Translate this page into:
Oncocytic follicular nodules of the thyroid with or without chronic lymphocytic thyroiditis: An institutional experience
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Oncocytic follicular (OF) cells can be a prominent component of fine needle aspiration (FNA) specimens from neoplasms (adenomas and carcinomas) and nodules arising in multinodular goiter and chronic lymphocytic thyroiditis (CLT). Because OF cells can be present in non-neoplastic and neoplastic thyroid lesions it can be challenging to differentiate between these two in FNA specimens. The aims of this study were to determine the risk of malignancy in cases diagnosed as either oncocytic follicular neoplasm (OFN) or hyperplastic/adenomatoid nodule with OF on FNA and to identify clinicopathologic features that may help in predicting malignancy in such cases, especially the presence or absence of CLT.
Design:
We retrospectively searched the computerized laboratory information system at our institution between 1998 and 2009 for thyroid US guided FNA specimens in which the term “oncocytic/oncocytes” was mentioned in the final cytopathologic diagnosis. A total of 340 cases were selected for this study. The following data points were collected: Patient demographics, site of thyroid biopsy, size of lesion, FNA diagnosis, histopathologic follow-up and presence of CLT. Surgical pathology follow-up (SPFU) was available in 269 (79%) cases.
Results:
Two hundred and sixty patients were females and 80 males (average age 53 years). The lesion size was <3.0 cm in 241 (71%) and ≥ 3.0 cm in 99 (29%) cases. Cytologic diagnoses included: Follicular neoplasm with oncocytic features (FNOF) 321 and suggestive of FNOF 19 cases; a secondary cytologic diagnosis of CLT was made in 20 cases. SPFU was available in 269 (79%) cases; it was benign in 213 (213/267 = 79%) and malignant in 56 (56/269 = 21%) cases. The background thyroid showed CLT in 67 (25%) cases; 24% (48/196) neoplasms occurred with versus 76% (147/196) without CLT. The rate of malignancy was lower in nodules measuring less than 3.0 cm as compared to those equal or greater than 3.0 cm in size (17% vs. 28% respectively). The presence of CLT did not significantly alter the rate of malignancy in both FNA and surgical pathology specimens.
Conclusions:
Based on this study, nodule size and not CLT appears to be an important clinicopathologic features in the management of thyroid FNA specimens diagnosed as OFN.
Keywords
Chronic lymphocytic thyroiditis
fine needle aspiration
neoplasm
oncocytic cells
thyroid
INTRODUCTION
Oncocytic cells of the thyroid, also known as Hürthle cells, Askanazy cells, or Oxyphilic cells, are derived from follicular cells and are microscopically characterized by abundant eosinophilic, granular cytoplasm, distinct cell borders, and large hyperchromatic nuclei with prominent nucleoli.[1] Thyroid oncocytic follicular (OF) cells are found in a variety of non-neoplastic and neoplastic pathologic conditions, including chronic lymphocytic thyroiditis (CLT), long-standing Graves’ disease, multinodular goiter, benign and malignant thyroid neoplasms.[12] Given the wide range of thyroid pathologies in which oncocytic cells may be encountered, it is not surprising that definitive classification of these lesions can be challenging.[23]
Interpreting fine needle aspiration (FNA) specimens of oncocytic lesions of thyroid is fraught with difficulty and diagnostic pitfalls.[3–7] Non-neoplastic thyroid lesions characterized by oncocytic metaplasia and/or hyperplasia may result in FNA smears with fairly monotonous cells, leading to a “misdiagnosis” of an oncocytic neoplasm.[478] It is not unusual to encounter cytomorphologic features of CLT in the background of FNA specimens containing a predominant population of OF cells.[39–11] Cytopathologists have long recognized the diagnostic challenge to differentiate between oncocytic metaplasia in a benign nodule versus oncocytic neoplasm arising in CLT.[11–13]
In this study, we sought to identify potential clinical and pathologic features predictive of malignancy in FNA specimens containing OF cells specifically in regards to the presence or absence of CLT.
MATERIALS AND METHODS
An electronic search was performed using the laboratory information system at the Hospital of the University of Pennsylvania, to identify all cases with the terms “oncocytic” or “oncocytes” in the final cytopathologic diagnosis during the years 1998-2009. Three hundred and forty patients were included in the study. Pathology reports were retrieved and the following data points were collected: Patient's age and sex, site of thyroid aspiration, ultrasound size of lesion, FNA diagnosis, histopathologic follow-up, presence or absence of CLT and other histopathologic findings. Additional, electronic medical records in all cases retrieved from pathology files were searched for clinical history of CLT (thyroid antibody titers and ultrasound findings).
At our institution, the following cytologic criteria are employed to diagnosis OF lesions in thyroid FNA specimens. A diagnosis of OFN is rendered when the 75% of the specimens consist of OF cells without or minimal evidence of CLT. These specimens usually show presence of thick colloid and lack watery colloid. In cases, where CLT is present; the neoplastic cell groups usually lack or contain few lymphocytes as compared to those in the background. A diagnosis of hyperplastic/adenomatoid nodule with oncocytic features arising in a background of CLT is made when a majority of oncocytic cell groups are infiltrated by lymphocytes (usually in the form of tangles). These cases also demonstrate a second population of non-OF cells in the background.
RESULTS
The clinic-pathologic features and surgical pathology follow-up (SPFU) is summarized in Tables 1 and 2.
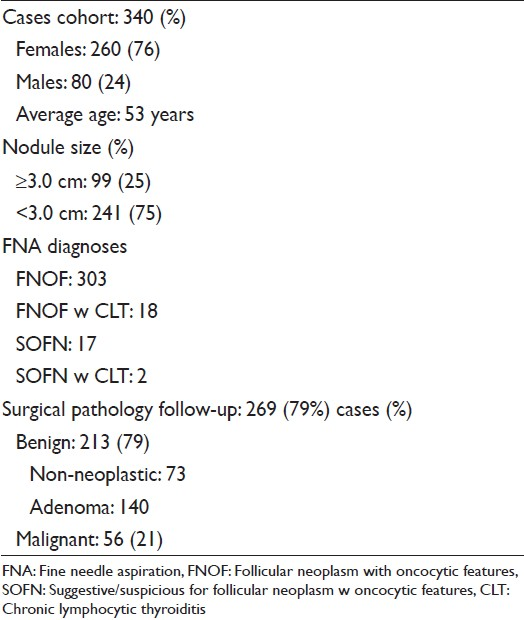
The case cohort consisted of 340 samples, collected from 260 (76%) women and 80 (24%) men. The average patient age was 53 years (range 13-86 years). By ultrasound, 173 nodules occurred in the right and 153 in the left lobe; 10 in isthmus, 3 in left and 1 in right side of isthmus. The aspirated nodule size was available in 268 cases; ≥3.0 cm in 99 (37%) and < 3.0 cm in 169 (63%) cases. A diagnosis of follicular neoplasm with oncocytic features (FNOF) was rendered in 303 (89%); FNOF with CLT in 18 (5%); and suggestive/suspicious of FNOF with and without CLT in 2 (1%) and 17 (5%) cases respectively.
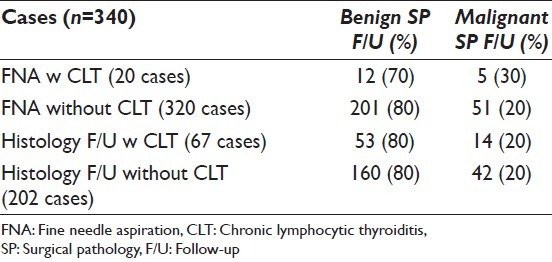
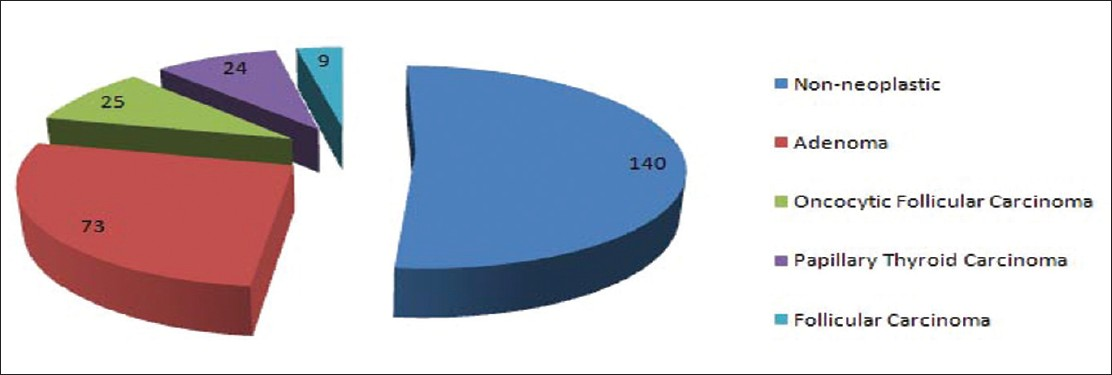
SPFU was available in 269 (79%) cases; 73 were diagnosed as non-neoplastic (27%), 140 (52%) as adenoma and 56 (21%) as malignant [Figure 1]. The malignant diagnoses included OF carcinoma 23, papillary thyroid carcinoma 24 (follicular variant 19 and classic variant 5) and follicular carcinoma 9 cases. CLT was present in 67 (25%) cases; of these 14 (25%) were diagnosed as malignant and 42 (75%) as benign. The SPFU was available in 18 FNA specimens, which showed cytomorphologic evidence of CLT; a malignant diagnosis was rendered in 5 (28%) and benign diagnosis in 13 (72%) cases. Of 320 FNA specimens without cytomorphologic evidence of CLT SPFU was available in 251 (78%) cases; it was benign in 200 (80%) and malignant in 51 (20%) cases.
- Surgical pathology follow-up of 296 diagnosed as oncocytic follicular neoplasm on fine-needle aspiration
The rate of malignancy was 22% in female and 16% in male patients (average age 53 years); and 28% in nodules measuring ≥3.0 cm and 17% in nodules measuring less than 3.0 cm.
DISCUSSION
OF cells can be encountered in thyroid FNA specimens of both benign and neoplastic lesions of the thyroid.[4614] As is widely known; thyroid FNA cannot distinguish between benign and malignant OFN, i.e., OF adenoma or OF carcinoma.[613] This distinction can only be accomplished by histopathologic examination of resected specimens to demonstrate invasive characteristics.[1–3] Some investigators have suggested that based on certain cytologic criteria such as, nuclear pleomorphism (enlargement, hyperchromasia, and prominent nucleoli), mitoses, solid and trabecular architecture and smear background one can distinguish between benign and malignant oncocytic lesions.[68] However, many have disputed this claim based on the fact that it is even difficult to make a diagnosis of oncocytic neoplasm in thyroid FNA specimens rather than suggest malignancy.[4715]
The most widely practiced cytologic criteria to render a diagnosis of Oncocytic follicular neoplasm (OFN) in FNA specimens includes: Cellular specimen showing a monotonous population of OF cells (75% or greater), prominent nucleoli, transgressing vessels and minimal background colloid.[15–18] The reported risk of the malignancy associated with a FNA diagnosis of OFN is 15-36%.[4719] Several authors have suggested that this risk malignancy can be correlated to the sex and age of the patient and size of the nodule.[47] Interestingly, a study by Roh et al. demonstrated a very low risk of malignancy in cases of OFN arising in CLT.[20] It is well known that the oncocytic cells are commonly encountered in thyroid affected by CLT/Hashimoto's thyroiditis.[2] Thus, it is likely that FNA specimens of these will pose problems in differentiating between a oncocytic hyperplastic nodule (OHN) versus OFN.
In our study cohort of 340 FNA cases diagnosed as OFN over a period of 10 years; SPFU was available in 269 (269/340) cases. Of these 196 (73%) were neoplasms (benign and malignant) including 56 (21%) malignant cases; the malignant diagnoses were OF carcinoma 23, papillary thyroid carcinoma 24 and follicular carcinoma 9 cases. The malignancy was more frequent in nodules measuring ≥3.0 cm as compared to nodules measuring <3.0 cm; 28% versus 17%. Appreciable CLT was identified affecting the background thyroid in 67/269 (25%) cases. Interestingly, 14/56 (25%) malignant cases were associated with CLT as compared to 42/56 (75%) without CLT on histologic examination. No statistically significant association was noted between risk of malignancy, age and sex of the patient.
Most published studies have focused on correlating the risk of malignancy in cases diagnosed as neoplasm on FNA with clinicopathologic features such as age and sex of the patient; and size of the nodule.[472122] Sippel et al. found a dramatic increase in the risk of malignancy from 13% to 55% in nodules measuring larger than 4 cm in size.[21] Giorgadze et al. found this to be the case in nodules measuring ≥2 cm in patients’ ≥40 years of age.[4] This study failed to find any statistically significant difference in risk of malignancy among male and female patients. Raparia et al. reported similar risk of malignancy for same sized nodules as in study by Giorgadze et al. however, in their study malignancy was more common in patients older than 50 years of age.[22] Similar findings with fewer differences in size of the nodules and age of the patients have been reported in studies by Strazisar et al. and Kim et al.[2324] The questions arise, are these variable results due to the difference in the histopathologic criteria applied to diagnose OF lesions of the thyroid or due to differences in the patient population seen at these referral centers? We believe the histologic criteria employed in diagnosing these lesions are usually uniform especially those based on presence or absence of vascular invasion, thus these variable observations are most likely due to the different patient population seen at these referral centers.
In a recent study by Roh et al. the provocative concept of comparing the risk of malignancy in cases diagnosed as OFN on FNA with presence or absence of CLT was explored.[20] A diagnosis of CLT was made based on the evaluation of clinico-radiological findings and SPFU. This study comprised a cohort of 401 cases with available SPFU in 287 cases. Of these 21 (7%) cases demonstrated CLT; interestingly, the neoplastic lesions were less common in cases with as compared to those without CLT (43% vs. 69% respectively). The rate of malignancy was 9.5% versus 25% in cases with and without CLT respectively. Based on the current study 24% (48/196) neoplasms occurred with versus 96% (147/196) without CLT. The number of malignant tumors were much lower in surgical specimens with as compared to those without CLT; 25% versus 75% respectively. These results do appear similar to those reported by Roh et al.[20] However, when we calculated the malignancy rates taking into account the number of specimens with and without CLT in FNA specimens only; no statistically significant difference was noted [Table 2]. Thus, in our case, cohort CLT in FNA specimens only has little or no effect on the malignancy rates as suggested by Roh et al.[20]
The malignant tumors seen in our study were not only OF carcinoma but also included papillary thyroid carcinoma (24 cases) and follicular carcinoma (9 cases). Similar results have been reported by other authors.[4720] In the study by Pu et al., the malignant tumors on the histologic follow-up were: Papillary thyroid carcinoma 58, OF carcinoma 15 and follicular carcinoma 12 cases.[7] Similar findings have been reported by Giorgadze et al.[4] (OF carcinoma 53, papillary thyroid carcinoma 19, follicular carcinoma 3 and medullary thyroid carcinoma 1 case) and Roh et al.[20] (OF carcinoma 41, papillary thyroid carcinoma 26 and poorly differentiated carcinoma 2 cases). These results from the above-mentioned studies including ours point to the fact that oncocytic cytoplasm can also be seen in PTC and other malignant tumors of the thyroid and mistaken for non-papillary OFN of the thyroid.[23]
Interestingly, in the study by Roh et al., only two (10%) malignant tumors were associated with CLT. Based on this low positive predictive value these authors entertained the possibility of diagnosing OF lesions with CLT as atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), which accurately reflects the 10% risk of malignancy rather than “neoplasm” to avoid surgery.[20] However, in our study, 14/56 (25%) malignant tumors arose in the background of CLT. This difference is most likely is due to the reflection of the high number of cases with CLT seen in our study (67 cases). We believe, it may be more appropriate to apply strict criteria to render the diagnosis of OFN in FNA specimens with CLT rather than classify most of these cases as AUS/FLUS, since under diagnosing these lesions could delay surgical excision and diagnosis of these aggressive tumors.[23]
In summary, based on this study, the morphologic evidence of CLT on FNA as well SPFU does not appear to significantly affect the rate of malignancy for thyroid nodules diagnosed as OFN. The thyroid nodule size remains one of the most important clinic-pathologic features for the clinical management of FNA specimens diagnosed as OFN.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors attest to having no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article.
SC, ACG, AG and HW carried out the data acquisition and analysis, ACG, HW and SC drafted the manuscript. ZWB, KTM and VAL equally participated in its design and coordination and helped to draft and finalize the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) (or its equivalent) of all the institutions associated with this study as applicable. Authors take responsibility to maintain relevant documentation in this respect.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/2/106686
REFERENCES
- The thyroid Hürthle (oncocytic) cell and its associated pathologic conditions: A surgical pathology and cytopathology review. Arch Pathol Lab Med. 2008;132:1241-50.
- [Google Scholar]
- Does the fine-needle aspiration diagnosis of Hürthle-cell neoplasm/follicular neoplasm with oncocytic features denote increased risk of malignancy? Diagn Cytopathol. 2004;31:307-12.
- [Google Scholar]
- Papillary hyperplastic nodule: Pitfall in the cytopathologic diagnosis of papillary thyroid carcinoma. Endocr Pract. 2008;14:863-8.
- [Google Scholar]
- Hürthle cell carcinoma is a better gold standard than Hürthle cell neoplasm for fine-needle aspiration of the thyroid: Defining more consistent and specific cytologic criteria. Cancer. 2002;96:261-6.
- [Google Scholar]
- Does Hurthle cell lesion/neoplasm predict malignancy more than follicular lesion/neoplasm on thyroid fine-needle aspiration? Diagn Cytopathol. 2006;34:330-4.
- [Google Scholar]
- Differential diagnosis of oncocytic lesions of the breast and thyroid utilizing a semiquantitative approach. Acta Cytol. 1999;43:544-51.
- [Google Scholar]
- Warthin-like papillary carcinoma of the thyroid. Arch Pathol Lab Med. 2000;124:1192-5.
- [Google Scholar]
- Incidence of neoplasia in Hashimoto's thyroiditis: A fine-needle aspiration study. Diagn Cytopathol. 1996;14:38-42.
- [Google Scholar]
- Histology and aspiration cytology of benign thyroid diseases. Rays. 1999;24:182-96.
- [Google Scholar]
- Diagnostic pitfalls in the evaluation of fine needle aspiration cytology of the thyroid: Correlation with histopathology in 260 cases. Cytopathology. 2009;20:103-8.
- [Google Scholar]
- Follicular thyroid lesions coexisting with Hashimoto's thyroiditis: Incidence and possible sources of diagnostic errors. Diagn Cytopathol. 2003;28:35-8.
- [Google Scholar]
- Flower-like colloid on thyroid fine needle aspiration. Diagn Cytopathol. 2008;36:607-8.
- [Google Scholar]
- Color Atlas of Differential Diagnosis in Exfoliative and Aspiration Cytopathology (2nd ed). Philadelphia: Wolters Kluwer/Lippincott Williams and Wilkins; 2011. p. :123.
- Diagnostic utility of intracytoplasmic lumen and transgressing vessels in evaluation of Hürthle cell lesions by fine-needle aspiration. Arch Pathol Lab Med. 2001;125:1031-5.
- [Google Scholar]
- Fine-needle aspiration biopsy of Hurthle cell lesions of the thyroid gland: A cytomorphologic study of 139 cases with statistical analysis. Cancer. 2006;108:102-9.
- [Google Scholar]
- Can abundant colloid exclude oncocytic (Hürthle cell) carcinoma in thyroid fine needle aspiration? Cytohistological correlation of 127 oncocytic (Hürthle cell) lesions. Cytopathology 2012 [EPUB ahead of print]
- [Google Scholar]
- Fine needle aspiration of the thyroid: Correlation with final histopathology in a surgical series of 797 patients. J Am Coll Surg. 2011;213:188-94.
- [Google Scholar]
- The predictive value of the fine-needle aspiration diagnosis suspicious for a follicular neoplasm, hurthle cell type in patients with hashimoto thyroiditis. Am J Clin Pathol. 2011;135:139-45.
- [Google Scholar]
- Tumor size predicts malignant potential in Hürthle cell neoplasms of the thyroid. World J Surg. 2008;32:702-7.
- [Google Scholar]
- Clinical outcomes for suspicious category in thyroid fine-needle aspiration biopsy: Patient's sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med. 2009;133:787-90.
- [Google Scholar]
- Predictive factors of carcinoma in 279 patients with Hürthle cell neoplasm of the thyroid gland. J Surg Oncol. 2010;101:582-6.
- [Google Scholar]
- Postoperative findings and risk for malignancy in thyroid nodules with cytological diagnosis of the so-called follicular neoplasm. Korean J Intern Med. 2003;18:94-7.
- [Google Scholar]








