma
H. pylori = Helicobacter pylori
GC = germinal center
PET/CT scan = Positron Emission Tomography – Computed Tomography
SCC = side scatter
K/L ratio = kappa-to-lambda light chain ratio
MALT lymphoma - Mucosa-associated lymphoid tissue lymphoma
MRI scan = magnetic resonance imaging
MZBL = marginal zone B-cell lymphoma
N = no
NK = Natural killer
PAP = Papanicolaou
RT-PCR = reverse transcriptase-PCR
T-ALL/LBL = precursor T lymphoblastic leukemia/ lymphoblastic lymphoma
Y = yes
References
- Flow cytometry as an adjunct to cytomorphologic analysis of serous effusions. Diagn Cytopathol. 2003;29:74-8.
- [CrossRef] [PubMed] [Google Scholar]
- Body cavity fluids In: Ramzy I, ed. Clinical Cytopathology and Aspiration Biopsy: Fundamental Principles and Practice (2nd edn). New York: McGraw-Hill; 2001. p. :205-23.
- [Google Scholar]
- Cytological diagnosis of lymphoma in serous effusions. J Clin Pathol. 1981;34:1311-25.
- [CrossRef] [PubMed] [Google Scholar]
- Immunologic methods in cytology: definitive diagnosis of non-Hodgkin's lymphomas using immunologic markers for Tand B-cells. Am J Clin Pathol. 1984;82:666-73.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical utility of flow cytometry in body fluid cytology: to flow or not to flow? That is the question. Diagn. 2001;24:305-6.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical utility of flow cytometry in pleural lymphocytosis. Mod Pathol. 1999;12:43.
- [Google Scholar]
- Can flow cytometry be a primary diagnostic tool for identifying positive lymphoid pleural effusions? Mod Pathol. 2004;17(Suppl 1):85a.
- [Google Scholar]
- Restricted kappa/lambda light chain ratio by flow cytometry in germinal center B cells in Hashimoto's thyroiditis. Am J Clin Pathol. 2006;125:42-8.
- [CrossRef] [PubMed] [Google Scholar]
- Characteristics of nonhematolymphoid neoplasms by flow cytometry. Mod Pathol. 2004;17(Suppl 1):364a.
- [Google Scholar]
- Lineage-specific identification of nonhematopoietic neoplasms by flow cytometry. Am J Clin Pathol. 2003;119:643-55.
- [CrossRef] [PubMed] [Google Scholar]
- Increased natural killer cells in fluids. A new, sensitive means of detecting carcinoma. Acta. 1996;40:1240-5.
- [CrossRef] [PubMed] [Google Scholar]
- Relation between natural killer cells and neoplastic cells in serous fluids. Diagn Cytopathol. 2000;22:347-50.
- [CrossRef] [Google Scholar]
- Serous effusions: diagnosis of malignancy beyond cytomorphology. An analytic review. Postgrad Med J. 2003;79:569-74.
- [CrossRef] [PubMed] [Google Scholar]
- Differential diagnosis between mesothelioma and adenocarcinoma: a multimodal approach based on ultrastructure and immunocytochemistry. Semin Diagn Pathol. 1992;9:124-40.
- [Google Scholar]
- Detection of numerical aberrations of chromosomes 7 and 9 in cytologic specimens of pleural malignant mesothelioma. Cancer. 2003;99:233-9.
- [CrossRef] [PubMed] [Google Scholar]
- Interphase fluorescence in situ hybridization improves the detection of malignant cells in effusions from breast cancer patients. Br J Cancer. 1997;75:403-7.
- [CrossRef] [PubMed] [Google Scholar]
- Analysis of chromosomes 9 and 11 aneuploidy frequency in pleural effusion of patients with and without malignancy: interphase FISH technique. Cancer Biol Ther. 2005;4:248-51.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitive detection of tumour cells in effusions by combining cytology and fluorescence in situ hybridisation (FISH) Br J Cancer. 2004;91:558-63.
- [CrossRef] [PubMed] [Google Scholar]
- Combination of cytology, fluorescence in situ hybridization for aneuploidy, and reverse-transcriptase polymerase chain reaction for human mammaglobin/mammaglobin B expression improves diagnosis of malignant effusions. J Clin Oncol. 2004;22:474-83.
- [CrossRef] [PubMed] [Google Scholar]
- Malignant cell detection by fluorescence in situ hybridization (FISH) in effusions from patients with carcinoma. Hum Pathol. 2000;31:448-55.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of hyperdiploid malignant cells in body cavity effusions by fluorescence in situ hybridization on ThinPrep slides. Cancer. 1997;81:299-308.
- [CrossRef] [Google Scholar]
- FISH in the evaluation of pleural and ascitic fluids. Cancer Genet Cytogenet. 1995;84:116-9.
- [CrossRef] [Google Scholar]
- International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. 1999;86:2668-73.
- [CrossRef] [Google Scholar]
- DNA polymerase chain reaction using fine-needle aspiration biopsy smears to evaluate non-Hodgkin's lymphoma. Acta Cytol. 1999;43:837-41.
- [CrossRef] [PubMed] [Google Scholar]
- The role of gene rearrangements for antigen receptors in the diagnosis of lymphoma obtained by fine-needle aspiration. A study of 63 cases with concomitant immunophenotyping. Am J Clin Pathol. 1991;96:479-90.
- [CrossRef] [PubMed] [Google Scholar]
- Polymerase chain reaction-based detection of B-cell monoclonality in cytologic specimens. Arch Pathol Lab Med. 1993;117:1099-103.
- [Google Scholar]
- Polymerase chain reaction detection of immunoglobulin gene rearrangement and bcl-2 translocation in archival glass slides of cytologic material. Diagn Mol Pathol. 1995;4:25-31.
- [CrossRef] [PubMed] [Google Scholar]
- Polymerase chain reactionbased detection of B-cell clonality in the fine needle aspiration biopsy of a thyroid mucosa-associated lymphoid tissue (MALT) lymphoma. Hum Pathol. 1997;28:989-92.
- [CrossRef] [Google Scholar]
- Automated molecular genetic DNA analysis for detecting B-cell non-Hodgkin's lymphoma in cytologic specimens. Anal Quant Cytol Histol. 1997;19:255-63.
- [Google Scholar]
- Analysis of clonality in cytologic material using the polymerase chain reaction (PCR) Cytopathology. 1997;8:114-21.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency and structure of t(14;18) major breakpoint regions in non-Hodgkin's lymphomas typed according to the Kiel classification: analysis by direct DNA sequencing. Cancer Res. 1991;51:3243-50.
- [Google Scholar]
- PCR analysis of IgH and BCL2 gene rearrangement in the diagnosis of follicular lymphoma in lymph node fine-needle aspiration. A critical appraisal. Diagn Mol Pathol. 1997;6:154-60.
- [CrossRef] [PubMed] [Google Scholar]
- Collection and processing of effusion fluids for cytopathologic evaluation. CytoJournal. 2022;19:5. HTML of this article is available FREE at:
- [CrossRef] [Google Scholar]
- Cell-blocks and hematolymphoid lesions. CytoJournal. 2021;18:7. HTML of this article is available FREE at:
- [CrossRef] [PubMed] [Google Scholar]
- Cell-blocks and other ancillary studies (including molecular pathology and proteomics) CytoJournal. 2021;18:4. HTML of this article is available FREE at:
- [CrossRef] [PubMed] [Google Scholar]
- Detection of immunoglobulin heavy chain gene rearrangements by polymerase chain reaction analysis on lymph node imprints and fine-needle aspirate smears: a comparison of five different imprint preparations. Diagn Cytopathol. 1999;20:333-8.
- [CrossRef] [Google Scholar]
- Best Practice No 185. Cytological and molecular diagnosis of lymphoma. J Clin Pathol. 2005;58:561-7.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of concurrent/recurrent non-Hodgkin's lymphoma in effusions by PCR. Hum Pathol. 1999;30:1361-6.
- [CrossRef] [Google Scholar]
- Application of molecular genetics to the diagnosis of lymphoid-rich effusions: study of 95 cases with concomitant immunophenotyping. Diagn Cytopathol. 2002;27:90-5.
- [CrossRef] [PubMed] [Google Scholar]
- The clinical utility of polymerase chain reaction for the diagnosis of pleural tuberculosis. Clin Infect Dis. 2005;41:660-6.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis of extrapulmonary tuberculosis by smear, culture, and PCR using universal sample processing technology. J Clin Microbiol. 2005;43:4357-62.
- [CrossRef] [PubMed] [Google Scholar]
- Rapid diagnosis of pleural tuberculosis by polymerase chain reaction. Am J Respir Crit Care Med. 1995;152:1977-81.
- [CrossRef] [PubMed] [Google Scholar]
- A comparative study of the polymerase chain reaction and conventional procedures for the diagnosis of tuberculous pleural effusion. Tuber Lung Dis. 1992;73:262-67.
- [CrossRef] [Google Scholar]
- Detection of mycobacterial DNA in pleural fluid from patients with tuberculous pleurisy by means of the polymerase chain reaction: comparison of two protocols. Thorax. 1992;47:265-9.
- [CrossRef] [PubMed] [Google Scholar]
- Polymerase chain reaction for the diagnosis of pleural tuberculosis in immunocompromised and immunocompetent patients. Clin Infect Dis. 1998;26:212-4.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of polymerase chain reaction for detection of Mycobacterium tuberculosis in pleural fluid. Chest. 2001;119:1737-41.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of nested polymerase chain reaction for detecting mycobacterial DNA in pleural fluid. Kansenshogaku Zasshi. 1995;69:175-180.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of polymerase chain reaction, adenosine deaminase, and interferon-gamma in pleural fluid for the differential diagnosis of pleural tuberculosis. Chest. 2000;118:1355-64.
- [CrossRef] [PubMed] [Google Scholar]
- Emerging cancer diagnostics. 'On slide' or 'off slide': that is the question. Am J Clin Pathol. 2003;120:822-4.
- [CrossRef] [Google Scholar]
- Routine DNA cytometry of benign and malignant pleural effusions by means of the remote quantitation server Euroquant: a prospective study. J Clin Pathol. 2000;53:760-4.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of Her-2/neu oncogene in breast carcinoma by chromogenic in situ hybridization in cytologic specimens. Diagn Cytopathol. 2005;33:376-80.
- [CrossRef] [PubMed] [Google Scholar]
- Comparison of conventional microscopy and digitized imaging for diagnosis in serous effusions. Anal Quant Cytol Histol. 1997;19:202-6.
- [Google Scholar]
- Use of proteomic patterns in serum to identify ovarian cancer. Lancet. 2002;359:572-7.
- [CrossRef] [Google Scholar]
- Proteomic profiling of human pleural effusion using two-dimensional nano liquid chromatography tandem mass spectrometry. J Proteome Res. 2005;4:1274-86.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomic analysis of malignant ovarian cancer effusions as a tool for biologic and prognostic profiling. Clin Cancer Res. 2006;12:791-9.
- [CrossRef] [PubMed] [Google Scholar]
- Molecular genetics of pediatric soft tissue tumors: clinical application. J Mol Diagn. 2003;5:143-54.
- [CrossRef] [Google Scholar]








