Translate this page into:
Utility of molecular tests in cytopathology
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
With the popularity of interventional radiology, diagnostic material obtained can be limited requiring critical decisions on making the best use of it. Molecular testing using nanogram amounts of tissue can add useful diagnostic information by improving sensitivity and/or specificity of the diagnosis. This review examines the use of molecular tests in cervical cytology, “indeterminate” thyroid cytology specimens, pancreatic cyst fluid, urinary tract and pulmonary adenocarcinoma cytologic material. Molecular human papillomavirus (HPV) testing combined with cervical cytology increases sensitivity of detection of high grade lesions. In cytologically negative cases, the HPV negative predictive value endorses longer screening intervals. With the high prevalence of benign thyroid nodules, cytology plays a vital role in screening. However, 10-40% of the specimens obtained are cytologically indeterminate. Molecular analysis of these specimens can predict the malignant risk in these cases. Increased detection of pancreatic cysts has necessitated accurate pre-operative diagnosis delineating non-mucinous from mucinous cysts, which have a potential for progression to adenocarcinoma. Multimodal diagnosis of pancreatic cysts and molecular analysis help to clarify neoplastic risk; and in cases of limited fluid, may be the only available diagnostic information. Urothelial carcinoma (UC) of the bladder, a common cancer with frequent recurrences, requires lifelong surveillance. The UroVysion ™ test kit can increase the sensitivity of detection of UC especially in cases of residual/recurrent carcinoma after therapy. Subsets of lung adenocarcinomas are now commonly targeted by therapies based on molecular mutation results of epidermal growth factor receptor, KRAS or echinoderm microtubule-associated protein-like 4-anaplastic lymphoma kinase re-arrangements. The move toward standardization of reporting of cytology specimens commencing with cervical smears and more recently, thyroid cytology specimens is also changing the practice of cytopathology. Combining the stringent cytology criteria with ancillary molecular testing is expected to yield more discrete and diagnostic categories for research and reporting. The profession is at an exciting point of implementing novel molecular markers to refine diagnostic criteria and create clinically relevant classification systems.
Keywords
Cytology
human papillomavirus
molecular tests
pancreatic fluid
pulmonary adenocarcinoma
thyroid
UroVysion
INTRODUCTION
The pathologist is increasingly faced with having to field queries on molecular tests. With the popularity of interventional radiology, the material obtained is often limited and decisions on making the best use of it are critical. The impact of this is especially felt in Cytopathology where, from the point of procurement of the material, the emphasis is on stretching the material to yield the best results which inform clinical decision.
Molecular tests are used as aids in diagnosis, prognosis and therapeutic decisions. In general, molecular tests may entail, with increasing complexity, polymerase chain reaction (PCR), chromogenic/fluorescent in-situ hybridization (C/F-ISH) for chromosomal re-arrangements, reverse transcription PCR (rt-PCR) for detection of messenger ribonucleic acid and, finally, exome or entire gene sequencing. The main areas whereby molecular testing impacts greatly are in the diagnosis of soft tissue tumors, therapeutics of metastatic malignant melanoma, therapeutics of adenocarcinomas of lung, colon and breast; and prognostication of thyroid carcinoma and oligodendrogliomas. Most of these tests are carried out on resection specimens containing many more lesional cells than cytology specimens. There are, however, specific areas in cytopathology where molecular tests have a valid role and contribute pertinent information. These are in cervical, thyroid, pancreatic, urine and lung cytology specimens [Table 1]. Each of these will be discussed below including the clinical context in which the molecular tests are ordered, relevant technological aspects impacting on interpretation and an algorithm for testing for lung adenocarcinoma. A brief section of potential areas of expansion of molecular testing, their further refinements and how laboratories such as ours are dealing with the challenges brought up by these requests will conclude this review.
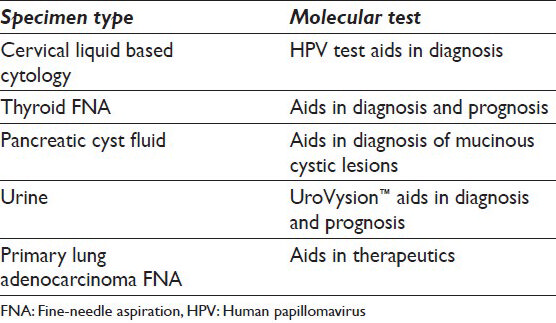
MOLECULAR TESTING IN CERVICAL CYTOLOGY – A DIAGNOSIS AID
There are three clinical contexts for human papillomavirus (HPV) test in cervical cytology. These are done on liquid based cytology samples as reflex test on patients with atypical squamous cells (ASC), normal cytology samples of women over the age of 30 years and in samples with suspected glandular lesions [Table 2]. There are five Food and Drug Administration (FDA) approved HPV tests using a hybrid capture assay, invader chemistry technology, real time PCR and transcription mediated amplification [Table 3]. No HPV test is FDA approved for primary stand-alone screening without cytology, although several large randomized studies are looking at HPV testing as a primary screening strategy.[1] HPV testing is more sensitive than cytology, whereas cytology is more specific. HPV test's high sensitivity and negative predictive values supports the endorsement of longer screening intervals.[1] This combination is now recommended by American Cancer Society, American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), American Congress of Obstetricians and Gynecologists (ACOG) and US Preventive Services Task Force (USPSTF) as a preferred strategy for screening women aged 30 and over due to the transient nature of infection in women in their early 20s compared to women in their 30's.[12] Women screened for HPV in conjunction with pap smears had a reduction of approximately 40% risk of grade 2 and 3 cervical intraepithelial neoplasia or cancer in subsequent screens compared with women screened with pap smears alone.[3] Combined testing is also more sensitive for detecting cervical adenocarcinoma comprising approximately 10% of cervical cancers.[1]
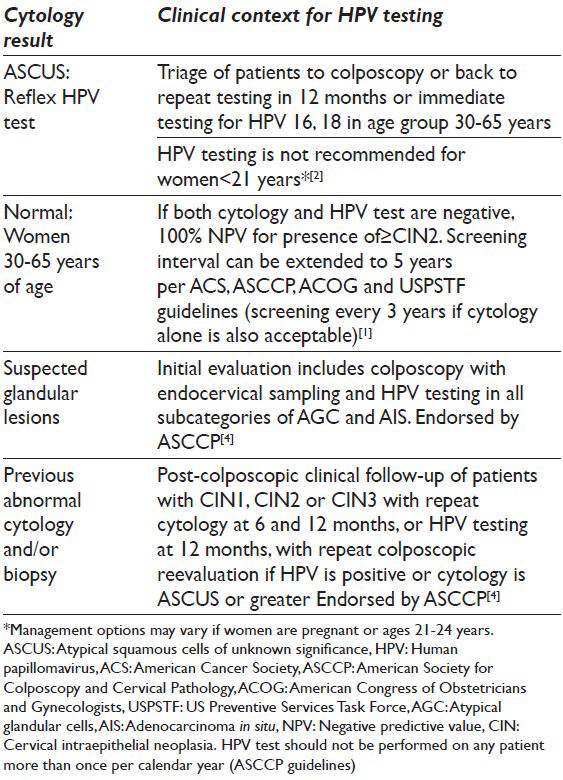

Recently ASC, ASCCP, ASCP and ACOG recommended HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing, but negative cytology results. The recommendation is for immediate referral to colposcopy if genotyping is positive with the alternative option of combined HPV and cytologic testing in 12 months.[12]
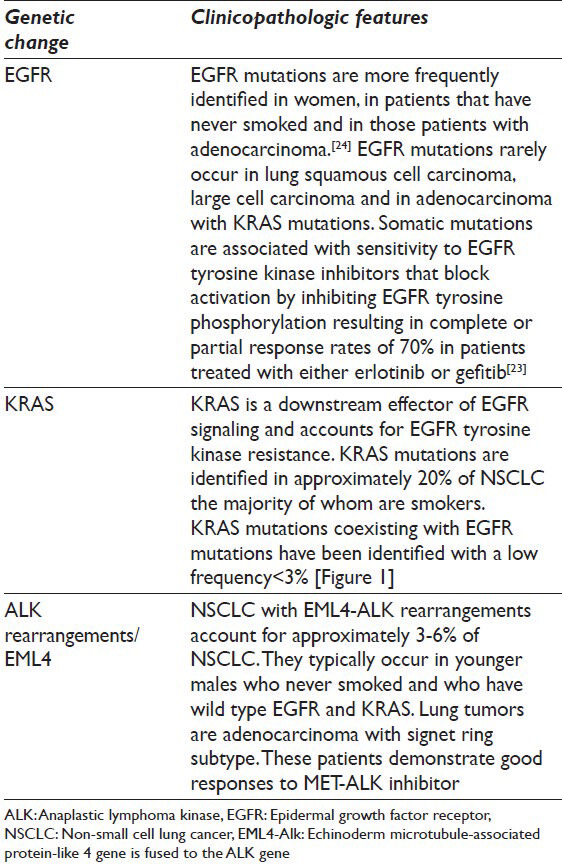
MOLECULAR TESTING IN THYROID CYTOLOGY – A DIAGNOSTIC AND PROGNOSTIC AID
Four non-overlapping genetic alterations are found in 70% of papillary and follicular carcinomas and can be detected in surgically resected samples and fine-needle aspiration (FNA) samples from thyroid nodules.[6] These are BRAF, RAS, rearranged during transfection/papillary thyroid cancer (RET/PTC) and PAX8/PPARγ genetic changes [Table 4]. BRAF, RET/PTC1 and RET/PTC3 are found almost exclusively in papillary thyroid carcinoma. RAS (NRAS, HRAS and KRAS) point mutations are more ubiquitous and are found in follicular neoplasms, follicular variant of papillary thyroid carcinoma (FVPTC) as well as cold adenomatous nodules and goiter nodules. Some believe that the presence of RAS mutation in the latter two lesions suggests that these lesions are likely true neoplasms, i.e. adenomas. PAX8/PPARγ occurs in conventional follicular carcinoma and rarely in oncocytic carcinoma.[6] Occasionally, FVPTC and follicular adenomas may also harbor the PAX8/PPARγ re-arrangement. RAS point mutations and PAX8/PPARγ are mutually exclusive and identified in 70-75% of follicular carcinomas.[6]
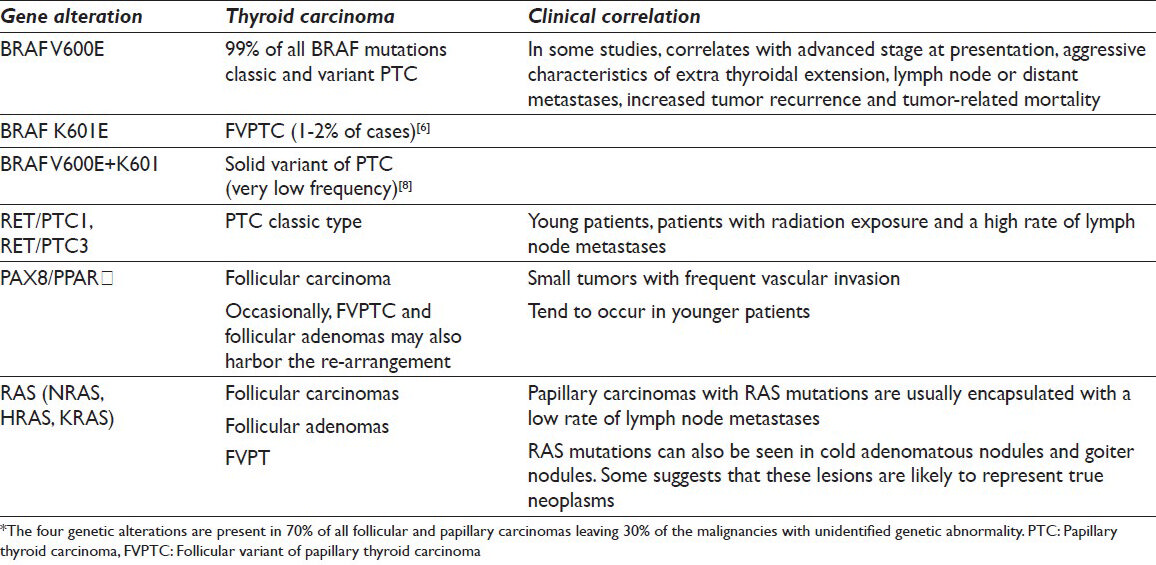
The clinical context for testing thyroid FNA sample is with specimens which are classified as atypia of unknown significance (AUS), follicular lesion of unknown significance (FLUS) and papillary thyroid carcinomas (PTC). Given that 10-40% of thyroid FNA samples may be indeterminate (AUS or FLUS) and 95% of these removed nodules prove to be benign,[7] molecular analysis has demonstrated improved clinical triage of these patients.[8] Detection of a RAS mutation in FNA sample correlates with malignancy in 74-88% of cases. Presence of BRAF mutation in a thyroid FNA sample indicates more than 99% probability of PTC and serves as a marker of aggressive behavior as well as radioiodine resistance (prognostic aids). Subgroups of BRAF mutations in PTC have been identified in addition to the predominant V600E mutation (conventional and variant PTC); the K601E (FVPTC) and BRAF V600E + K601 (solid variant of PTC).[8] The RET/PTC1 and RET/PTC3 rearrangements in papillary thyroid carcinomas are typically present in young patients and those with a history of radiation exposure. These tumors present with classic papillary histology and have a high rate of lymph node metastases.
Two commercially available, non-FDA cleared, thyroid FNA molecular tests are the Affirma Gene Expression Classifier (GEC)™ and the Asuragen miRInform (miRI)™. These molecular tests may allow for more conservative, surgery sparing clinical approach for patients with indeterminate nodules. The GEC determines the presence of 142 genes related to the metabolism and biology of the thyroid gland through microarray hybridization and the results are reported as either benign or suspicious. The test has a high negative predictive value (95% for AUS and 94% for FLUS) and sensitivity (95%); however, the specificity and positive predictive value are 52% and 47%, respectively.[9] In practical terms, the test is best at establishing a benign diagnosis.
The Asuragen miRI test consists of 17 deoxyribonucleic acid (DNA) and ribonucleic acid markers (BRAF [V600E], KRAS, HRAS, NRAS, RET/PTC1, RET/PTC3, PAX8/PPARγ), detecting 95% of all genetic changes occurring in cancer nodules.[10] The presence of any mutation conveys a greater cancer risk suggesting total thyroidectomy in all categories of indeterminate cytologic samples. This Asuragen test has not been validated as extensively as the GEC test; however, one study shows for AUS a sensitivity of 63%, specificity of 99%, positive predictive value (PPV) of 88% and negative predictive value (NPV) of 94%. For FLUS the sensitivity was 57%, specificity of 97%, PPV of 87% and NPV of 86% respectively.[10] The test has the distinct advantage of ambient temperature transport medium.
MOLECULAR TESTING IN PANCREATIC CYTOLOGY – A DIAGNOSTIC AID
The particular niche for molecular testing is in cystic pancreatic mucinous neoplasms which include intraductal papillary mucinous neoplasm and mucinous cystic neoplasms. As a result of increasing use of enhanced abdominal imaging in standard medical practice, more pancreatic cystic lesions are being identified with the majority being cystic neoplasms. Pre-operative diagnosis of non-mucinous cysts (with intrinsically low malignant potential) from mucinous cystic lesions which have a potential to progress to invasive adenocarcinoma, is important for patient management and has led to increased awareness for accurate classification of the FNA specimens.[11]
In one study, cytologic examination of cyst fluid had low sensitivity (34.5%), high specificity (83%) and low accuracy (59%) for detecting mucinous cysts.[12] Table 5 summarizes the accuracy of various modalities in predicting neoplastic cysts. Detection of KRAS mutation is very specific (96%) for a mucinous neoplasm, but lacks sensitivity (45%). Elevated carcinoembryonic antigen (CEA) of > 192 ng/ml is considered to be one of the most accurate tests to distinguish a mucinous cyst (specificity of 83% and sensitivity of 64%). A multimodal approach using KRAS, CEA, fluid viscosity and cytology further increases the sensitivity to 91%, although with reduced specificity of 56%.[11]
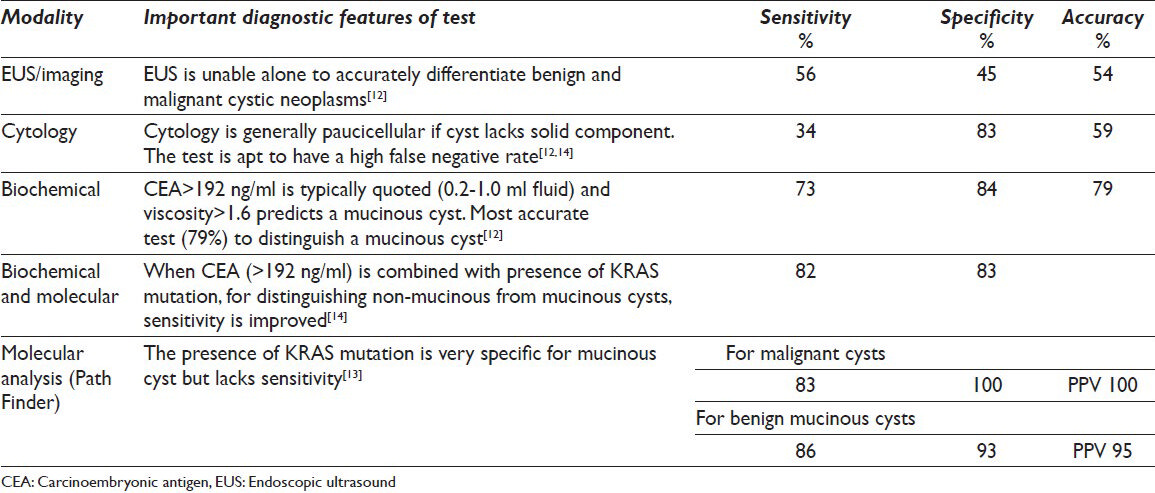
A molecular test available through RedPath Integrated Pathology™ for pancreatic cyst fluid utilizes k-ras gene point mutation, loss of heterozygosity (LOH) analysis of 15 genomic loci associated with tumor suppressor genes and the determination of DNA quantity/quality in the cyst.[13] Each of the three tests is defined as “abnormal” when the following are identified: k-ras gene point mutation, LOH mutations in 2 or more genomic loci and a high quantity/quality of DNA content. When none of the above defined abnormalities are present, a diagnosis of benign non mucinous cyst is rendered. When one of three abnormal findings is identified, a benign mucinous cyst is reported. When there is a high amplitude of K-ras or LOH mutations (>75% of total DNA), a malignant diagnosis is rendered. A small field study (n = 45) compared the clinical consensus diagnosis (histology or combination of endoscopic ultrasound features, cyst fluid CEA and cytology) with RedPath molecular results. The results of RedPath assay showed a sensitivity of 83%, specificity of 100%, PPV of 100% in identification of malignant mucinous cysts. Benign mucinous cysts were identified with a sensitivity of 86%, specificity of 93% and PPV of 95%. Benign non mucinous cysts were equally well identified (sensitivity 93%, specificity 86%, PPV 81%).[14] The manufacturer web site quotes a NPV of 97% based on an ongoing study over 2 years and with 492 patients.[15]
MOLECULAR TESTING IN URINE CYTOLOGY – A DIAGNOSTIC AND PROGNOSTIC AID
Cytology is best at detecting high grade urothelial carcinoma (UC) with a sensitivity of 80% and specificity of 97%.[16] Cytology is poor at detecting low grade and post-treatment UCs with sensitivity and specificity of around 30%.[17]
The Urovysion Kit™ is an FDA approved molecular test, which uses FISH technique for detecting copy number variation of chromosomes 3, 7, 17 and deletion at the 9p21 locus. The test is scored as positive if 4 or more of 25 urothelial cells show two or more abnormalities (amplification) in chromosomes 3, 7 or 17 in the same cell. The test is also scored as positive if there is homozygous deletion of 9q21 in 12 of 25 urothelial cells. If only tetrasomy is present, then 10 or more abnormal cells are required before scoring the sample positive for abnormality. Notably, the results do not take into account the cytological appearance of the specimen analyzed.
UroVysion™ is licensed in two clinical settings: Patients with hematuria and surveillance of bladder UC. Other non-approved uses are reflex testing of cytology samples reported as atypical and prediction of likelihood of recurrence after Bacille Calmette Guerin (BCG) therapy of bladder UC. Field studies have reported that the incidence of finding UC in a work-up for microscopic hematuria in unselected patient groups is 2.5%. For surveillance of bladder UC and upper tract UC, the overall sensitivity of UroVysion is 71% versus 26% with specificity of 70% versus 94%.[1819] Further segregation of the results shows that, similar to cytology, the UroVysion test is optimized towards detection of high grade lesion hence arguably showing not much improvement over cytology in diagnosing recurrent tumors. The overall specificity and sensitivity of the test precludes it from being used as a reflex test where the cytologic results are equivocal (Ferra et al., 2009). UroVysion is, however, helpful in detecting residual/recurrent carcinoma after BCG therapy with a negative predictive value of 85%.[2021]
The most important pitfalls with UroVysion are directly due to the fact that the cytological appearance is not taken into account. Hence, a false negative UroVysion test may be due to a sample that is heavily contaminated by squamous cells and is paucicellular of urothelial cells. Viral cytopathic changes and abundance of umbrella cells may result in a false positive UroVysion test. A difficult and controversial clinical scenario is where the cytology sample is “negative” and the UroVysion test is positive, but the urinary tract does not contain any lesions. These “anticipatory positive” cases were examined in an article by Ferra et al., 2009[22] which concluded that no additional workup was warranted in such patients. However, in another study which followed patients over a period of 29 months, 65% of these patients developed recurrent tumor and only a small percentage showed disappearance of abnormal cells.[20]
Molecular testing in non-small cell carcinoma (nscc) of the lung-a therapeutic aid
The incidence of smoking is declining in the USA and parallels the drop of cases of squamous cell carcinoma. Proportionally, there is an increase in cases of adenocarcinoma against which therapeutic targets are available, mainly anaplastic lymphoma kinase (ALK) and epidermal growth factor receptor (EGFR) inhibitors. The molecular tests that are presently available test for echinoderm microtubule-associated protein-like 4-ALK re-arrangement, EGFR and KRAS mutations, which are commonly mutually exclusive. The clinical and pathological characteristics associated with these changes are illustrated in Table 6. In cases whereby there is an EGFR mutation, the presence of KRAS mutation needs to be evaluated since its presence will negate the effect of EGFR inhibitors downstream and render the treatment ineffective. The algorithmic approach to testing of cytology specimens is illustrated in Figure 1.[23]
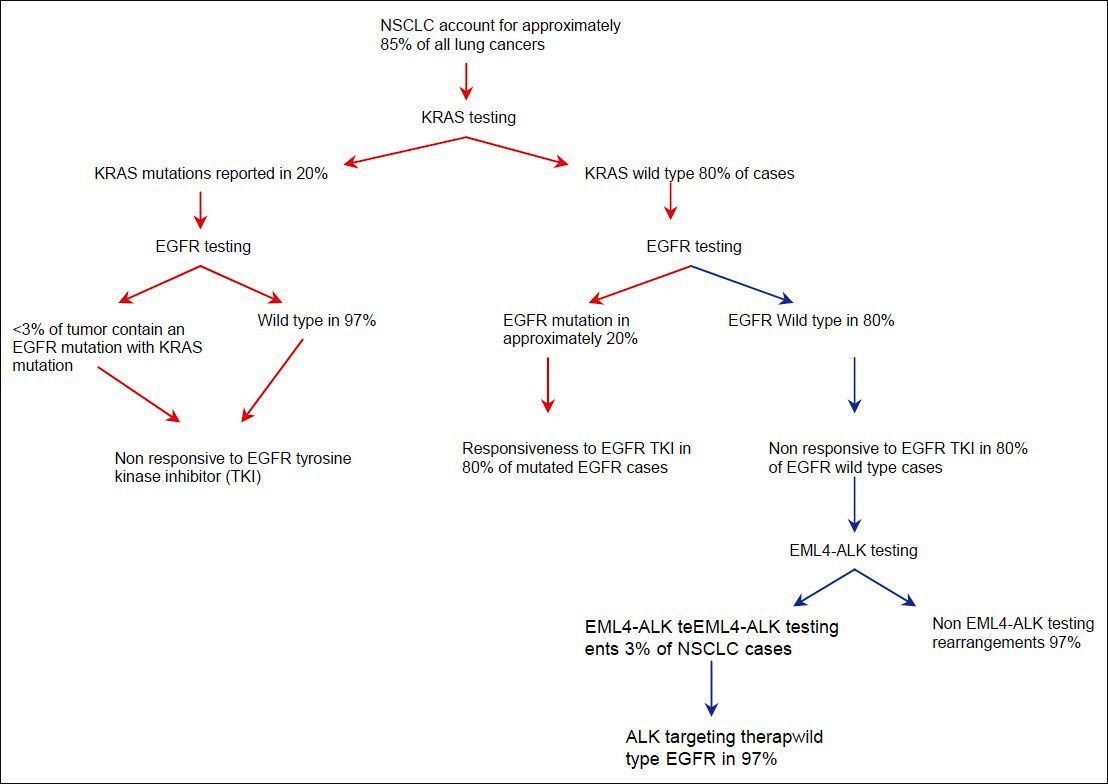
- Algorithmic approach to testing of cytology specimens in non-small cell lung cancer
There are unresolved issues regarding the utility of testing in samples obtained from metastatic lung NSCC, post-treatment recurrence samples and in the presence of mixed adenosquamous elements. Reflex testing of NSCCs is also not currently addressed.
Refinement and potential areas of expansion of molecular testing
There is no doubt that molecular testing will undergo further refinements and intrude into the traditional pathology practice. Specifically, future developments in molecular diagnostics are in the pipeline for analysis of pancreatic solid and cystic neoplasms. Addition of GNAS mutation testing is proposed to imply the development of intraductal papillary mucinous neoplasia; and evaluation of the altered expression of miR-155 and miR-21 has been shown to have the highest fold change in pancreatic cancer.[25]
Another potential area of change in molecular analysis is in urinary tract specimens. The evaluation of UroVysion FISH is time consuming and costly requiring numeration of variations in copy number at four sites in one cell. A recent study concluded that DNA gains using just one chromosome instead of all three (3, 7 and 17) may be just as sensitive in detecting bladder UC. Loss of 9p21 was less predictive of carcinoma and did not occur in any of their subjects. The authors propose that a simpler and more cost effective method could utilize detection of only chromosomal gain in any one of the chromosomes 3, 7 or 17.[26]
The imminent recommendations on reporting of cytologic specimens from the pancreatobiliary system are expected to take into account ancillary molecular testing of the specimen. This will conceivably result in expansion of molecular testing and refinements to the diagnostic specificity and/or sensitivity.
Other avenues of development include validation of molecular abnormalities in cytologic specimens such as that carried out with HPV in oropharyngeal carcinomas[27] and ALK re-arrangements in lung adenocarcinoma.[28]
CONCLUSION AND SUMMARY
Diagnostic challenges in cytopathology have always required the most information out of precious little material. The molecular advances in diagnosis, prognosis and therapeutics have impacted greatly on cytopathology and require substantial changes all the way from procurement of specimen to the final diagnosis.
Apart from in house HPV testing, we have been using outside reference laboratories for molecular testing of cytologic specimens. At the time of procurement, all specimens potentially requiring therapy related molecular testing are assessed for diagnostic adequacy as well as material for further testing. As a rule, after achieving a diagnostic sample, one dedicated pass is performed for each test that might be requested with the express aim of getting a cell block with enough cellularity. Some reference laboratories will only accept samples that have been fixed in formalin; however, others will test on cytology cell blocks that were initially collected in alcohol preservative. As academic pathology laboratories like ours acquire molecular expertise, in house assays will increase in number. The laboratories will be faced with challenges including validation of their results against reference material, which in cytology will probably mean working with suspended cell lines. The variety of testing (PCR, F/C-ISH, RT-PCR, DNA sequencing) will also need to be standardized to allow for meaningful interpretation of the results across all laboratories.
The move toward standardization of reporting of cytology specimens, commencing with cervical smears and more recently, thyroid FNA specimens[29] is also changing the practice of cytopathology. Combining the stringent cytology criteria with ancillary molecular testing is expected to yield more discrete and clinically relevant diagnostic categories for research and reporting. Similar to cervical cytology, the categories may be further refined as evidence accrues for their clinical validity.
Cytopathologists are increasingly relevant partners with endocrinologists, gastroenterologists and oncologists in efficient planning of management of patients. The profession is at an exciting point of implementing novel molecular markers to refine diagnostic criteria and create clinically relevant classification systems.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The authors have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Both authors contributed towards this manuscript.
ETHICS STATEMENT BY ALL AUTHORS
No ethical conflicts towards preparation of the manuscript.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Cervical cancer screening: What's new and what's coming? Cleve Clin J Med. 2013;80:153-60.
- [Google Scholar]
- American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137:516-42.
- [Google Scholar]
- Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357:1589-97.
- [Google Scholar]
- Update on ASCCP consensus guidelines for abnormal cervical screening tests and cervical histology. Am Fam Physician. 2009;80:147-55.
- [Google Scholar]
- Thyroid. In: Text Cytology Diagnostic Principles and Clinical Correlates (3rd ed). Philadelphia, PA19103-2899: Saunders Elsevier; 2009. p. :255.
- [Google Scholar]
- Cytological and molecular diagnosis of solid variant of papillary thyroid carcinoma: A case report. Cytojournal. 2008;5:2.
- [Google Scholar]
- Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705-15.
- [Google Scholar]
- Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390-7.
- [Google Scholar]
- Integration of KRAS testing in the diagnosis of pancreatic cystic lesions: A clinical experience of 618 pancreatic cysts. Mod Pathol. 2013;26:1478-87.
- [Google Scholar]
- Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330-6.
- [Google Scholar]
- Molecular analysis of pancreatic cyst fluid: A comparative analysis with current practice of diagnosis. Cancer. 2009;117:217-27.
- [Google Scholar]
- Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: A report of the PANDA study. Gastrointest Endosc. 2009;69:1095-102.
- [Google Scholar]
- RedPath™ the national pancreatic cyst registry. Available from: http://www.npcnregistry.com/#
- [Google Scholar]
- Evaluation of urovysion and cytology for bladder cancer detection: A study of 1835 paired urine samples with clinical and histologic correlation. Cancer Cytopathol. 2013;121:591-7.
- [Google Scholar]
- Reflex UroVysion testing of bladder cancer surveillance patients with equivocal or negative urine cytology: A prospective study with focus on the natural history of anticipatory positive findings. Am J Clin Pathol. 2007;127:295-301.
- [Google Scholar]
- UroVysion compared with cytology and quantitative cytology in the surveillance of non-muscle-invasive bladder cancer. Eur Urol. 2007;51:1275-80.
- [Google Scholar]
- Clinical utility of fluorescent in situ hybridization for the surveillance of bladder cancer patients treated with bacillus Calmette-Guérin therapy. Eur Urol. 2007;52:752-9.
- [Google Scholar]
- A systematic approach to identifying urothelial cells likely to be polysomic by fluorescence in situ hybridization. Anal Quant Cytol Histol. 2005;27:317-22.
- [Google Scholar]
- The prognostic value of cytology and fluorescence in situ hybridization in the follow-up of nonmuscle-invasive bladder cancer after intravesical Bacillus Calmette-Guérin therapy. Int J Cancer. 2009;124:2899-904.
- [Google Scholar]
- Reflex UroVysion testing in suspicious urine cytology cases. Cancer. 2009;117:7-14.
- [Google Scholar]
- The landscape of EGFR pathways and personalized management of non-small-cell lung cancer. Future Oncol. 2011;7:519-41.
- [Google Scholar]
- Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958-67.
- [Google Scholar]
- Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3:92ra66.
- [Google Scholar]
- Chromosomal instability and bladder cancer: The UroVysion (TM) test in the UroScreen study. BJU Int. 2013;112:E372-82.
- [Google Scholar]
- Application of the hybrid capture 2 assay to squamous cell carcinomas of the head and neck: A convenient liquid-phase approach for the reliable determination of human papillomavirus status. Cancer Cytopathol. 2012;120:18-25.
- [Google Scholar]
- The use of stained cytologic direct smears for ALK gene rearrangement analysis of lung adenocarcinoma. Cancer Cytopathol. 2013;121:489-99.
- [Google Scholar]








