Translate this page into:
Increased mast cells in endocervical smears of women with dysmenorrhea
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Mast cells are observed in peritoneal endometriosis which causes dysmenorrhea. However, there is no report about the relationship between endocervical mast cells and dysmenorrhea. The aim of this study is to evaluate the relationship using endocervical smears.
Materials and Methods:
Between January 2016 and June 2016, patients filled out a questionnaire regarding dysmenorrhea and were classified into the dysmenorrhea or the control group (without dysmenorrhea). Patients underwent endocervical brushing and endocervical smears were obtained. The smears were stained with methylene blue to detect mast cells. The number of mast cells per slide was counted by microscopy and recorded.
Results:
Eighty-nine patients were enrolled in this study (dysmenorrhea group, 34; control group, 55). The median number of mast cells present in the endocervical one slides was 35 (interquartile range, 17–58) and 2 (interquartile range, 0–6) in the dysmenorrhea and control groups, respectively. There was a significant difference in the number of mast cells between the two groups (P < 0.0001).
Conclusion:
More mast cells were observed in the endocervical smears of women with dysmenorrhea than in those of women without dysmenorrhea.
Keywords
Dysmenorrhea
endocervical smear
mast cells
INTRODUCTION
Dysmenorrhea is a common gynecological complaint among reproductive age females, characterized by a painful cramping sensation in the lower abdomen or back during menstruation.[1] The reported prevalence of dysmenorrhea varies from 16% to 91%. There is no way to diagnose them as dysmenorrhea objectively. Primary dysmenorrhea is common among young women, and secondary dysmenorrhea conditions, such as endometriosis, are common among older women.[2] Although classified differently, these two types of dysmenorrhea are caused by the same chemical mediators.[2]
Mast cells are known to contain various mediators such as histamine, prostaglandin, serotonin, and nerve growth factor. These mediators can stimulate nociceptive neurons. Furthermore, the activation of mast cells indirectly causes pain through the recruitment of neutrophils and macrophages, which also releases pain-related mediators.[3] Therefore, mast cell activation is considered one of the mechanisms of dysmenorrhea.[45]
According to a report about mast cell distribution in the human uterus, there was no change in the number of mast cells between the proliferative and secretory phases of the menstrual cycle in the endometrium, myometrium, or cervix.[6] However, little is known about the relationship between dysmenorrhea and mast cells in the uterus. In this study, we investigated the association between dysmenorrhea and uterine mast cells using endocervical smears, which are simple and minimally invasive tests commonly performed in the gynecologic clinic.
MATERIALS AND METHODS
Patients
This study was approved by the Ethics Committee of Shiga University of Medical Science (approval number 26-217), and written informed consent was obtained from all the participants. The study protocol conforms to the provisions of the Declaration of Helsinki (as revised in Tokyo 2004). This study was performed from January 2016 to June 2016 at Shiga University Hospital and Tsuji Ladies clinic. Patients were excluded from the study if they had hormonal therapy, previous cervical surgery, any cervicitis and vaginitis, or an irregular menstrual cycle. Eligible patients completed a questionnaire to assess symptoms of dysmenorrhea and underwent a cervical smear for cancer screening followed by pelvic ultrasound examination. Cases of suspected endometrial cysts were confirmed by magnetic resonance imaging. All patients were classified into the dysmenorrhea group or the control group (without dysmenorrhea).
Smears obtained using endocervical brushing
We excluded the cervical mucosa using a dry swab and inserted an Endo-Cervex-Brush® (Rovers Medical Devices B.V., Oss, The Netherlands) into the cervical canal to collect endocervical cells. The brush was slowly turned four times and then smeared onto two glass slides gently and uniformly. One slide was screened for cancer. The other slide was dried and immersed in 99.9% methanol for 3 min. After fixation, the slide was bathed in Eosinostain-Torii® (Torii Pharmaceutical Co. Ltd., Tokyo, Japan) for 1 min. This stain contains methylene blue, which stains mast cells in the same fashion as toluidine blue. After gentle washing, the slides were rinsed with methanol for 1 min and dried.
Analysis
All patient slides were evaluated using a light microscope (400-fold magnification), and the number of mast cells was counted. The count was performed by two investigators blinded to the patient group assignments. Statistical analysis was performed with GraphPad Prism, version 6 (GraphPad Software, Inc., La Jolla, CA, USA). Because data were not normally distributed, we used nonparametric methods. Data were analyzed using the Mann–Whitney U-test, and values are expressed as medians and interquartile ranges. A P < 0.05 was considered statistically significant.
RESULTS
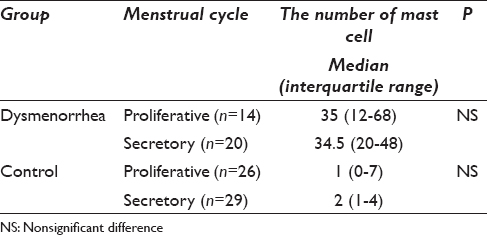
Eighty-nine patients were enrolled in this study: 34 in the dysmenorrhea and 55 in the control group (mean age, 36.1 and 39.1 years, respectively). No malignancy was identified in any patient. A methylene blue-stained slide with mast cells is shown in Figure 1. The median number of mast cells per slide was 35 (17–58) in the dysmenorrhea group and 2 (0–6) in the control group and this difference was statistically significant (P < 0.0001) [Figure 2]. There was no significant difference in the number of mast cells during the menstrual cycle in each group [Table 1].

- Mast cells stained by methylene blue on a glass slide observed at high-power (×400). Granules in mast cells are clearly observed
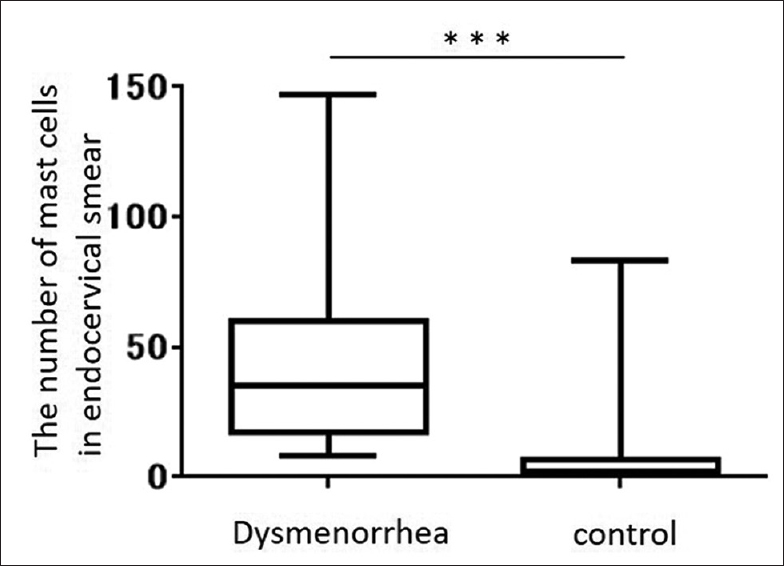
- Box-and-whisker plot comparing the number of mast cells in endocervical smears between patients with dysmenorrhea and controls without dysmenorrhea. The number of mast cells in the dysmenorrhea group (n = 34) was significantly higher than the number in the control group (n = 55) (P < 0.0001).
DISCUSSION
We investigated the association between dysmenorrhea and uterine mast cells by comparing the endocervical smears of patients with and without dysmenorrhea. This study revealed that an increased number of mast cells are present in the endocervical smears of women with dysmenorrhea. Women with any cervicitis and vaginitis were excluded from this study because one report indicated an increased number of mast cells in association with vaginal trichomoniasis.[7] Although the association between mast cells in cervical smears and uterine cervix neoplasia is not clear, none of our patients had a smear showing dysplastic or malignant cells. There is a reported relationship between mast cells and pain in diseases other than dysmenorrhea. Barbara et al.[8] documented that colonic mast cell infiltration and mediator release in proximity to mucosal innervation contribute to abdominal pain in patients with irritable bowel syndrome. Furthermore, mast cells are located in the central nervous system and modulate the nociceptive process; they perform a similar function in other regions of the body.[9] Of equal importance, the release of mast cell mediators such as histamine and serotonin in the uterine myometrium evokes strong contractions.[10] Furthermore, the prostaglandin which causes the uterus to contract is produced by activated mast cells.[11] Therefore, the association between increased mast cells in the uterine cervix and dysmenorrhea identified in this study is consistent with previous findings. Because no change was observed in the number of mast cells during the menstrual cycle, these cells may be activated especially during menstruation.
One of the limitations of this study is to be unclear the type of dysmenorrhea, which is primary or secondary dysmenorrhea. However, 11 patients had endometrial cyst in the dysmenorrhea group of this study. Endometrial cyst clearly indicated the secondary dysmenorrhea and endometriosis. There was no significant difference in the number of mast cells in the smears of dysmenorrhea patients with an endometrial cyst (n = 11) versus those without an endometrial cyst (n = 23). In general, the severity of endometriosis increases in the presence of an endometrial cyst according to the revised American Society for Reproductive Medicine classification.[12] This result suggested that the number of mast cells in an endocervical smear had no association with the severity of endometriosis and dysmenorrhea.
On the other hand, several reports suggest a relationship between mast cells and endometriosis.[1314] An association between atopic disease and endometriosis has also been reported.[1516] However, we could not demonstrate an association between allergic disease and mast cells in the cervix in this study. Further studies are also needed in patients with more precise diagnoses; i.e., radioallergosorbent-confirmed allergic disease and laparoscopy-confirmed endometriosis to examine the type of dysmenorrhea.
To the best of our knowledge, this study is the first to report a higher number of mast cells in the endocervical smears of patients with dysmenorrhea compared to those of patients without dysmenorrhea. Our findings are preliminary, and it is unclear whether the increased number of mast cells we found is the cause or result of dysmenorrhea. However, the evidence of increased mast cells in association with dysmenorrhea may contribute to the establishment of a novel method for the objective evaluation of this condition.
CONCLUSION
In this study, we performed endocervical smears and found that patients with dysmenorrhea had a higher median number of mast cells compared to patients without dysmenorrhea, and this difference was statistically significant. To the best of our knowledge, this is the first study to implicate mast cells in dysmenorrhea. These findings may contribute to the establishment of a novel method for the objective evaluation of this condition.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. ST, KT and HO acquired the data. ST performed statistical analysis and drafted the article. KT, HO and MT contributed to conception and design of this study. MT developed a concept for this study and revised the manuscript. All authors approved that the final version was read and published.
ETHICS STATEMENT BY ALL AUTHORS
This study was approved by the Ethics Committee of Shiga University of Medical Science (approval number 26-217).
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- The role of mast cells and their mediators in reproduction, pregnancy and labour. Hum Reprod Update. 2011;17:383-96.
- [Google Scholar]
- Endometriosis and mechanisms of pelvic pain. J Minim Invasive Gynecol. 2009;16:540-50.
- [Google Scholar]
- Mast cells in chronic inflammation, pelvic pain and depression in women. Gynecol Endocrinol. 2014;30:472-7.
- [Google Scholar]
- Distribution and heterogeneity of mast cells in the human uterus. Hum Reprod. 1997;12:368-72.
- [Google Scholar]
- Association of mast cells with vaginal trichomoniasis in endocervical smears. Acta Cytol. 1983;27:133-7.
- [Google Scholar]
- Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693-702.
- [Google Scholar]
- Distribution of mast cells and the effect of their mediators on contractility in human myometrium. Br J Obstet Gynaecol. 1993;100:1125-30.
- [Google Scholar]
- Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J Immunol. 1987;138:2611-5.
- [Google Scholar]
- Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil Steril. 1997;67:817-21.
- [Google Scholar]
- Increased mast cell density in peritoneal endometriosis compared with eutopic endometrium with endometriosis. Am J Reprod Immunol. 1998;40:291-4.
- [Google Scholar]
- Localization of mast cells in endometrial cysts. Am J Reprod Immunol. 2004;51:341-4.
- [Google Scholar]








