Translate this page into:
Metastatic urachal carcinoma in bronchial brush cytology
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Urachal carcinoma is rare comprising less than 1% of all bladder carcinomas. Metastases of urachal carcinoma have been reported to meninges, brain, ovary, lung, and maxilla. Cytologic features of metastatic urachal carcinoma have not been previously reported. We present a case of metastatic urachal adenocarcinoma in bronchial brushings and review the use of immunohistochemistry in its diagnosis. A 47-year-old female was seen initially in 2007 with adenocarcinoma of the bladder dome for which she underwent partial cystectomy. She presented in 2011 with a left lung mass and mediastinal adenopathy. Bronchoscopy showed an endobronchial lesion from which brushings were obtained. These showed numerous groups of columnar cells with medium sized nuclei and abundant cytoplasm. The cells were positive for CK20 and CDX2 and negative for CK7. The cytomorphological findings were similar to those in the previous resection specimen and concurrent biopsy. This is the first case report of bronchial brushings containing metastatic urachal carcinoma. No specific immunohistochemical profile is available for its diagnosis. The consideration of a second primary was a distinct possibility in this case due to the lapse of time from primary resection, absence of local disease, and lack of regional metastases.
Keywords
Bronchial brushings
cytology
immunohistochemistry
metastasis
urachal carcinoma
INTRODUCTION
The urachus is an embryologic remnant of the urogenital sinus and allantois. Involution usually occurs before birth and the urachus persists as a median umbilical ligament. Urachal carcinoma is rare and comprises less than 1% of bladder neoplasms.[1] The pathogenesis of urachal tumors is not understood. The vast majority of urachal epithelial neoplasms are adenocarcinomas with several morphologic subtypes including enteric, signet ring, mucinous, mixed cell subtype as well as adenocarcinoma not otherwise specified.[2] Adenocarcinoma from several other primaries may mimic any of these urachal adenocarcinoma subtypes in the bladder or at distant sites. We present a case of metastatic urachal adenocarcinoma in bronchial brushings and review the use of immunohistochemistry in its diagnosis.
CASE REPORT
A 47-year-old female was seen initially in August 2007 with urinary frequency and hematuria. Cystoscopy and biopsy of a dome lesion revealed adenocarcinoma. She was treated by partial cystectomy with en bloc resection of the median umbilical ligament and umbilicus. The surgical specimen revealed a 1.1 cm moderately differentiated adenocarcinoma with focal mucin extravasation invading deep muscle. The closest margin was 7 mm from the invasive front. No lymphovascular invasion was identified. Subsequent cystogram and imaging studies showed no residual tumor. Follow-up over the next 4 years were negative for signs of recurrence.
In July 2011, the patient presented with new onset of shortness of breath and stridor. Imaging studies showed a left lung mass with mediastinal adenopathy. Bronchoscopy showed an endobronchial obstructive lesion. A bronchial brush and tissue biopsies were obtained.
Thin Prep slide of bronchial brushings showed small sheets of columnar cells with medium size nuclei, mildly irregular nuclear membranes, coarse chromatin pattern, and abundant vacuolated cytoplasm in a background of mucin. These cells lacked cilia and were the predominant population when compared with the sparse presence of ciliated bronchial cells. The lesional cells were positive for CK20 and CDX2 but negative for CK7 (performed on Thin Prep slides, Figure 1). Due to insufficient cytologic material, TTF-1 was performed on the biopsy and was negative.
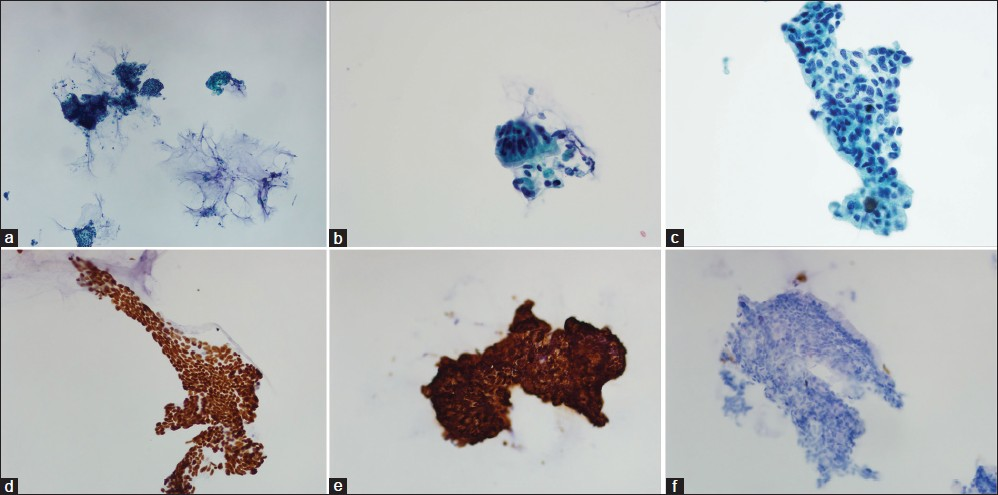
- Bronchial brushings in Thin Prep (a) Scanning view showing columnar cell clusters in mucoid background (Papanicolau stain ×40) (b) Rare cluster of bronchial epithelial cells with terminal bars and cilia (Papanicolau stain ×400) (c) Many clusters composed of disorganized columnar cells with abundant vacuolated cytoplasm and irregular nuclear contours (Papanicolau stain ×400) (d) CDX2 nuclear positive stain (×400) (e) CK20 positive stain (×400) (f) CK7 negative stain (×200)
The concurrent biopsy and the previous resection specimens showed similar cytomorphologic features and immunohistochemical profiles [Figure 2]. Based on these findings, a final diagnosis of metastatic urachal adenocarcinoma was made.
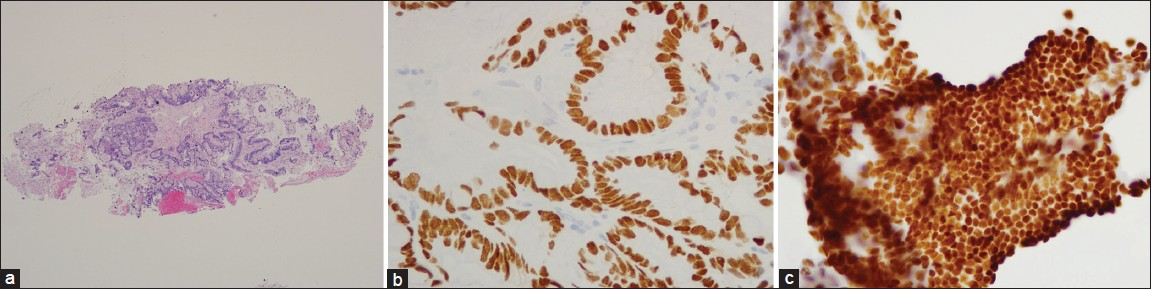
- Concurrent bronchial biopsy specimen showing well differentiated mucin secreting adenocarcinoma with vacuolated cytoplasm and irregular nuclear outlines (a) H and E, ×100 (b) CDX2 and (c) CK20 are positive (×400)
DISCUSSION
Urachal carcinoma is an extremely rare tumor comprising less that 1% of bladder cancer.[1] The criteria for diagnosis of urachal carcinoma are somewhat controversial.[34] Less stringent criteria than previously suggested have been proposed[5] and include location of tumor in the dome/anterior wall, epicenter of carcinoma in the bladder wall, absence of widespread cystitis cystica/glandularis beyond the dome/anterior wall, and absence of a known primary elsewhere.
Our patient fulfilled all these criteria and was diagnosed with urachal adenocarcinoma in 2007.
Metastases from urachal carcinoma have been reported to meninges,[6] brain,[78] maxilla,[9] ovary,[1011] and abdominal wall.[12] The most common sites of metastases were regional lymph nodes and lungs followed by peritoneum, anterior abdominal wall, bone, soft-tissue, and ovary. The time interval between initial diagnosis and metastasis was up to 132.8 months with a mean of 28.7 months.[5] Our patient presented 4 years after diagnosis with a lung mass, mediastinal adenopathy, and an endobronchial lesion. At this time, the diagnosis of a second primary was a distinct possibility given the time lapse and lack of regional disease or metastases.
The tumor cells were immunoreactive with CK20 and CDX2 and were negative for CK7 and TTF-1. Lack of TTF-1 staining in a well differentiated adenocarcinoma is unusual for a primary pulmonary neoplasm. Our differential diagnosis at this stage included metastasis from the patient's known urachal carcinoma or a second primary from the colorectum. To date, only two studies have addressed the immunohistochemical profile of urachal adenocarcinomas. Gopalan et al.,[5] noted that all 15 urachal adenocarcinomas were positive for CK20 and variably positive for CK7 and 34BE12. The majority showed cytoplasmic membranous staining pattern for beta-catenin and in one case, there was focal nuclear immunoreactivity. Paner et al.,[2] studied 34 urachal adenocarcinomas using a panel of markers (p63, CK7, CK20, CDX2, nuclear beta-catenin, claudin-18 and Reg IV) and found that nuclear beta-catenin was the only one that had some value in differentiating urachal adenocarcinoma of enteric morphology from colonic adenocarcinoma. All urachal adenocarcinomas had membrano cytoplasmic staining and only 6% had focal to moderate nuclear staining. In contrast, metastatic colonic adenocarcinoma had both membrano cytoplasmic and nuclear positivity. The authors caution that moderate nuclear staining can rarely occur in urachal adenocarcinoma and that the frequent membrano-cytoplasmic staining pattern may make nuclear staining evaluation difficult. From these two studies, the most helpful differentiating marker would appear to be beta-catenin. However, the presence of nuclear staining in up to 6% of urachal adenocarcinomas and somewhat problematic evaluation of the stain compromise its specificity.
In conclusion, this is the first case report of urachal adenocarcinoma presenting in bronchial brushings. The differential diagnosis in this patient included metastasis from a second primary and metastatic urachal carcinoma. There is no specific immunochemical profile to confirm the diagnosis of urachal carcinoma. The diagnosis in this case would have been difficult to make and required knowledge of the patient's history of urachal carcinoma, comparison with the patient's previous material, and lack of gastrointestinal pathology.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
No competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors were involved in the preparation of this manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is case report without identifiers, our institution does not require approval from Institutional Review Board (IRB) (or its equivalent).
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/1/106684
REFERENCES
- Immunohistochemical analysis in a morphologic spectrum of urachal epithelial neoplasms: Diagnostic implications and pitfalls. Am J Surg Pathol. 2011;35:787-98.
- [Google Scholar]
- Urachal carcinoma: A clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol. 2009;33:659-68.
- [Google Scholar]
- Carcinomatous meningitis from urachal carcinoma: The first reported case. J Neurooncol. 2006;76:171-4.
- [Google Scholar]
- Metastatic brain tumor originating from urachal carcinoma: Case report. No Shinkei Geka. 2005;33:1015-9.
- [Google Scholar]
- Urachal carcinoma with metastasis to the maxilla: the first reported case. J Oral Pathol Med. 2001;30:378-80.
- [Google Scholar]
- Urachal adenocarcinoma metastatic to the ovaries resembling primary ovarian mucinous carcinoma: A case report with the immunohistochemical study. Int J Clin Exp Pathol. 2010;4:118-23.
- [Google Scholar]
- Alpha-methylacyl- coenzyme a racemase-expressing urachal adenocarcinoma of the abdominal wall. Korean J Urol. 2010;51:498-500.
- [Google Scholar]








