Translate this page into:
Paraganglioma with unusual presentation in parotid gland: A diagnostic dilemma in fine needle aspiration
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Paragangliomas (PGLs) are uncommon tumors. Although PGLs are known to occur in the head and neck region, especially the carotid body, middle ear, and larynx, involvement of the parotid glands has not been reported. In this article, we report the fine needle aspiration features of tumor in an unusual location, presenting as a parotid gland mass, submitted to pathology for initial diagnosis. The clinical presentation, cytomorphology, and the immunohistochemical features for the diagnosis are described. To our knowledge, this is the first case of paraganglioma of the parotid gland reported in the literature.
Keywords
Fine needle aspiration
paragangliomas
parotid gland
INTRODUCTION
Paragangliomas (PGLs) are uncommon tumors (incidence 1-2 per 100,000) and based on their locations, the tumors are often given special designations.[1] Only three percent (3%) of all PGLs occur within the head and neck of which the majority are located in the carotid body (carotid body tumors), temporal-bone/middle-ear (glomus jugulare), and the vagus nerves in the neck (vagal PGLs).[23] Ninety percent of the head and neck PGLs are sporadic, only 10% are hereditary in nature.[2] Parasympathetic extra-adrenal PGLs are located predominantly in the head and neck; and about 95% of such tumors are nonsecretory in nature. In contrast with the sympathetic PGLs, the parasympathetic extra-adrenal PGLs are more often familial and less likely to be malignant.[24] The diagnosis of hereditary PGL syndrome is based on physical examination, family history, imaging studies, biochemical testing, and molecular genetic testing for the mutations, involving the subunits of succinate dehydrogenase, a mitochondrial enzyme.[5] In this article, we describe a patient with PGL presenting as a parotid gland mass which turned out to be a diagnostic dilemma in the initial fine needle aspiration (FNA) due to its unexpected location. To our knowledge, PGL in the parotid gland has never been previously reported.
CASE REPORT
The patient was a 56-year-old woman, with a history of unrepaired Tetralogy of Fallot and Eisenmenger's syndrome, who was admitted to our Medical Center with atrial flutter/atrial fibrillation, palpitations, and possible cerebrovascular accident due to the congenital heart disease. Magnetic resonance angiography (MRA) evaluation of the head and neck showed an incidental right parotid gland mass. Subsequent ultrasound of this area showed a 2.0 × 1.5 cm solid tumor located in the deep within the parotid gland, which was presumed to be a pleomorphic adenoma. Ultrasound-guided FNA was then performed by a radiologist. Cytological evaluation of the initial FNA smears showed moderate cellularity consisting of atypical epithelioid cells in disorganized groups, strips, and single cells. The atypical cells showed enlarged nuclei with variably granular chromatin, occasional nucleoli, anisonucleosis, and scant delicate cytoplasm [Figure 1a and b]. Rare salivary gland acinar and ductal cells were noted as small groups in the blood-tinted background. Since the cell block was paucicellular, two of the Papanicolaou-stained slides were re-stained for synaptophysin and keratin by immunohistochemistry (IHC). The positive immunostain of the cells for synaptophysin [Figure 1c] was compatible with a tumor of neuroendocrine origin. The cytokeratin stain showed negative on the re-stained smear. Subsequently, a diagnosis of “atypical cells present, suspicious for neuroendocrine tumor” was rendered.
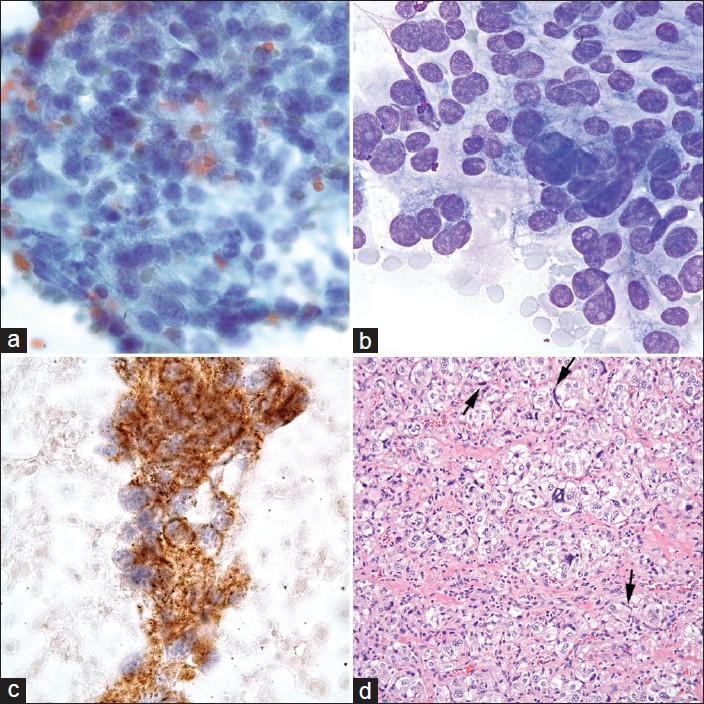
- Composite photomicrographs of the tumor showing epithelioid cells arranged in a group with granular chromatins (a, FNA Pap stain, ×40 Obj.); pleomorphic nuclei with nucleoli (b, FNA Diff-Quick stain, ×100 Obj.); and cells with positive staining for synaptophysin (c, FNA, ×100 Obj.). The histological section of the tumor (d, H and E, ×40 Obj.) shows epithelioid or chief cells which are arranged in a pseudoalveolar pattern (Zellballen) nested in a vascular stroma which were positive for synaptophysin but negative for cytokeratin. Hyperchromatic spindle shaped sustentacular cells are at the periphery of the chief cell nests (arrows). They were only positive for S-100
On the basis of diagnosis of neuroendocrine tumor by FNA, a total parotidectomy and neck dissection was performed. During the surgery, the 2.0 cm mass was confirmed to be in the central portion of the deep lobe of the parotid gland. On gross examination, the tumor was a red-pink, firm, well-circumscribed mass, measuring 2.2 × 1.5 × 1.4 cm.
Histological evaluation of the surgical specimen [Figure 1d] showed epithelioid cells arranged in an alveolar pattern in nests surrounded by capillary networks. These cells showed considerable nuclear atypia, enlargement, and hyperchromasia. The cell cytoplasms varied from pink to clear and often vacuolated. A second type of cell was seen at the periphery of the nests which was more of a spindle shape. No mitoses were identified. The nested (chief) cells were positive for chromogranin and synaptophysin, while negative for keratin and S-100. The spindle cells (sustentacular) at the periphery were positive only for S-100. A final histological diagnosis of PGL was made based on the characteristic morphology and immunohistochemical stains.
An incidental metastatic papillary thyroid carcinoma was identified in one of twenty-one dissected lymph nodes. This finding led to a subsequent ultrasound of the thyroid gland which revealed a previously unknown sub-centimeter mass with calcifications suspicious for papillary thyroid carcinoma. Due to the urgent cardiac problem of the patient, the surgical intervention of the thyroid gland was postponed indefinitely.
DISCUSSION
Most of the parotid gland tumors (80%) are benign comprising of pleomorphic adenoma and Warthin's tumor, both in solid and cystic forms.[67] Remaining entities include oncocytoma, basal cell adenoma, and ductal papilloma in the benign category. Mucoepidermoid carcinoma, polymorphous low-grade adenocarcinoma, acinic cell carcinoma, adenoid cystic carcinoma, malignant mixed tumor, and squamous cell carcinoma are amongst the malignant category.[6] Neuroendocrine carcinomas have also been reported in the parotid gland.[8]
PGLs most commonly present as an asymptomatic palpable mass in the anterior triangle of the neck. The mass is usually slow growing and can easily be confused by the clinicians for a lymph node or other head and neck tumors.[12] The extra-adrenal PGLs can be divided into sympathetic and parasympathetic types. Sympathetic extra-adrenal PGLs are generally confined to the thorax, abdomen, and pelvis, and are typically secretory which produce catecholamines.[24]
PGLs in the head and neck usually present as mass lesions in specific locations. The classic radiographic features are homogeneous or heterogeneous hyper-enhancing soft-tissue mass as shown by computed tomography (CT) scan; multiple areas of signal void interspersed with hyperintense foci (salt-and-pepper appearance) within tumor mass as shown by magnetic resonance imaging (MRI); and as an intense tumor blush with enlarged feeding arteries by angiography.[9] The preferred term for tumors of the extra-adrenal paraganglia is “paraganlioma,” prefaced by the anatomic site of the origin.[210] Tumors arising from the paraganglia of the adrenal medulla are called pheochromocytoma.[2]
PGLs are highly vascular tumors composed of two types of cells that are arranged in a pseudoalveolar pattern known as Zellballen or literally “cell balls” in German. Type I cells, the chief cells, are predominate in PGLs and contain catecholamine-bound granules (positive for synaptophysin and chromogranin). Type II or sustentacular cells stain positive for S-100, devoid of granules (negative for synaptophysin and chromogranin), and are located at the periphery of the small pseudoalveolar clusters of Type I cells.[2] PGLs are classified as either benign or malignant. Malignancy cannot be determined by cytology or even by histological findings and is only defined when the tumor metastasizes to regional lymph nodes or more distant sites. Nuclear polymorphism, neurovascular invasion, high mitotic indices, and necrosis have been described in both benign and malignant types of the tumors.[2]
On cytology, the cellularity may range from highly cellular to almost acellular, depending on the amount of contaminating red blood cells during sampling. The neoplastic cells may have a variable morphology including classically described acinar arrangements with salt-and-pepper chromatin, but can also have spindle and pleomorphic forms with dense granular chromatin.[1112] The differential diagnosis for this cytological pattern varies widely and depends on the morphological pattern of the tumor. Aside from the other salivary gland lesions as previously mentioned, given the location, one may consider malignant melanoma, follicular variant of thyroid carcinoma and/or adenocarcinoma with an acinar pattern, whereas, a more spindled cell pattern could be suspected for a medullary thyroid carcinoma or even a sarcoma.[1113] A combination of history, tumor location, clinical impression, radiology findings, and confirmatory IHC will assist in identifying the majority of these tumors.
FNA biopsy has been performed on numerous occasions to establish a diagnosis of PGL.[11–16] However, the exact role of FNA in diagnosis of these tumors has not been currently established. The main concern in performing a pre-operative FNA on suspected PGLs is the risk of hemorrhage and hematoma.[1416] In this article not only are we reporting the first PGL of the parotid gland in the literature, but also would like to emphasize the cytological features of the tumor in FNA aiding in the diagnosis.
This case report illustrates the importance of a broad differential diagnoses when confronted with unexpected cytology in an otherwise routine parotid gland FNA with emphasis on morphological evaluation and immunohistochemical confirmation. First criterion is the epithelioid appearance of the cells with pleomorphic morphology. The second criterion is positivity for chromogranin-A and synaptophysin with negativity for cytokeratin by IHC. In contrast, the cells in the classical neuroendocrine tumors are positive for cytokeratin in a punctate form.[8] Third, presence of a highly-bloody FNA background is a characteristic and helpful feature. The fourth is S-100 positivity of only the spindle shaped cells. It is needless to say that always malignant melanoma should be in the differential diagnoses list. A simple IHC stain with HMB45 and/or Melan-A antibodies will aid to rule out the latter. The findings in this patient extend the spectrum of the PGLs to the parotid and perhaps to all major salivary glands. Close interaction between the pathologists, radiologists, and the surgeons is required to avoid aggressive and unnecessary surgical procedures, such as the lymph node dissection, as it was in this case. Such collaboration will ensure careful evaluation and accurate diagnosis of such tumors.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
Competing interests are not present in this case.
AUTHORSHIP STATEMENT MADE BY ALL AUTHORS
AV Cytopathology fellow, collected all the data, participated in cytological evaluation, and drafting of manuscript. CKL performed histological evaluation and manuscript review.
JYR performed cytological evaluation and manuscript review. SKA performed cytological evaluation and manuscript review. NAM performed conceptual organization, cytological-histological evaluation, drafted and reviewed the manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without patient identifiers, approval from the Institutional Review Board (IRB) is not required at our institution.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/26/105119
REFERENCES
- Head and neck paragangliomas: Revision of 89 cases in 73 patients. Acta Otorrinolaringol Esp. 2007;58:94-100.
- [Google Scholar]
- Tumors of paraganglionic system: Introduction. In: Barnes L, Eveson J, Reichart P, Sidranskey D, eds. WHO Clasification of Tumours. Lyon: IARC Press; 2005. p. :361-70.
- [Google Scholar]
- Hereditary paraganglioma-pheochromocytoma syndromes. 1993. GeneReviews™. Seattle (WA): University of Washington, Seattle;
- [Google Scholar]
- SDH mutations in tumorigenesis and inherited endocrine tumours: Lesson from the phaeochromocytoma-paraganglioma syndromes. J Intern Med. 2009;266:19-42.
- [Google Scholar]
- Salivary gland. In: Cibas ES, Ducatman BS, eds. Cytology, Diagnostic Principles and Clinical Correlates. Philadelphia: Saunders; 2009. p. :285-318.
- [Google Scholar]
- A correlation study of diagnostic fine-needle aspiration with histologic diagnosis in cystic neck lesions. Diagn Cytopathol. 2009;37:720-6.
- [Google Scholar]
- Neuroendocrine carcinoma of the head and neck: A 20-year case series. Ear Nose Throat J. 2012;91:E20-4.
- [Google Scholar]
- Extraadrenal paragangliomas of the body: Imaging features. AJR Am J Roentgenol. 2006;187:492-504.
- [Google Scholar]
- Recommendations for the reporting of extra-adrenal paragangliomas. The Association of Directors of Anatomic and Surgical Pathology. Hum Pathol. 2003;34:112-3.
- [Google Scholar]
- Interconnecting cytoplasmic processes on fine-needle aspiration smears of carotid body paraganglioma. Diagn Cytopathol. 2010;38:507-8.
- [Google Scholar]
- Pitfalls in fine needle aspiration cytology of extraadrenal paraganglioma.A report of 2 cases. Acta Cytol. 2003;47:1082-6.
- [Google Scholar]
- Bilateral carotid body tumor: The role of fine-needle aspiration biopsy in the preoperative diagnosis. Diagn Cytopathol. 2008;36:178-80.
- [Google Scholar]
- Carotid body paraganglioma manifesting as a malignant solitary mass on imaging: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:e54-8.
- [Google Scholar]








