Translate this page into:
Prevalence of human papilloma virus in cytological abnormalities: Association of risk factors and cytomorphological findings
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Previous studies demonstrated the etiological role of human papilloma virus (HPV) in cervical carcinogenesis. Assessing the distribution of HPV may elucidate these observations.
Materials and Methods:
In total, we examined 3839 specimens, of which 187 abnormally classified cervical smears were immunostained using the p16INK4A assay. DNA was extracted from 182 specimens, and polymerase chain reaction (PCR) was performed. Participants’ socio-demographics, sexual and reproductive history, HIV status, contraceptive use, and Pap smear history were recorded.
Results:
Subject ages, number of sexual partners, and age at first sexual encounter ranged from 15 to 49 years, from 1 to 37 partners, and from 13 to 34 years, respectively. P16 immunoreactivity was detected in 60.4% of cases. The distribution of epithelial lesions and P16 overexpression (bracketed) was: 28 (5) atypical squamous cells of undetermined significance (ASC-US), 96 (50) lower grade squamous intraepithelial lesion (LSIL), 9 (7) atypical squamous cells-cannot exclude HSIL (ASC-H), and 54 (51) higher grade squamous intraepithelial lesion (HSIL). Ninety-four percent of HSIL expressed P16. Fifty-two percent of LSIL expressed P16. P16 expression declined from 61% (25–34 year age group) to 5% (45–49 year age group) for different age groups. HPV-DNA by PCR was detected in 94.5% of P16-positive samples. Type-specific PCR (HPV 16 and 18) was found in 12.2% and 14.5% of abnormal lesions, respectively. Younger age at first sexual encounter and HIV infection predominated in HPV type(s) 16 and/or 18 positive subjects.
Conclusion:
This study reinforced the value of the p16INK4A surrogate marker in identifying women with progressive cervical disease.
Keywords
Cervical cancer
human papilloma virus
Namibia
p16INK44A stain
INTRODUCTION
The association of human papilloma virus (HPV) with cervical precancer and invasive cancer has been corroborated by studies in which the majority of cervical cancers (99.7%) were reported to be related to persistent exposure to oncogenic HPVs.[1] An overwhelming presence of HPV types 16 and 18 was reported in approximately 70% of cervical cancers, with HPV type 16 more commonly implicated in rapid cervical disease progression.[2] Based on a meta-analysis of 42 studies, the presence of HPV 16 and 18 in cervical cancer was found to be 10% less in developing countries than in developed nations.[3] Risk of progression 10 years after exposure to the HPV increases from 13.5% to 17% for HPV types 18 and 16, respectively.[4] Even though most women become infected with the virus following a sexual experience, the infection resolves spontaneously, with a small proportion of women developing precancerous and cancerous lesions of the cervix over a period of time, usually not less than 10 years. This knowledge has led scientists to believe that subsidiary factors may further contribute to the development of cervical cancer.[5] Although HPV testing has been considered as a diagnostic indicator in detecting the presence of the HPV genome, its reduced specificity, particularly in women younger than 30 years of age, has triggered researchers to search for alternative screening strategies.
Namibia embarked on a cervical screening initiative (Pap smear) in 1992 and has since been successful in reducing the incidence of cervical cancer. In spite of this achievement, the incidence of cervical cancer, being the second most common cancer among women in Namibia, remains high (18.4% of all cancers among women in Namibia), especially in the presence of the Human Immunodeficiency Virus (HIV) epidemic. Published data showed that the age standardized ratio (ASR) for cervical cancer in Namibia progressed from 10.6 per 100,000 women (all age groups) in 2001 to 12.1 per 100,000 women (all age groups) in 2005, peaking to 52.8 per 100,000 women (60–64 years) from 2001 to 2005.[6] In another review,[7] the Pap smear demonstrated a sensitivity of 60–80% in detecting a higher grade squamous intraepithelial lesion (HSIL), declining further in detecting a lower grade squamous intraepithelial lesion (LSIL). Through the implementation of the Pap smear method and a well-organized cervical screening program, a considerable reduction in the number of invasive cervical cancer rates of up to 75% has been observed.[8] Despite the success of such a program, a significant number of cervical cancers and precursor lesions still occur in women who have been adequately screened.[9] This finding demonstrates the poor sensitivity of the Pap smear technique (if not repeated) in detecting cervical abnormalities. A meta-analysis of 94 studies demonstrated that the sensitivity and specificity of a Pap smear ranged from 30% to 87% and from 87% to 100%, respectively.[7] Seemingly, even with the implementation of novel strategies such as Liquid Based Cytology (LBC), sensitivity does not improve.[10]
Diagnostic adjuncts such as P16INK4A, a marker that detects the presence of p16, a tumor suppressor gene expressed during disruption of the pathways of p53, and the retinoblastoma gene (pRb) by high-risk human papilloma virus (HR-HPV) oncoproteins E6 and E7, might have clinical value in detecting cervical lesions where expression of HPV oncogenic activity has taken place. Contrary to this, HPV DNA detection by polymerase chain reaction (PCR) merely serves as an indicator for the presence of the HPV genome and not its activity.
This study used a specific marker (p16INK4A) which is able to identify cells in the early stages of the transformation process initiated by HPV integration. Through the application of this biomarker along with the Pap smear, we aimed to report on the prevalence rate of HPV-induced cervical lesions among Namibian women attending gynecological facilities in Windhoek and the associated risk factors.
MATERIALS AND METHODS
This study was conducted in Windhoek, the capital city of Namibia, from 1 February 2006 until 31 March 2007. Women between the ages of 15 and 49 years attending public health care facilities for gynecological screening were invited to participate. After getting written informed consent and ethical approval, 3839 patients were interviewed and questionnaires were completed which focused on patient demographics, sexual and reproductive histories, HIV status, cervical screening practices, contraceptive use, and cigarette smoking. All study subjects were interviewed by trained health care workers and replies were personally completed by the respective study participants. To ensure standardization of responses, participants who had already completed the questionnaires at the respective sites were approached by the researcher to complete the questionnaire for the second time. This generally occurred during their scheduled follow-up visits (at a colposcopy clinic) after which both questionnaires were compared for the correctness of information provided.
The current cervical screening initiative was embedded as part of the Reproductive Health Policy of Namibia, with its focus on women and children's health. Despite this initiative, no clear guidelines as to who is eligible for cervical screening exist at present in Namibia. Consequently, Pap smears are collected from women on a “need to have” basis and not as per the World Health Organization (WHO) guidelines. Recently, and in collaboration with Centers for Disease Control and Prevention (CDC) and the Ministry of Health-Namibia, Pap smear screening in HIV-positive women has become a preference.
Using an Ayre spatula or cytobrush, a standardized sample of ectocervical and endocervical cells was collected by either a clinician/gynecologist or a registered nurse. Cells were then smeared onto a glass slide and fixed with either commercially available ether/alcohol (Fencott Spray fix) or a 50% ethanol solution, and then forwarded to the laboratory for microscopic assessment. Cervical smears were subjected to Papanicoloau staining[11] and microscopically assessed for abnormalities using the Bethesda system (TBS) of 1991 combined with the Cervical intraepithelial neoplasia (CIN) classification.[12] Unfortunately TBS of 1991 is still employed in the Namibian set-up as many rural clinics are managed by nurses who are unfamiliar with the new Bethesda terminology of 2001. Cervical smears were reviewed and re-grouped by two cytoscreeners into TBS 2001 to ascertain standardization of cervical diagnosis.
Failure to collect a duplicate slide resulted in the decolorization of the primary slide, after which it was immunostained using the Cintec P16INK4A cytology kit (Dako, Glostrup, Denmark). Fourteen cervical smears with a benign diagnosis (negative for intraepithelial lesion or malignancy) were included as negative controls. Following incubation with the primary mouse monoclonal antibody (clone E6H4) to human P16, visualization of cellular material was optimized using a visualization reagent. Brown colorization of the nucleus and/or cytoplasm in abnormal cells was considered as positive for immunoexpression. At least five abnormal cells displaying P16 overexpression in the nucleus and/or cytoplasm were considered as positive for P16 immunoexpression.
Later, cellular material from slides were scraped off with a sterile scalpel blade and collected into a sterile Eppendorf tube containing 100 μl lysis buffer. DNA was extracted using the High Pure PCR Template Preparation kit (Roche Diagnostics GmbH Mannheim, Germany). Initial gel electrophoresis to determine the presence of genomic DNA was performed on 182 samples. Primer sets GP5+/GP6+ with an expected amplicon size of 150 basepairs (bp) were used to identify genital HPV infection found in the L1 region of the HPV genome.[13] GP5+/GP6+ positive samples were further processed for the presence of HPV types 16 and 18 situated in the E7 667–686 and 753–774 regions of the HPV genome, respectively.[14] Amplicon sizes of 108 bp and 191 bp were expected for HPV types 16 and 18, respectively.
A control group (n = 205), residing in the area of Windhoek, with documented histories of normal Pap smears was also subjected to similar interviews, after which properly completed questionnaires as for the study subjects were collected. Each duly completed and signed questionnaire (n = 3839) was co-signed by the specific interviewer and allocated a unique identification number to preserve anonymity during analysis.
RESULTS
Of the study population (n = 3839), 187 demonstrating cervical abnormalities by the Papanicoloau technique were classified as atypical squamous cells of undetermined significance (ASC-US; 28), LSIL (96), atypical squamous cells-cannot exclude HSIL (ASC-H; 9), and HSIL (54). The mean age of the Namibian cohort was 33 years (range 18–49 years). Women with cervical abnormalities were stratified into seven groups, namely, 15–19 years, 20–24 years, 25–29 years, 30–34 years, 35–39 years, 40–44 years, and 45–49 years [Table 1].
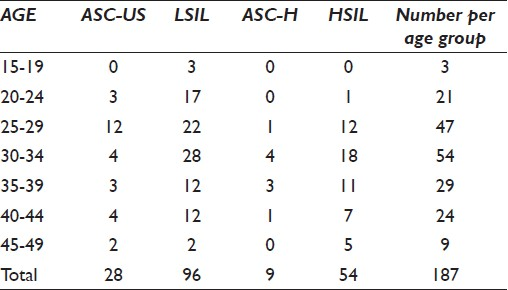
The highest peak of ASC-US classified lesions was observed among women of 25–34 years of age (8.6%), followed by a high prevalence of LSIL categorized lesions (26.7%) among women in this age group. Similarly, ASC-H (2.7%) as well as HSIL (16%) classified lesions predominated among women in this age group, with a second less pronounced peak of HSIL classified lesions (5.9%) observed among women 35–39 years of age. Notably, the rate of cervical abnormalities progressively declined to 4.8% among women aged 45–49 years.
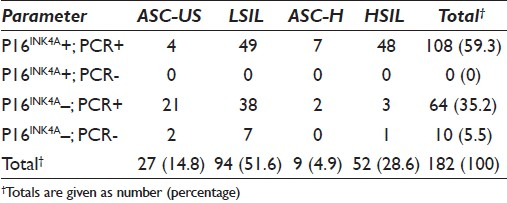
To assess whether the p16INK4A assay can be used as a predictor for cervical disease progression, we subjected 187 (n = 3839) cytologically diagnosed cervical smears for immunostaining. Altogether, 60.4% (n = 113) demonstrated the P16 antigen [Table 2].
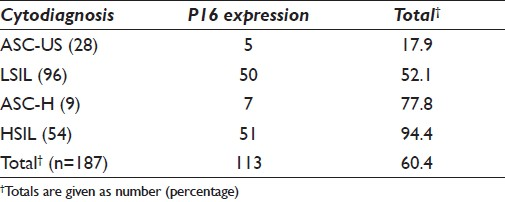
P16 immunostaining was detected in 17.9% and 52.1% of ASC-US and LSIL classified lesions, respectively. Concomitantly, P16 expression increased with the degree of abnormality to 77.8% and 94.4% in the ASC-H [Figure 1] and HSIL [Figure 2] groups, respectively.
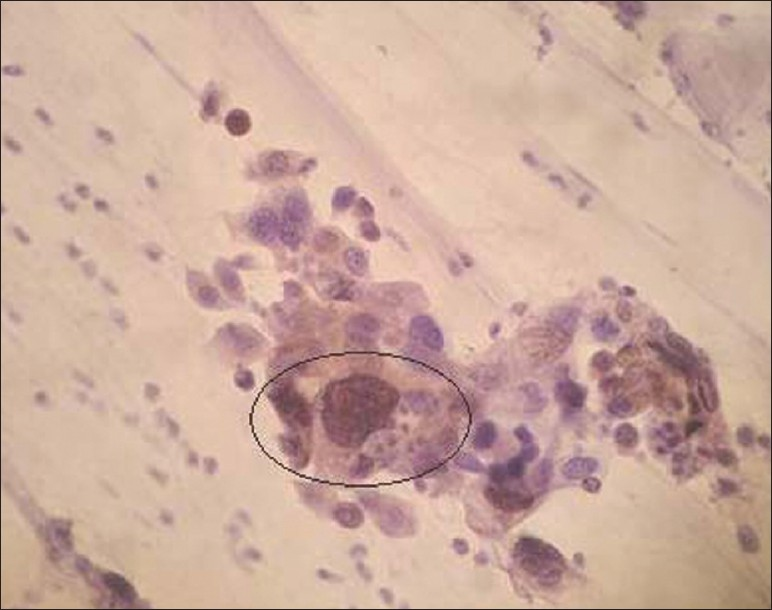
- Positive P16INK4A immunoexpression seen in ASC-H (×400 magnification)
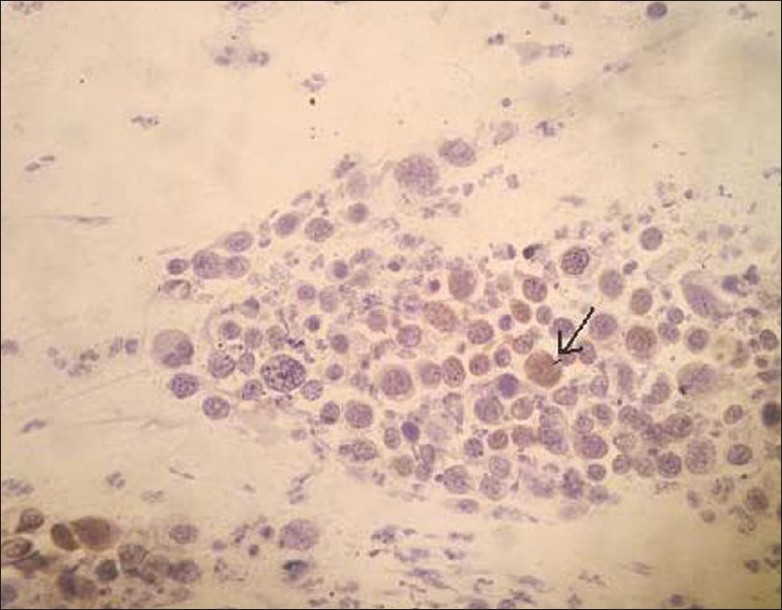
- Positive P16INK4A staining (as depicted by arrow) in a cytologically diagnosed HSIL (CIN III) (×200 magnification)
The number of abnormal cells displaying P16 expression in each case was not less than five, with the majority of higher grade lesions having strong immunoexpression in their nucleus. The 15 controls with benign diagnoses did not display P16 expression.
The mean age of women with P16 expression was 32 years (range 20–9 years). The distribution of P16 immunoexpression in women aged 20–24 years was 9 (8.0%), with a high of 69 (61.1%) among women aged 25–34 years. A high prevalence of P16 immunoexpression was observed among women aged 30–34 years, strongly correlating with the high rate of higher grade lesions (9.6%). Expression of the P16 protein was absent among women aged 15–19 years as corroborated from the lack of higher grade lesions.
Of the 187 P16 positive specimens eligible for HPV DNA detection using GP5+/6+ primer pairs, five yielded inadequate DNA for subsequent amplification and were excluded. A final sample count of 182 was ascertained. Of these, 172 tested positive for GP5+/6+ and were further subjected to type-specific PCR. Thirty-one percent of the GP5+/6+ HPV positive samples tested positive for the presence of HPV types 16 and 18 [Table 3].
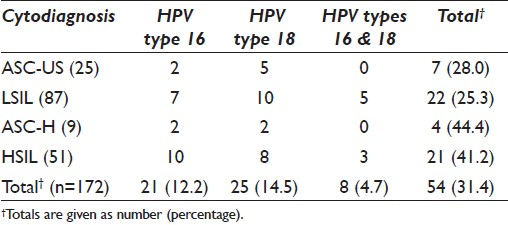
HPV types 16 and 18 accounted for 21 (12.2 %) and 25 (14.5%) of all HPV infections, respectively. Infection with both HPV types 16 and 18 was found in 8 (4.7%) participants. Concurrently, the HPV 16 genome was observed in 2 (8%) ASC-US classified lesions, while HPV type 18 was noted in 6 (20%) of such lesions.
HPV types 16 and/or 18 were further detected in 22 (25.3%) of LSIL classified lesions (n = 87) positive for GP5+/6+. Of these lesions, 7 (8.0%) demonstrated the presence of HPV type 16, whereas HPV type 18 was observed in 10 (11.5%) of these lesions. Five (5.7%) lesions demonstrated infection with both HPV types 16 and 18.
The presence of HR-HPV (types 16 and/or 18) amplicons was observed in 4 (44.4%) of ASC-H and 21 (41.2%) of HSIL classified lesion (s). HPV type 16 was detected in 10 (19.6%) of HSIL classified lesions, with HPV type 18 seen in 6 (15.7%) of these lesions. Concurrent infection of both HPV types 16 and 18 was seen in 3 (5.9%) of HSIL classified lesions.
Table 4 demonstrates the comparison of P16 immunocytochemical and PCR test results for 182 patients who had cytologically classified abnormal Pap smears. Of the 52 cytologically diagnosed HSIL (CIN II/III) specimens with histologically confirmed biopsies, 92.3% (48/52) were positive for the presence of the P16 protein and HPV by PCR analysis. Two HSIL classified lesions that were positive for HPV amplification by PCR were negative for the P16INK4A immunoassay. One HSIL classified cervical lesion was negative for both P16 and HPV DNA detection by PCR. Forty-nine (52.1%) of the specimens displaying LSIL (n = 94) on cytology were positive for both P16 and PCR, as compared to 38/94 (40.4%) that were negative for P16 and positive for HPV detection by PCR. Seven (7.4%) of the cytologically classified LSIL lesions were negative for both P16 and HPV by PCR analysis. Of the 27 ASC-US classified lesions, 4 (14.8%) were positive for both P16 and PCR, with 21 (77.8%) found to be positive for HPV amplification by PCR but lacking the P16 protein. Two (7.4%) ASC-US categorized lesions that tested negative for P16 immunoexpression were negative for HPV DNA detection by PCR. Seven (77.8%) of ASC-H classified samples (n = 9) that were positive for HPV DNA detection by PCR expressed the P16 protein.
Of the 25 ASC-US cytology specimens which were positive for GP5+/6+, 8% was positive for both the P16 protein and the presence of HPV types 16 and 18, with lack of P16 expression observed in 64% of HPV type 16 negative specimens [Table 5].
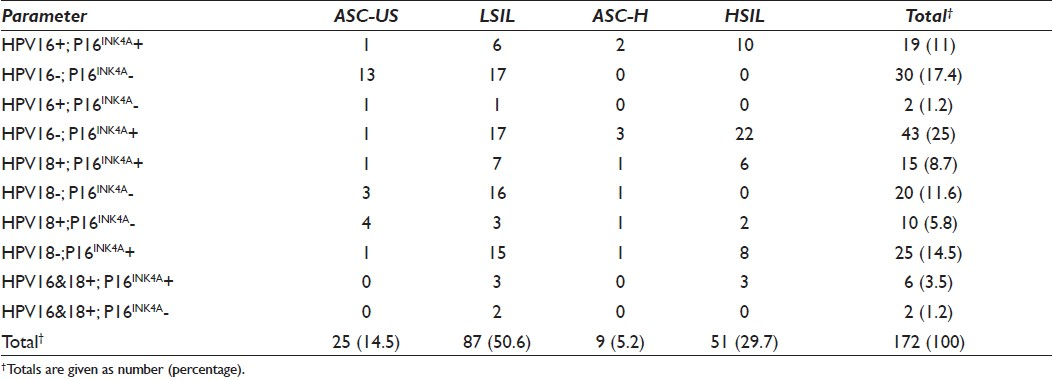
P16 expression was observed in 8% of HPV type 18 positive ASC-US classified lesions, with 16% of P16 negative ASC-US classified lesions being positive for the presence of HPV type 18. Amplifiable HPV type 16 DNA was detected in 6.9% of LSIL classified lesions expressing the P16 protein. More than 19% of LSIL classified lesions which were negative for HPV type 16 lacked the P16 protein. P16 expression strongly correlated with 8% HPV of type 18 positive LSIL lesions. Over 18% of HPV type 18 negative LSIL classified lesions lacked the P16 protein. The P16 protein was expressed in 18.4% of HPV types 16 and 18 positive LSIL lesions. Infection with both HPV types 16 and 18 was observed in 5.7% of LSIL categorized lesions. P16 expression was associated with the presence of HPV type 16 in 22.2% of ASC-H classified lesions. Forty-four percent of ASC-H classified lesions exhibiting P16 expression were negative for HPV types 16 and 18 by PCR. More than 19% of HPV type 16 amplifiable DNA HSIL classified lesions showed P16 expression. In 58.8% of P16 positive HSIL classified lesions, HPV types 16 and 18 were not detected. P16 expression and detectable HPV type 18 were observed in 11.8% of HSIL classified lesions. The presence of both HPV types 16 and 18 was demonstrated in 5.9% of P16 positive HSIL classified cervical lesions.
The mean age of women harboring either HPV type 16 or 18 was 32 years (range 21–47 years). HR-HPV types 16 and 18 were detected in 11.1% of HPV types 16 and 18 positive women younger than 25 years old, increasing to 64.8% in women 25–34 years old, and 30–34 years showing the highest peak [Table 6].
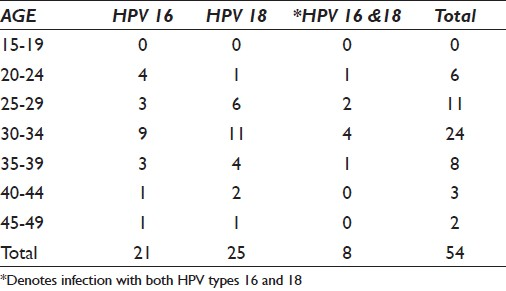
A sudden decline was observed among women 35 years and older. HPV types 16 and 18 predominated among women 30–34 years old with a prevalence rate of 16.6% and 20.4% for HPV types 16 and 18, respectively. Notably, prevalence of HPV type 16 was elevated only in women 20–24 years of age, with HPV type 18 more commonly found in women 25 years and older.
Risk for HPV acquisition and subsequent cervical disease progression were also examined. Participants ranged in age from 18 to 49 years for control subjects and from 19 to 49 years for study subjects, with a mean age of 33 years in both groups. More than 75% of the study subjects had never been married. Women having had more than five pregnancies (3.4%) were more at risk of having HPV-associated lesions. Study subjects (42.8%) were more likely to have a history of injectable contraceptive use. Neither cigarette smoking nor alcohol consumption was found to be a significant risk factor for HPV-induced lesions. Most women reported to have had more than three sexual partners (53.4%), with the age at first sexual encounter between 16 and 19 years (58.3%) and an average of 13.5 years of sexual activity (median 13; range 1–32 years). The mean age at first sexual encounter was 19.9 years (median 19; range 10–31 years) for controls and 19.5 years (median 18; range 13–29 years) for study subjects.
Most participants (56.7%) attended gynecological facilities only when they encountered vaginal problems. The mean age of women who presented for cervical screening based on a history of vaginal problems and/or first time attendants was 36.3 years (median 32) for control subjects and 35 years (median 33) for study subjects. The mean age at first sexual encounter for first time attendants and women who presented for cervical screening (Pap smear) due to vaginal problems was 21.7 years (median 19) for control subjects and 19 years (median 18) for study subjects.
By deduction, cervical disease development induced by HPV in the Namibian cohort was more commonly associated with an unmarried status (>70%), women aged 25–34 years (>50%), HIV positivity (>40%), younger age at first sexual encounter (16–19 years) (>50%), number of sexual partners (>60%), and a history of injectable contraception use (>40%), except for HPV type 18 which does not appear to have a link to contraceptive use.
Patients with cytological aberrancies were followed up for 48 months to assess whether histological confirmation was carried out. Of the 172 GP5+/6+ positive patients, histological assessment was performed on 91 (52.9%) patients. Fifty-two of these patients with a cytology result of HSIL had a histological outcome of CIN II (11/52) and CIN III (41/52). Of the 37 biopsied patients with an LSIL diagnosis on cytology, 27 coincided with a histological diagnosis of CIN I and/or HPV-associated changes, while 4 lesions demonstrated cellular changes associated with CIN III with/without HPV on histology. In addition, two LSIL cytologically diagnosed lesions demonstrated a progression risk to a CIN II histologically confirmed lesion, with four lesions showing changes associated with benign cellular processes. Considering the cytological ASC-H (1/9) and ASC-US (1/27) categories, both patients had a histological outcome of CIN II.
Overall, 26 (28.6%) and 57 (62.6%) of p16INK4A negative and p16INK4A positive cervical abnormalities (n = 91) coincided or regressed with histologic follow-up, while 3 (3.3%) and 5 (5.5%) of p16INK4A negative and p16INK4A positive abnormal cervical lesions progressed to CIN II/III with/without HPV, respectively. Regarding p16 immunostaining as a predictor for cervical disease progression, 51/52 (98.08%) biopsy confirmed HSILs were p16INK4A positive. In the histologically evaluated LSIL category, 10 (27%) specimens expressed P16, of which 6 (16.2%) P16+ specimens were associated with CIN I ± HPV-associated changes on histology, 4 (10.8%) P16+ specimens showed CIN II/III progression, while P16 was absent in specimens with benign proliferative cellular changes. P16 expression was not observed in the cytologically diagnosed ASC-US specimen having a histological outcome of CIN II. The one ASC-H diagnosed specimen with a CIN II outcome on histology expressed the P16 protein.
DISCUSSION
The results of this study demonstrated the value of the p16INK4A biomarker as a means of measuring HPV disease progression in cervical lesions. In line with our hypothesis, the results confirm that the detection of the P16 tumor suppressor protein in cervical smears may be linked to an enhanced expression of high-risk HPV oncogenes which are observed during cervical carcinogenesis. Our findings further suggest that identification of ASC-US and LSIL classified cervical lesions induced by HPV offers useful information for specific management strategies on the condition that histological confirmation is ascertained. Nevertheless, the need for appropriately formulated cervical screening guidelines for Namibia is reinforced.
The proportion of diagnosis among positives was: 15% as ASC-US, 51.3% as LSIL, 4.8% as ASC-H, and 28.9% as HSIL. Okonda et al,[15] showed an incidence rate of 19.8% as ASC-US, 9.0% as LSIL, 8.8% as ASC-H, and 4.6% as HSIL. The number of cervical abnormalities in women of Windhoek may be an underestimation in the capital as we only targeted women attending public health facilities. Our study was limited to women in their reproductive years (15–49 years) and as such may not be a true reflection of the number of cervical aberrancies in the country of Namibia. Due to the lack of a well-organized screening program in Windhoek, at-risk groups may have been excluded and this figure could potentially rise to much higher numbers.
While cervical abnormalities are perceived as deviations more commonly encountered among elderly women, our study demonstrated that aberrancies were the highest among sexually active young women between the ages of 25 and 34 years (54%). We speculate that this specific age group is more susceptible to multiple sexual experiences, in turn increasing their chances of being exposed to HPV. Similarly, several authors[1516] reported a high prevalence of cervical abnormalities among young Swazi (20%) and South African (20%) women. These findings corroborated with studies in which HSILs and LSILs were frequently observed in younger women.[1217]
Our results demonstrate good concordance between P16 expression and severity of the cervical lesion stratified by HPV status. Several studies[18–20] have observed P16 expression in HSILs, suggesting that expression of the p16 gene commonly occurs in cervical carcinogenesis. The sensitivity of the P16INK4A assay in HSIL classified lesions was found to be high akin to a high specificity for HPV-negative benign cells, suggesting that p16INK4A might be a useful marker in identifying potentially malignant transformation of cervical cells.
The reported prevalence of P16 expression in LSILs differs somewhat from others,[1419] possibly because they used histologically confirmed samples as opposed to the use of conventional cervical cytology without histological confirmation as used in our study. As such, the inter-observer reproducibility might have been controlled for in other studies. Conversely, P16 expression is less pronounced in ASC-US classified lesions (17.9%); however, positive immunoexpression was demonstrated in more than half of ASC-US lesions.[2122] Histological confirmation was sought and as such the potential of screener subjectivity was drastically reduced. Even though 5–17% of ASC-US lesions harbor an underlying HSIL,[7] management of such lesions in Namibia is rooted on a conservative approach where a 6 months follow-up Pap smear is advised until the lesion has resolved. Should it progress, colposcopy is recommended. Clearly, this demonstrates the need for properly constructed cervical screening guidelines appropriate for the Namibian population.
While the reproducibility of cervical cytology has been well delineated in a context-dependent manner,[7] we meticulously assessed specific morphological characteristics in single-lying abnormal cells to avoid the common diagnostic difficulties frequently encountered. Three corporate areas comprising collection, processing, and interpretation of cervical smears have been considered as potential sources for diagnostic errors in cervical cytology.[23] Though we endeavored to scrutinize these diagnostic mimics very carefully, a small proportion of cervical smears from the target population displayed cellular features falsely associated with HSILs. Cellular features resembling those of hyperchromatic crowded groups (HCGs)[12] were commonly misclassified as HSIL; however, unparalleled cellular characteristics coupled with the patient's clinical history were collectively considered in the final interpretation. Similarly, the poor diagnostic accuracy (35.3%) of cervical cytology in detecting cellular changes associated with HPV in our study has been well supported by findings from several researchers,[171924] suggesting the crucial need for more sensitive markers in supplementing cervical cytology methods, e.g. Pap smear.
Distinctive morphological characteristics coupled with immunocytochemical staining intensity were collaboratively regarded as a predictor for cervical disease progression. Presence of strong positive immunostaining in the nucleus of abnormal cells was found in 55.5% and 83.3% of ASC-H and HSIL classified lesions, respectively, suggesting that immuno-intensity in the nucleus may be indicative of a developing HSIL. A disparity exists with regard to the stain location whether it is the nucleus and/or cytoplasm and the number of P16 positive cells,[2526] with some investigators not considering staining distribution and P16 immunostaining cell count as reflective of disease severity.[2728] We demonstrated strong P16 immunostaining in the nucleus of abnormal cells in 26% of LSIL classified lesions, but there was also varying intensity in both the nucleus and cytoplasm of other cases of LSIL. We employed a threshold of at least five abnormal cells exhibiting P16 immunoexpression to confirm immunoreactivity. Sahebali et al.[29] considered the number of cells displaying P16 to be immunoreactive and predictive of cervical disease severity, as also suggested by other authors,[2830] in that the number of positive cells is insignificant as it relates to the cervical abnormality.
An overall presence of HPV types 16 and 18 was noted in 31.4% of cervical lesions via PCR. Though the prevalence of HPV types 16 and 18 appears to be low in the Namibian setting, it merely denotes the presence of the two most common HR-HPV types associated with cervical abnormalities. Additionally, this indicates that potential HR-HPV types other than HPV types 16 and 18 may be present and a need for further studies is warranted. More importantly, in our study, HPV type 16 predominated in higher grade cervical lesions, with HPV type 18 more commonly found in less severe dysplastic lesions and to a lesser extent in HSILs.[8] The decreased incidence of HPV type 18 in HSILs was similarly noted, suggesting that HPV type 18 induced cervical lesions elicit a more aggressive behavior with a short progression time in HSILs. This finding correlates with the high prevalence in ASC-US and LSIL classified lesions in our study, suggesting that these lesions may already harbor an underlying higher grade lesion.[8] It is noteworthy to speculate that HPV 18 associated cervical lesions will progress at a faster rate than those caused by HPV type 16; however, HPV 16 is also found to be distinctive of early-onset cancers.[3132] Interestingly, women with LSIL infected by HPV type 18 are approximately 4 years younger than those with ASC-US classified lesions who have been infected with the same virus. This finding could potentially constitute a specific moiety caused by HPV.
In the present cohort, number of sexual partners (mean 3.488) was the highest among women aged 25-34 years.[33] An increasing rate of sexual partners is directly related to a higher HPV positivity stratified by cervical disease severity. Most women between the ages of 25 and 34 years appear to have had at least three sexual partners (one person reported to having had 37 sexual partners) during their lifetime, suggesting that they are at greater risk of HPV exposure which could possibly develop into cervical abnormalities.[34] Contrary to this, another study reported a higher prevalence of HPV infections in older women, suggesting that these women's sexual behavior correspond with an elevated risk of HPV infection.[35]
While HPV infection is common, it is more prevalent among women with an HIV-positive status. In this study, we found that an HIV-positive status highly correlates with the presence of the HPV. A high preponderance of HIV positivity among women of 25–34 years of age was found (40.8%), underscoring the importance of HPV acquisition and persistence in immune suppressed women. Jay et al.[36] concluded that HIV-seropositive women have a greater risk for progressive cervical lesions with a lesser probability of full recovery.
Although the current management of women with a cytologically diagnosed HSIL and ASC-H necessitates colposcopy and biopsy, those with low-risk (LSIL or ASC-US) cervical lesions are subjected to a three-monthly and six-monthly follow-up, respectively. Following three successive diagnoses of LSIL and/or ASC-US, a colposcopy and biopsy is then recommended. As reflected by either the regression of ASC-US and LSIL cervical lesions or non-adherence to recommended follow-ups, limited histology was performed in this low-risk category. This may be as a result of poor comprehension of cervical carcinogenesis. Some women who succumb to seek gynecological examination do so due to financial difficulties as they have to travel several hundred kilometers from desolated areas to the colposcopy clinic. Additionally, being custodians for grandchildren and having no one to watch over them while away for colposcopic examination further exacerbated the low rate of histologic assessments. More importantly, Bose et al.[37] demonstrated P16 expression in 21% of LSIL and ASC-US classified cervical lesions developing into HSIL, suggesting that p16INK4A might be a valid marker for further evaluating this proportion of women. In agreement with our findings, several authors[3839] highlighted an increased risk of progression of P16+ LSIL categorized lesions to an HSIL, as compared to P16– LSIL lesions. Based on these findings, it therefore seems that p16INK4A immunostaining shows potential to identify HPV-associated cervical disease instead of infection.
The use of positive P16 immunostaining on abnormally classified cervical smears clearly demonstrated its usefulness in identifying those lesions that will progress to higher grade lesions. Using P16 as an adjunctive testing to particularly the gray areas (ASC-US, LSIL, and ASC-H) in cervical cytology will potentially reduce the morbidity and cost implications linked to repeated follow-ups (Pap smears) and therapeutic procedures. Moreover, positive P16 immunoexpression in cervical aberrancies could direct effective management regimens with a considerable reduction in costs.
At present, procedures such as HPV testing (not favored in Namibia because of the high cost), colposcopy, and biopsy are used in the treatment of a range of abnormally classified cervical lesions as proposed by the American Society for Colposcopy and Cervical Pathology ASCCP.[40] These techniques require adequately qualified and skilled personnel and the personnel may not be readily available at some of the major public health centers in Namibia. In fact, there are only two qualified gynecologists/colposcopists who perform the aforesaid procedures in the public health sector of Namibia. This presents a serious burden on the public health sector as patients have to wait for several weeks before the procedure can be performed. Cognizant that HPV testing may be desirable, it lacks specificity, particularly in women less than 30 years of age, and merely identifies the presence of HPV without presenting any evidence as to whether the lesion will progress or regress. Contrary to this, P16 as an adjunct testing serves as an excellent choice in the diagnostic decision-making process since it reduces invasive procedures such as colposcopy and biopsy. This technique produces a cost-effective management algorithm which could lead to a substantial saving in cost with the possibility to minimize morbidity.[41]
We recognize several limitations in this study. Firstly, the results may be confounded as mostly at-risk participants (women in their reproductive ages with multiple sexual partners and an HIV-positive status) attended health care facilities, with only a small proportion attending gynecological clinics annually or as recommended. Candidates with potential HPV-induced cervical lesions may have been missed as some women fail to visit health facilities due to the absence of apparent clinical symptoms. Furthermore, this study may be underrepresentative of the Namibian women as most European women and women with medical aid insurance preferably visit private gynecological facilities. As a result, the findings may not be extrapolated to the whole population.
Secondly, type-specific primers used may not reflect the true status of the cohort as the presence of other oncogenic HPV types was not determined. These results may bias interpretation as only the two most common HR-HPV types were tested for, excluding the possibility of detecting other HR-HPV types. This presented a weakness, possibly restricting our inference in establishing the true incidence of HR-HPV induced lesions of the cervix in Namibian women. A need for further assessment is warranted to determine the distribution of all HR-HPVs in cervical specimens from Namibian women.
Thirdly, histological evaluation as the preferred diagnostic tool to validate cytological diagnosis was not performed on all specimens, particularly with respect to LSIL and ASC-US classified lesions. This presented a dilemma as some true lower grade lesions may have been potentially misclassified as ASC-US and vice versa. Even though histological confirmation is not required for first time ASC-US classified lesions, follow-up smears displaying “same” or “worse” diagnosis were treated as if this was a first time aberrant diagnosis due to improper and inadequate record keeping procedures. Additionally, LSIL and ASC-US classified lesions presented a subset of issues in that detection and correlation were not as corroborative as was hoped. However, we could not assess the robustness of the P16INK4A assay in these lesions as molecular testing for all known HR-HPV types was not done.
The results obtained at enrollment were based on cervical samples and were restricted to the cytological diagnosis only. This is considered as a potential limitation of this study as it may be affected by inter-observer reproducibility of results. We collected histologically proven diagnosis for most HSIL classified lesions, where possible, to improve sensitivity; however, since current cervical screening guidelines in Namibia do not advise for a biopsy for LSIL and ASC-US classified cervical lesions, histological confirmation was not obtained. Conversely, P16 immunostaining was applied to cervical smears with/without a biopsy-proven diagnosis.
Considering all factors, we conclude that p16INK4A does have the potential to detect precancerous cervical lesions induced by HPV and that advancement of abnormal lesions of the cervix can be monitored through the use of the p16INK4A assay.[42] Its utility in the field of gynecologic cytopathology may further serve as a guide in reconstructing appropriate management strategies for women who present with cervical aberrancies. Taking the sexual history and HIV positivity status into account, specific target groups who are at a higher risk of developing progressive cervical lesions can be identified. In this way, unique screening guidelines that may further enhance the sensitivity of the Pap smear can be formulated and implemented.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that they qualify for authorship as defined by ICMJE. All authors participated in its design and coordination, and worked collaboratively to draft the manuscript. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board (IRB) of the Cape Peninsula University of Technology (CPUT).
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGMENTS
We thankfully acknowledge the study interviewers for their contribution and, in particular, the study participants for their involvement and cooperation in this research.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/19/100123
REFERENCES
- Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12-9.
- [Google Scholar]
- GLOBOCAN: Cancer Incidence, Mortality, and Prevalence Worldwide. In: IARC Cancer Base 2002. Vol 5. Lyon: IARC Press; 2004. Version 2.0
- [Google Scholar]
- Worldwide comparisons of HPV types in invasive cervical cancer and cervical intraepithelial neoplasia grade 3 (CIN 3) Poster presented at: International Papilloma virus Conference, Vancouver 2006 May
- [Google Scholar]
- A study of the impact of adding HPV types to cervical cancer screening and triage tests. J Natl Cancer Inst. 2005;97:147-50.
- [Google Scholar]
- Epidemiology of HPV infection among Mexican women with normal cervical cytology. Int J Cancer. 2001;91:412-20.
- [Google Scholar]
- Cancer in Namibia 2000-2005. Namibia: Cancer Association of Namibia; 2009.
- Accuracy of the Papanicoloau test in screening for and follow-up of cervical cytologic abnormalities: a systematic review. Ann Intern Med. 2000;132:810-9.
- [Google Scholar]
- Cervical Cancer Facts and Figures-2006, Vol. 2006: American Cancer Society, 2006
- Prevention strategies of cervical cancer in the HPV vaccine era. Gynecol Oncol. 2006;103:21-4.
- [Google Scholar]
- Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006;118:957-62.
- [Google Scholar]
- Diagnostic Cytology and its Histopathological basis Vol 2. (3rd ed). Philadelphia: JB Lippincott Company; 1979. p. :1211-5.
- The Art and Science of Cytopathology: Exfoliative cytology Vol 1. (1st ed). Hong Kong: Carlson and Rogers: ASCP Press; 1996. p. :155.
- Detection of human papillomavirus from archival tissues in cervical cancer patients in Mauritius. J Clin Virol. 2006;35:173-8.
- [Google Scholar]
- Cervical human papillomavirus screening among older women. Emerg Infect Dis. 2005;11:1680-5.
- [Google Scholar]
- The status of cervical cytology in Swaziland, Southern Africa: a descriptive study. Cytojournal. 2009;6:14.
- [Google Scholar]
- Opportunistic testing of medically underserved women for cervical cancer in South Africa. Acta Cytol. 2001;45:313-6.
- [Google Scholar]
- Gynecological Cytology Vol 1. (1st ed). Stuttgart, Germany: Georg Thieme Verlag; 2001. p. :167.
- Overexpression of p16(INK4A) as a specific marker for dysplastic and neoplastic epithelial cells of the cervix uteri. Int J Cancer. 2001;92:276-84.
- [Google Scholar]
- Procedure for immunocytochemical detection of P16INK4A antigen in thin-layer, liquid-based specimens. Acta Cytol. 2002;46:25-9.
- [Google Scholar]
- Human papilloma virus genotyping and p16INK4a expression in cervical intraepithelial neoplasia of adolescents. Mod Pathol. 2005;18:267-73.
- [Google Scholar]
- Is p16(INK4a) expression more useful than human papilloma virus test to determine the outcome of atypical squamous cells of undetermined significance-categorized Pap smear. A comparative analysis using abnormal cervical smears with follow-up biopsies. Gynecol Oncol. 2005;97:35-40.
- [Google Scholar]
- Immunocytochemical staining of p16INK4A protein from conventional Pap test and its association with human papillomavirus infection. Diagn Cytopathol. 2004;31:235-42.
- [Google Scholar]
- Cytological Pitfalls. A review of five areas of diagnostic difficulty. J Gynecol Surg. 2009;1:253-69.
- [Google Scholar]
- Accuracy of cervical cytology in patients with Condyloma. J Gynecol Surg. 2009;4:9-14.
- [Google Scholar]
- Expression status of p16 protein is associated with human papillomavirus oncogenic potential in cervical and genital lesions. Am J Pathol. 1998;153:1741-8.
- [Google Scholar]
- p16INK4A expression and progression of low grade squamous intraepithelial neoplasia of the uterine cervix. Virchows Arch. 2003;445:616-20.
- [Google Scholar]
- Role of p16 (INK4a) expression in identifying CIN2 or more severe lesions among HPV-positive patients referred for colposcopy after abnormal cytology. Cancer. 2006;108:119-23.
- [Google Scholar]
- Evaluation of a nuclear score for p16INK4a-stained cervical squamous cells in liquid-based cytology samples. Cancer. 2005;105:461-7.
- [Google Scholar]
- p16INK4a as an adjunct marker in liquid-based cervical cytology. Int J Cancer. 2004;108:871-6.
- [Google Scholar]
- Evaluation of p16INK4A as a diagnostic tool in the triage of Pap smears demonstrating atypical squamous cells of undetermined significance. Cancer. 2007;114:34-48.
- [Google Scholar]
- Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048-56.
- [Google Scholar]
- Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101:475-87.
- [Google Scholar]
- Risks for incident human papilloma virus infection and low-grade squamous intraepithelial lesion development in young females. JAMA. 2001;285:2995-3002.
- [Google Scholar]
- Determinants of sexual activity and its relation to cervical cancer risk among South African women. BMC Public Health. 2007;7:341.
- [Google Scholar]
- Age distribution of human papilloma virus infection and cervical neoplasia reflects caveats of cervical screening policies. Int J Cancer. 2009;126:297-301.
- [Google Scholar]
- Human papilloma virus infections in women with HIV disease: Prevalence, risk, and management. AIDS Read. 2002;10:659-68.
- [Google Scholar]
- p16(INK4A) is a surrogate biomarker for a subset of human papilloma virus-associated dysplasias of the uterine cervix as determined on the Pap smear. Diagn Cytopathol. 2005;32:21-4.
- [Google Scholar]
- The use of p16INK4A in further defining atypical squamous cells cannot exclude a high grade lesion on cervical cytology (ASC-H): results of a pilot study. South Afr J Epidemiol Infect. 2007;22:4.
- [Google Scholar]
- p16INK4A is a useful marker for the diagnosis of adenocarcinoma of the cervix uteri and its precursors: an immunohistochemical study with immunocytochemical correlations. Am J Surg Path. 2003;27:187-93.
- [Google Scholar]
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin number 66. September 2005. Management of abnormal cervical cytology and histology. Obstet Gynecol. 2005;106:645-64.
- [Google Scholar]
- p16 immunocytochemistry on cell blocks as an adjunct to cervical cytology: Potential reflex testing on specially prepared cell blocks from residual liquid-based cytology specimens. CytoJournal. 2011;8:1.
- [Google Scholar]
- Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536-45.
- [Google Scholar]








