Translate this page into:
Pre-analytic steps for molecular testing on thyroid fine-needle aspirations: The goal of good results
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Fine-needle aspiration cytology (FNAC) represents a valid alternative to biopsy in a variety of clinical settings mainly based on its simplicity and less invasive clinical approach. In some cases, morphology evaluation alone is not sufficient to manage the patients, so that the application of ancillary techniques can contribute to diagnosis, prognosis and prediction of tumor behavior. These techniques include polymerase chain reaction (PCR), fluorescence in situ hybridization (FISH), in situ PCR, direct Sequencing, microarrays and proteomic methodologies. Although several recent experiences underline the superior value of deoxyribonucleic acid (DNA) quality mainly for advanced genomic high throughput platforms, very scant literature studied the role of the pre-analytical or analytical phases. Despite the high specificity of molecular techniques as a support for diagnosis, there is a need for an increased standardization of pre-analytical/analytical steps such as providing appropriate clinical history, proper collection of laboratory specimens and proper preparation of samples, adequate fixative/reagent concentrations and technical equipments. All these requirements are crucial according to the results from 42 American laboratories, which reported 0.33% of significant molecular errors with 60% of them in the pre-analytical phase. The most common error is to forget that cytological preparation requires specific molecular variables, which are different from histological specimens. Cytological samples offer the advantage of a well preserved DNA, readily extractable and reasonably stable (from 6 months to 5 years) avoiding pitfalls due to formalin-fixation. Freshly prepared, unstained direct, alcohol-fixed papanicolaou, air-dried diff-quick smears are all suitable for DNA extraction and preservation. In the specific field of thyroid FNAC, molecular analysis has been supported by the growing evidence that papillary thyroid carcinoma (PTC), the most common thyroid cancer, frequently is a diploid lesion and can display non-overlapping mutations of the v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) in 46% to 70%, cases, ret proto-oncogene (RET) in 3 to 85% and Rat Sarcoma oncogene (RAS) in 0-21% cases. Recently, several cytological papers demonstrated that the combination of morphology and molecular analysis can increase the diagnostic accuracy allowing more precise prediction of malignancy regardless of the diagnostic categories. In conclusion, the correct use of the pre-analytical-analytical steps might lead to optimal results on cytology and empower the prognostic value of molecular techniques as strong indicators of cancer for their high specificity and positive predictive value.
Keywords
Cytology
molecular testing
pre-analytical steps
INTRODUCTION
In the last decades, cytology emerged as an essential diagnostic and prognostic tool based on its morphological role in the evaluation of cells. Although morphology continues to solve the majority of cytological diagnoses, the increasing and accurate knowledge of the molecular mechanisms of cancers, has opened to the application of ancillary techniques (both molecular and immunocytochemistry) on exfoliated and aspirated cytology with diagnostic and prognostic intents.[12]
Besides some difficulties in the diagnostic evaluation of specimens, which is encountered also on histology, some impressive issues are represented by the technical problems in different phases of performing laboratory testing, which include pre-analytical, analytical and post-analytical steps. Each of these phases plays an essential role in achieving good and correct results.[3] Even though all these three phases should be applied correctly, few data from literature clearly underline the crucial role of a correct pre-analytical phase. This was assessed by the data from a recent certificated study in which 84.5% are errors in the pre-analytical step and the majority due to an inaccurate quality of specimens.[3] The results of survey data from 42 American laboratories reported significant errors in 0.33% of tests performed with 60% of them in the pre-analytical phase.[3]
Not only must these errors be avoided for the morphological evaluation of the specimen, but also for the application of ancillary techniques.
The latter, mainly defined by the molecular analysis on cytology, is still a challenge demanding an increase in standardization and optimization of pre-analytical and analytical requirements. Numerous papers highlighted the use of molecular testing on cytology, but very scant literature is available on the pre-analytical requirements used for its cytological application.[456789101112]
The first component of assessing a new test is to ensure the accuracy. The analytical validity of an assay refers to how precisely and how reliably this test can be performed in detecting the resulting product.
Several papers have shown the application of a variety of different ancillary techniques for diagnostic, prognostic and therapeutic purposes. These papers referred to a range of assays such as immunocytochemistry, immunohistochemistry, fluorescence in situ hybridization (FISH), polymerase chain reaction (PCR), reverse trascription-Polymerase chain reaction (RT-PCR), and sequencing in order to assess proteins, deoxyribonucleic acid (DNA) or messenger ribonucleic acid (RNA) alterations on different tissue samples including exfoliated cells, serum liquid, fine-needle aspiration cytology (FNAC) and histology[1111213141516] [Table 1].
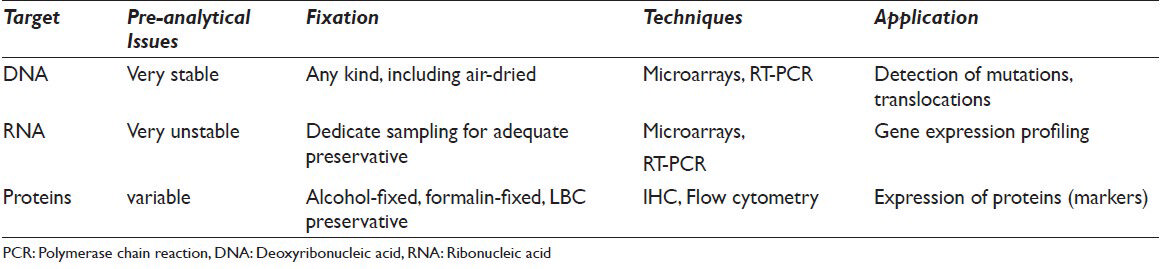
Although histology has been the leading method for molecular application, in these last years it is also becoming evident that cytology offers similar results with better preserved material and with less invasive procedures. Furthermore, in the histological specimens, the cross-linking action of formalin, which is useful for the morphological evaluation, creates some structural damage and fragmentation in the process of DNA extraction.[45] And even, Hematossilin and Eosin-stained slides may produce more variable PCR results due to the interaction between nuclear stain and histones with the final increasing resistance to digestion by proteinase K.
All these issue supported the statement that cytology can be a well-established alternative diagnostic technique also supported by a higher proportion of cells with less stromal component as well FNAC offers the advantage of rapid on situ evaluation of the adequacy[1]
Furthermore, these emerging horizons of molecular application on cytological samples were highlighted by several studies showing the applicability and feasibility of these techniques even on FNAC in cases with very scant diagnostic material.[78910]
On the other hand, the main challenges for the application of any ancillary technique in cytology are: (1) to select the appreciate test for a limited sample quantity; (2) to avoid jumping from a technique adapted from histology directly to cytology and (3) to use appropriate controls for cytological material. Although the technical validation on cytological material is essential, this point has not been always and accurately described in the literature probably as a consequence of the high number of different cytological preparations such as fresh cells, liquid-based cytology (LBC), previously stained alcohol-fixed or air-dried smears and cell-blocks.[4] The introduction of these techniques pointed to the important challenge of how to preserve good-quality material maintaining cellular morphology and DNA/RNA or protein integrity. All these cytological techniques should be always differentiated and chosen in order to obtain the more adequate material, the correct protocol, the more suitable molecular method.
The main technical issues linked to cytological application are: (1) the need to develop and validate criteria for standardized interpretation of molecular results using cytological specimens and (2) the minimal number of tumor cells for successful analyses.[4]
To the best of our knowledge, very few papers have been entirely focused on the evaluation of the pre-analytical and analytical steps. As reported in a meta-analytical paper by Correia et al., the majority of papers do not mention the pre-analytical and analytical steps and even the controls used.[6]
Both the recent editorial by da Cunha Santos[11] and the paper by Dejmek et al.,[4] evaluated the variable results in DNA yield and quality obtained in molecular cytology with different cytological preparation, fixation, mounting media and staining. The results stated that cytospin and liquid preparations are superior to direct smears as well liquid preparations with different preservative media.[12] The authors compared CytoLyt or PreservCyt solution with CytoRich Red collecting fluid and they found that the formers were superior in terms of suitable DNA yield. Another critical point is ascribed to the different mounting media. Although Pertex media is a xylene-based media, Eco Mount is a polymer-based, which shows better DNA yield.
Another point of the evaluation was the use of alternative types of fixation, such as ethanol-fixed or spray-fixed samples, which did not seem to alter the reliability of the molecular results.
Otherwise in the analysis of the staining used, they underlined that May Grunewald Giemsa reached poor results while pap-stained or even archived-pap-stained slides led to adequate and well preserved DNA extraction.[456789101112]
Several authors assessed that, in order to detect gross chromosomal abnormalities as deletions, translocation, but also point mutation in genes, PCR methods are ideal for FNAC material.[12] They can be performed with good results on fresh cell, liquid based preparations and from stained slides.[9] Furthermore, based on the better nuclear definition, also in situ hybridization, using fluorescent or chromogenic markers, can be applied on FNAC with excellent results in order to detect numerical or architectural chromosomal abnormalities.[1314]
The goal of a valid application for molecular analysis is in the quantification of the mutant alleles which can vary between 1% and 5% in amplification refractory mutation system ARMS or PCR-pyrosequencing and 20-30% in dideoxysequencing. As suggested by Dejmek, all the assays should be designed for a DNA quality measured by fragment lengths varying between 100 base pairs (bp) and 400 bp with a DNA quantity between 1 ng and 50 ng per analysis.[4] The standard is in assessing 50-100 cells as the adequate amount for obtaining good PCR results.
Our experience deals with application of ancillary techniques on LBC. In a previous paper Arcila and even Filhodemonstrated that liquid based preparations were suitable for the preservation of cells and DNA and adequate in obtaining valid material for molecular analyses even with archival liquid preparations.[11516]
For this reason, in this review, we also focused on our experience with the pre-analytical and analytical requirements for the detection of v-Raf murine sarcoma viral oncogene homolog B1 (BRAF) in the cytological evaluation of thyroid nodules belonged to the categories of follicular proliferations, suspicious for malignancy and malignant diagnoses.
Activating mutations of BRAF have been frequently defined in many benign and malignant tumors.[17] The most frequent activating mutation consists on a missense substitution of valine to glutamic acid in a mutational hotspot at amino acid position 600, which ends in a constitutive kinase activation of BRAF and phosphorylation of downstream targets.[18]
Recent studies supported the evidence that BRAF, RET/PTC or RAS mutations are found in more than 70% of PTC specially in those with more aggressive clinical-pathological features such as lymph-nodes involvement, extra capsular invasion, multifocality and failure of radioiodine treatment.[21718192021]
Traditionally, the gold standard for molecular (including BRAF) analysis has been represented by direct-DNA sequencing mainly obtained from histological samples although a variety of additional DNA-based techniques have been suggested and applied.[151619]
The promising diagnostic and prognostic role of thyroid FNAC has recently addressed the adoption of genetic and molecular tests in order to improve the diagnostic accuracy for thyroid nodular evaluation even though with some limitations (i.e. paucity of neoplastic cells and lesion heterogeneity).[4]
In general, we experienced the BRAF evaluation on the remaining liquid based material stored in the Preservcyt™ solution at room temperature. Our first critical step was the definition of the lower limit for the cellular adequacy, which was established, according to the British RC Path classification, in six groups of thyroid follicular epithelial cells with at least 10 well-visualized epithelial cells. Hence that this analysis can be performed with even 2 ml remaining material eluted in 5 ml of Preservcyt solution. The molecular analysis is performed even when these parameters are not satisfied and in cases of negative results, a note for the inadequacy is added on the molecular report.
DNA extraction was performed on fine-needle cytological sample ThinPrep 2000 (Hologic, Marlborough, MA) and paraffin block using the QIAamp tissue kit (Qiagen, Hilden, Germany). The percentage of disease specific cells for molecular analysis was at least 50% in all LBC samples. We assessed the quantity and quality of the DNA spectrophometrically (E260, E260/E280 ratio, spectrum 220-320 nm; Biochrom, Cambridge, UK) and by separation on an Agilent 2100 Bioanalyzer (Palo Alto, CA, USA). BRAF gene (exon 11 and exon 15) were amplified using the following primers: For exon 11, forward 5’-TTA TTG ATG CGA ACA GTG AAT AT-3’ and reverse 5’- TTA CAG TGG GAC AAA GAA TTG-3’; for exon 15, forward 5’-TCA TAA TGC TTG CTC TGA TAG GA-3’ and reverse 5’- GGC CAA AAA TTT AAT CAG TGG A-3’. Briefly, DNA (100-200 ng) was amplified in a mixture containing 1X PCR buffer (20 mM TRIS [pH 8.3], 50 mMKCl, 1.5 mM MgCl2], deoxyribonucleoside triphosphates dNTPs (200 mM each), primers (20 pM each) and 0.5U of GoTaq polymerase (Promega, Milan, Italy) in a final volume of 25 l. PCR conditions were: Initial denaturation at 95°C for 8 min, followed by 35 cycles at 95°C for 40 s, 55°C for 40 s and 72°C for 40 s. After visualization onto agarose gel, PCR products were treated with ExoSAP-IT (USB Corp, Cleveland, Ohio) following the manufacturer's protocol, amplified with BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Milan, Italy) using forward and reverse primers and sequenced with an ABI PRISM 3100-Avant Genetic Analyzer (Applied Biosystems). Water was used as a negative control.
It is remarkable to underline that in the majority of cases, our results and intents for the molecular analysis did not add important information for the diagnosis, for which morphology alone led to the correct diagnosis, but are mainly important for prognostic purposes.[19] Our results clearly show that the activating mutation of BRAF was particularly frequent in papillary carcinoma and in its more aggressive variant (tall cell variant-TCV). Furthermore, we demonstrated that the presence of BRAF mutation was significantly associated to two parameters of aggressiveness in thyroid tumor such as lymph-nodes metastasis (P = 0.0007) and bilateral localization (P = 0.0007).
In our experience, the application of molecular analyses on LBC is feasible, highly reproducible and provide high yield with informative molecular results also some (up to 4) months after FNAC.[19] In agreement with our results, we also found a complete concordance between the cytological and histological evaluation of BRAF mutational analysis. Furthermore, in our opinion, the quality on LBC slide, is comparable with that of conventional smears in terms of accuracy of the morphologic details and purity of background, as some studies have demonstrated.[16171819212223242526]
In conclusion, the pre-analytical step can significantly interfere in the molecular analysis and although good result might be obtained with all different cytological preparations, we need to remember that the method, fixation, staining and mounting medium can affect the quality. Cytology represents an optimal method for obtaining better DNA and avoiding artifacts due to formalin-fixed preparation.[24] From our experience, LBC represents a valid and feasible method for these molecular approaches.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
None of the authors had conflict of interest.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Both the authors contributed equally to the paper.
ETHICS STATEMENT BY ALL AUTHORS
Both the authors followed all the ethics statement required.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind-model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENT
The authors are thankful to Mrs. Beth Boyle for the extensive English support and revision.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2013/10/1/24/122300
REFERENCES
- Molecular cytopathology and flow cytometry: Pre-analytical procedures matter. Cytopathology. 2011;22:355-7.
- [Google Scholar]
- Molecular diagnostics and the training of future tissue- and cell-based pathologists. Cytopathology. 2012;23:283-5.
- [Google Scholar]
- Errors in pathology and laboratory medicine: Consequences and prevention. J Surg Oncol. 2004;88:161-81.
- [Google Scholar]
- Preparation of DNA from cytological material: Effects of fixation, staining, and mounting medium on DNA yield and quality. Cancer Cytopathol. 2013;121:344-53.
- [Google Scholar]
- Molecular pathology in anatomic pathology practice: A review of basic principles. Arch Pathol Lab Med. 2008;132:248-60.
- [Google Scholar]
- Use of molecular markers in samples obtained from preoperative aspiration of thyroid. Endocr J. 2012;59:417-24.
- [Google Scholar]
- Molecular fine-needle aspiration biopsy diagnosis of thyroid nodules by tumor specific mutations and gene expression patterns. Mol Cell Endocrinol. 2010;322:29-37.
- [Google Scholar]
- Contribution of molecular testing to thyroid fine-needle aspiration cytology of “follicular lesion of undetermined significance/atypia of undetermined significance”. Cancer Cytopathol. 2010;118:17-23.
- [Google Scholar]
- A prospective study evaluating the accuracy of using combined clinical factors and candidate diagnostic markers to refine the accuracy of thyroid fine needle aspiration biopsy. Surgery. 2010;148:1170-6.
- [Google Scholar]
- Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg. 2010;34:2589-94.
- [Google Scholar]
- Standardizing preanalytical variables for molecular cytopathology. Cancer Cytopathol. 2013;121:341-3.
- [Google Scholar]
- Reply to ancillary techniques on direct-smear aspirate slides: A significant evolution for cytopathology techniques. Cancer Cytopathol. 2013;121:276.
- [Google Scholar]
- Possible use and role of molecular techniques in fine needle aspiration cytology (FNAC) practice. Diagn Histopathol. 2011;17:286-92.
- [Google Scholar]
- Role of fluorescence in situ hybridization in lung cancer cytology. Acta Cytol. 2012;56:611-21.
- [Google Scholar]
- Simple protocol for DNA extraction from archival stained FNA smears, cytospins, and thin preparations. Acta Cytol. 2012;56:632-5.
- [Google Scholar]
- Liquid-based cytology in DNA-based molecular research: Viability and potential application. Anal Quant Cytol Histol. 2009;31:395-400.
- [Google Scholar]
- Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol. 2011;122:11-9.
- [Google Scholar]
- Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855-67.
- [Google Scholar]
- BRAF (V600E) mutation analysis on liquid-based cytology-processed aspiration biopsies predicts bilaterality and lymph node involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2013;121:291-7.
- [Google Scholar]
- BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742-62.
- [Google Scholar]
- Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092-8.
- [Google Scholar]
- Fine-needle aspiration biopsy of thyroid lesions processed by thin-layer cytology: One-year institutional experience with histologic correlation. Thyroid. 2006;16:975-81.
- [Google Scholar]
- Thyroid fine needle aspiration: The morphological features on ThinPrep slide preparations. Eighty cases with histological control. Cytopathology. 2003;14:343-9.
- [Google Scholar]
- Role of ancillary studies in fine-needle aspiration from selected tumors. Cancer Cytopathol. 2012;120:145-60.
- [Google Scholar]








