Translate this page into:
Pleural fluid metastases of salivary duct carcinoma: A case report and review of the literature
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Salivary duct carcinoma (SDC) comprises a small proportion of salivary gland tumors; however, it is known to be aggressive with a high rate of metastasis. Although frequent references are made to pulmonary dissemination, metastases in the pleural fluid have not been described. In this article, we report the cytologic features of metastatic SDC in the pleural fluid. The clinical history, cytomorphology and immunohistochemical features used for diagnosis are described. To the best of our knowledge, this is the first case of pleural fluid involvement by salivary duct carcinoma reported in the literature.
Keywords
Cytology
metastases
pleural
salivary duct carcinoma
INTRODUCTION
Salivary duct carcinoma (SDC) is a relatively uncommon salivary gland tumor, with a high stage on diagnosis and a high rate of metastasis over the course of the disease.[123] Multiple sites of metastasis have been reported, including lung, liver, bone and rarely gingiva, eye, vagina and even distant inguinal lymph nodes.[14567] While multiple other salivary gland tumors have been reported in pleural fluid, our review of the literature found no prior reference to SDC metastasis. In this article, we describe a patient with extensively metastatic salivary duct carcinoma presenting with a pleural effusion, providing a previously unreported finding of SDC in the pleural fluid.
CASE REPORT
The present case report is about a 76-year-old male patient, with a 9-year history of SDC metastatic to the right testicle and brain, who presented at our institution for cryoablation of suspicious lung nodules and subsequently had a recurrent left pleural effusion. He was originally diagnosed at age 67 with a 1.5 cm right submandibular SDC, which showed large polygonal epithelioid cells with prominent nucleoli and abundant granular eosinophilic cytoplasm, arranged in a cribriform pattern with central necrosis [Figure 1]. Perineural and lymphovascular invasion were noted. Immunohistochemical stains showed positivity for human epidermal growth factor receptor-2/neu (HER2/neu) and gross cystic disease fluid protein-15 (GCDFP-15) and negativity for estrogen receptor (ER) and progesterone receptor (PR). The patient was treated with local radiation and chemotherapy. At 4 years later, pulmonary micronodules were noted on positron emission tomography and computed tomography (CT) scan, as well as a recurrence in his right testis. He underwent orchiectomy and received additional chemotherapy. The following year, a right occipital lobe metastasis was irradiated and resected. Serial radiographic imaging showed slow progressive growth of the pulmonary nodules.
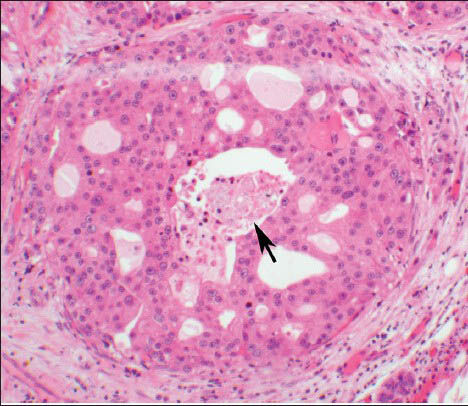
- The histologic section of the parotid gland tumor shows a malignant proliferation of a duct, resembling ductal carcinoma in situ of the breast. There is a cribriform architecture with central necrosis (arrow). The cells have prominent nucleoli with apocrine type cytoplasm (H and E, ×10 Obj)
At the time of current admission, the patient was receiving radiation therapy, but not systemic therapy. The most recent chest CT angiogram showed bilateral pulmonary metastases up to 5.4 cm, pleural implants and a nodular rind of metastatic disease along the left mediastinal reflection. Following cryoablation of the three largest nodules, ultrasound-guided thoracentesis of a left pleural effusion yielded 350 cc of bloody, opaque fluid. Cytological evaluation of cytospin preparations and cell block revealed large, pleomorphic epithelioid cells in small clusters with subtle cribriform features [Figure 2]. The cells displayed large oval nuclei with prominent nucleoli and coarsely granular chromatin. A moderate amount of finely granular apocrine-type cytoplasm was present.
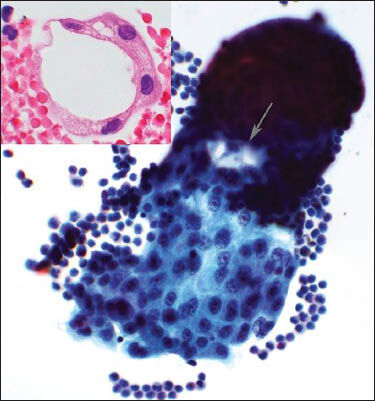
- Composite photograph of the pleural fluid shows smears and cell block. The smear shows a single cluster of epithelioid cells with prominent nucleoli and apocrine cytoplasm (Papanicolaou stain, ×40 Obj). A subtle cribriforming feature is noted (arrow). The inset is a photomicrograph of the cell block showing a cluster of malignant epithelioid cells making a lumen (H and E, ×60 Obj)
Immunohistochemical stains [Figure 3] performed on the cell block were positive for androgen receptor (AR) (panel A), HER2/neu (panel B), Moc31 (panel C) and GCDFP-15 (panel D). These cells were also positive for BerEp4 and were negative for ER and PR. The malignant cells were not present on deeper cuts for evaluation of mammaglobin and B72.3. The morphologic and immunohistochemical profile was similar to the original submandibular pathology and a diagnosis of metastatic SDC was rendered.
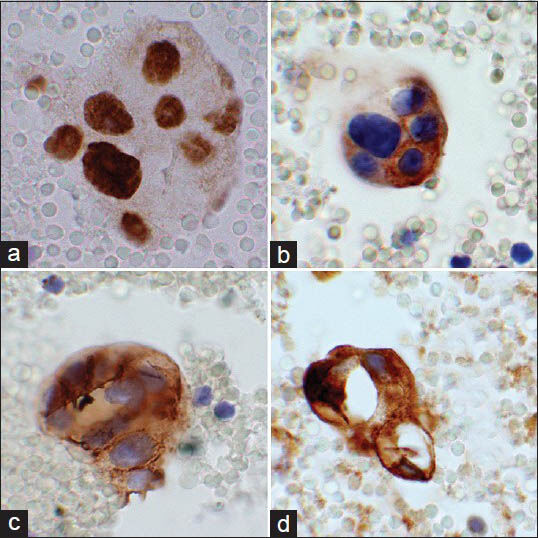
- Composite photograph of the immunohistochemical stains performed on the cell block. Panel A is a photomicrograph of androgen receptor showing positive nuclear staining of the malignant cells. Panel B shows human epidermal growth factor receptor-2/neu staining cell membrane of the malignant cells. Panel C illustrates cell membrane and cytoplasmic staining of these cells with MOC-31. Panel D shows cytoplasmic staining of this cluster with gross cystic disease fluid protein-15 (×60 Obj)
DISCUSSION
Malignancy is a common cause of pleural effusion, accounting for 9-44% of cases.[8] The likelihood of pleural involvement varies according to tumor type, with adenocarcinomas comprising two-thirds of all malignant effusions. Among men, lung cancer is the most frequent etiology, followed by lymphoproliferative lesions and gastrointestinal tract malignancies. Breast, lung and lymphoid neoplasms make up the majority of malignant pleural effusions in women.[8]
Pleural fluid involvement by salivary gland tumors is rare. Occasional case reports exist of adenoid cystic carcinoma metastasizing to the pleural fluid,[91011] as well as a rare case of benign metastasizing pleomorphic adenoma[12] and a case of mucoepidermoid carcinoma involving peritoneal and pericardial fluid.[913] However, this appears to be the first case report of metastatic SDC in pleural fluid.
SDC accounts for approximately 1.8% of all salivary tumors.[1214] It is more common in men (8:1, male: female) between the ages of 60-80, though an age range of 22-91 has been reported.[121415] Most arise in the parotid gland (72-88%), but submandibular, sublingual, minor salivary gland, maxillary and laryngeal primaries have all been described.[1214] The clinical presentation usually involves rapid growth of a painful mass with possible facial paralysis secondary to perineural invasion.[1] Treatment is primarily surgical, with radiation and chemotherapy playing a limited role.[14] Hormone therapy has not yet been shown to be effective.[16] Local recurrence occurs in one-third of patients.[1] Lymph node involvement in 56-60% of patients at initial presentation[121517] and distant metastases occur in 46% of patients.[1] The lung is a frequent site of metastatic disease, although lesions in liver, bone, thyroid, skin, vagina, brain and eye have also been described.[14567] The 10-year mortality rate due to the tumor is 36-65% respectively.[1214]
The cytologic characteristics of SDC on a fine-needle aspiration (FNA) specimen have been well-described, though the ability to adequately make the primary diagnosis on FNA alone is challenging. Hypercellular smears are often seen, with broad flat sheets, tightly-cohesive three-dimensional clusters, papillary fragments and rarely distinctive cribriform configurations.[318] In general the cells are large and polygonal with eccentric round to oval nuclei, coarse chromatin, prominent nucleoli and mild to moderate nuclear atypia. A moderate to an abundant amount of vacuolated or oncocytic cytoplasm is seen.[318] Some cases describe abundant mitoses, intranuclear cytoplasmic inclusions, squamoid cells and a background of necrosis.[318]
The histologic similarity of SDC and ductal carcinoma of the mammary gland has been noted since the initial description in 1968 by Kleinsasser et al.[19] Cell blocks may show variable-sized nodules of epithelioid cells, arranged in a cribriform pattern with central comedonecrosis. Solid, cystic or papillary architecture may also be seen.[219] Rarely, spindle cell or sarcomatoid growth patterns, mucin-rich variants, squamous metaplasia, or micropapillary features can be observed.[12] The tumor can present in a background of a pleomorphic adenoma in approximately 20% of cases.[1220]
The differential diagnosis on FNA can include high grade mucoepidermoid carcinoma or acinic cell carcinoma, as well as oncocytic neoplasms.[18] Metastatic breast carcinoma should always be ruled out, as the two entities are so alike.[1] Other metastatic adenocarcinomas or squamous cell carcinoma may also be considered, if the lesion is poorly differentiated or lacks the characteristic cribriform pattern.
SDC tends to stain positive for epithelial markers (BerEp4, MOC31, low and high molecular weight cytokeratins) and negative for mucicarmine. These results should prompt closer assessment of the specimen and clinical history to elicit the location of a primary malignancy. Confirmation of the diagnosis can be achieved with special stains. HER2/neu is positive in most SDC's (47-67% of cases), with overexpression in approximately 20% of patients.[121721] Other predominately positive stains include AR, prostate specific antigen, prostatic acid phosphatase, GCDFP-15, p53 and p16.[123172122] Cytoplasmic staining for mucin is rare and helps exclude mucoepidermoid carcinoma. Negative stains include myoepithelial markers and ER and PR, which aids in distinguishing it from breast carcinoma.[1231721]
SUMMARY
Given the propensity of SDC to metastasize to the lung, the paucity of case reports describing pleural fluid involvement is unexpected. Considerations for the rarity of this finding may include the aggressive nature of the disease, a low clinical yield of performing thoracentesis, or possibly a strong cohesive nature of the malignant cells. A similar behavioral pattern can be seen in other tumors such as squamous cell carcinoma or other salivary gland tumors, which may involve lung parenchyma but not the pleura. The cohesive nature of these cells may discourage metastases to body fluids, when compared to the often discohesive appearance of lobular breast carcinoma, adenocarcinomas and lymphoproliferative lesions. Elements of this case which contributed to the diagnostic material stem from the patient's length of disease and the significant lung involvement seen on imaging, as well as the recent disruption of lung parenchyma by cryoablation. Our case illustrates an unusual spread of SDC into the pleural fluid and highlights the challenges in rendering a diagnosis of metastatic SDC on a cytology specimen.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author (s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that we qualify for authorship as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the work and takes public responsibility for appropriate portions of the content of this article. JH, pathology resident, collected all the data, participated in cytological evaluation and drafted the manuscript. RC, cytopathology fellow, performed cytologic evaluation and manuscript review. SH performed cytologic evaluation and manuscript review. NAM performed conceptual organization, cytological-histological evaluation, drafted and reviewed the manuscript. All authors have read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without patient identifiers, approval from the Institutional Review Board (IRB) is not required at our institution.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Salivary duct carcinoma. In: Barnes L, Eveson JW, Reichart P, Sidransky D, eds. World Health Organization Classification of Tumors: Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. p. :236-7.
- [Google Scholar]
- Update on selected salivary gland neoplasms. Arch Pathol Lab Med. 2009;133:1763-74.
- [Google Scholar]
- Gingival metastasis from salivary duct carcinoma of the parotid gland. J Periodontol. 2008;79:748-52.
- [Google Scholar]
- Salivary duct carcinoma metastatic to eyelid and orbit-a case report. Graefes Arch Clin Exp Ophthalmol. 2008;246:1185-8.
- [Google Scholar]
- Salivary duct carcinoma presenting with vaginal metastasis: Case report. Eur J Gynaecol Oncol. 2007;28:415-7.
- [Google Scholar]
- Salivary duct carcinoma metastatic to inguinal lymph node: A case report of salivary duct carcinoma with distant metastasis diagnosed by fine-needle aspiration. Diagn Cytopathol. 2006;34:41-4.
- [Google Scholar]
- The cytologic diagnosis of malignant neoplasms in pleural and peritoneal effusions. Acta Cytol. 1987;31:85-97.
- [Google Scholar]
- The cytomorphologic spectrum of salivary gland type tumors in the lung and mediastinum: A report of 16 patients. Diagn Cytopathol. 2012;40:1062-70.
- [Google Scholar]
- Massive pleural effusion as isolated manifestation of metastatic spread of salivary adenoid cystic carcinoma. Respir Med. 1997;91:169-70.
- [Google Scholar]
- Extra-salivary gland presentations of adenoid cystic carcinoma: A report of three cases. Diagn Cytopathol. 2006;34:491-4.
- [Google Scholar]
- Fine-needle aspiration (FNA) and pleural fluid cytology diagnosis of benign metastasizing pleomorphic adenoma of the parotid gland in the lung: A case report and review of literature. Diagn Cytopathol. 2009;37:828-31.
- [Google Scholar]
- Mucoepidermoid carcinoma of the parotid gland with ovarian and peritoneal metastasis. J Obstet Gynaecol Res. 2008;34:271-3.
- [Google Scholar]
- Survival rates and prognostic factors for infiltrating salivary duct carcinoma: Analysis of 228 cases from the Surveillance, Epidemiology and End Results database. 2013. Head Neck. Available from http://onlinelibrary.wiley.com/doi/10.1002/hed 23350/abstract; jsessionid = 5A251B1D116C3162150C7482DB9537F8f04t01
- [Google Scholar]
- SEER Cancer Statistics Review 1975-2010. Available from: http://www.seer.cancer.gov/csr/1975_2010/browse_csr.php?section=20 and page=sect_20_table.28.html
- [Google Scholar]
- Salivary duct carcinoma of the parotid gland: Case report and review of the literature. J Oral Maxillofac Surg. 2008;66:1708-13.
- [Google Scholar]
- Clinical and immunohistologic typing of salivary duct carcinoma: A report of 50 cases. Cancer. 2005;103:2526-33.
- [Google Scholar]
- Salivary duct carcinoma: Correlation of morphologic features by fine needle aspiration cytology and histopathology. Indian J Pathol Microbiol. 2011;54:37-41.
- [Google Scholar]
- Salivary duct carcinoma. A group of salivary gland tumors analogous to mammary duct carcinoma. Arch Klin Exp Ohren Nasen Kehlkopfheilkd. 1968;192:100-5.
- [Google Scholar]
- Invasive salivary duct carcinoma ex pleomorphic adenoma of the parotid gland: A teaching case giving rise to the genuine diagnostic difficulty on an inadequate cytology specimen. Diagn Pathol. 2012;7:61.
- [Google Scholar]
- Immunohistochemical analysis of salivary gland tumors: Application for surgical pathology practice. Acta Histochem Cytochem. 2012;45:269-82.
- [Google Scholar]
- Expression of androgen receptor and prostatic specific markers in salivary duct carcinoma: An immunohistochemical analysis of 13 cases and review of the literature. Am J Surg Pathol. 2000;24:579-86.
- [Google Scholar]








