Translate this page into:
Pulmonary artery intimal sarcoma diagnosed using endobronchial ultrasound-guided transbronchial needle aspiration
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Intimal sarcoma of the pulmonary artery is a rare intraluminal malignant neoplasm that has an aggressive biological behavior, and early diagnosis may improve patient outcome. We describe a case of pulmonary artery intimal sarcoma diagnosed on cytologic material obtained by endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) biopsy with rapid on-site evaluation (ROSE). The aspirate showed loosely cohesive clusters of pleomorphic malignant spindled and epithelioid cells. An immunostain panel did not demonstrate any definitive mesenchymal or epithelial differentiation. The tumor's intraluminal origin was supported by radiographic imaging studies. Subsequently, the patient received preoperative chemotherapy and underwent tumor resection with reconstruction. This report describes the cytomorphologic features of this rare intravascular tumor and demonstrates how EBUS-TBNA with ROSE was instrumental in obtaining optimal cytologic sampling for ancillary studies, thus expediting the management.
Keywords
Cytology
endobronchial ultrasound-guided transbronchial needle aspiration
fine-needle aspiration
intimal sarcoma
pulmonary artery sarcoma
INTRODUCTION
Intimal sarcomas are malignant mesenchymal tumors that have intraluminal growth and obstruct the large systemic and pulmonary arteries.[1] The majority of intimal sarcomas arise in the pulmonary system, usually in the pulmonary trunk and also have been called pulmonary artery sarcomas (PAS).[23456] PAS are often misdiagnosed clinically as chronic pulmonary thromboemboli or right heart failure since these conditions have similar presentations and are much more common than PAS.[7] Consequently, in many cases diagnosis is delayed or made postmortem. However, advances in imaging technology have facilitated earlier detection and diagnosis. These diagnostic advances are important since the clinical treatment of PAS differs from those of pulmonary thromboemboli and right heart failure.[8]
Diagnosing PAS from tissue samples, especially with limited samples, also can be difficult. The majority of these tumors are poorly differentiated malignant mesenchymal tumors.[1] However, these tumors occasionally show specific cellular differentiation on morphology with or without immunophenotyping.[345] Only a few case reports describe the use of fine-needle aspiration (FNA) in evaluating these lesions,[91011] and only one study describes the cytomorphologic features in a metastatic lesion.[9]
We present a case of PAS that was diagnosed using a sample obtained by EBUS-TBNA with rapid on-site evaluation (ROSE) in a patient with a history of three prior primary tumors.
CASE REPORT
A 78-year-old man with three prior malignancies was referred to our institution for further evaluation of a hilar mass. He had been diagnosed with large B-cell lymphoma 12 years prior to his presentation to our institution. Two years after the diagnosis, the patient had been diagnosed with prostate adenocarcinoma and melanoma of the ear. Eight months prior to his presentation at our institution, the patient was seen by his local physician for persistent back pain. A thoracic spine-computed tomography (CT) showed a pathologic fracture of T-4 vertebra that was associated with a soft tissue mass as well as a hilar mass. An abdominal CT revealed a mesenteric mass and biopsy showed large cell lymphoma. The patient received palliative radiotherapy to the spine and chemotherapy. A restaging positron emission tomography (PET)-CT scan revealed a persistent hypermetabolic hilar mass.
The patient then sought consultation at our institution. A CT scan showed a 5-cm mass involving the right main pulmonary artery. A PET-CT scan showed a hypermetabolic mass involving the right perihilar space [Figure 1]. The patient was referred for an endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) with ROSE. The findings were in keeping with a PAS. Subsequently, the patient underwent preoperative chemotherapy followed by resection of the right pulmonary artery tumor with reconstruction, followed by a right thoracotomy. Two months later, a PET-CT scan revealed a fluorodeoxyglucose (FDG)-avid mass within the right hemithorax consistent with recurrent disease. The patient returned home transitioning into hospice care out of state 9 months after diagnosis.
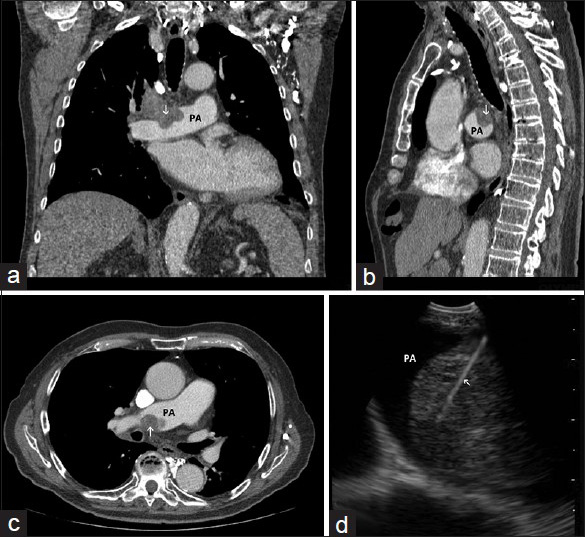
- (a) Coronal, (b) sagittal, and (c), axial images of chest computed tomography demonstrating a mass (arrow) invading the right pulmonary artery (PA). (d) Image obtained during endobronchial ultrasound-guided transbronchial needle aspiration showing the sampling needle inside the intra-arterial mass (arrow) within the lumen of the pulmonary artery
The smears [Figures 2 and 3] and cell block [Figure 4] primarily showed loosely cohesive clusters of pleomorphic spindled and epithelioid cells. The size of the tumor nuclei varied, but the nuclei were often large with irregular nuclear membranes, coarse chromatin and nucleoli. Occasional binucleated and multinucleated cells were observed. The tumor cells had varying amounts of cytoplasm and ill-defined cell borders.
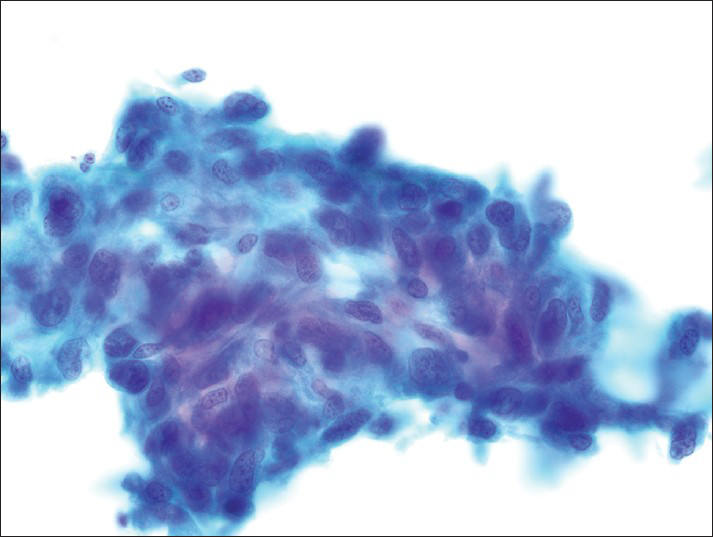
- The fixed smears demonstrate loosely cohesive clusters of spindled cells with irregular elongated nuclei (Papanicolaou)
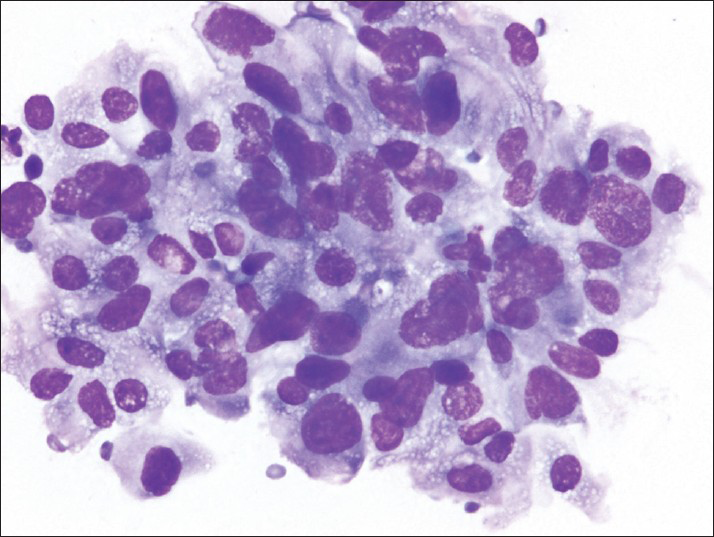
- The air-dried smears show pleomorphic cells; many have large, irregular nuclei and finely vacuolated cytoplasm (Diff-Quik)
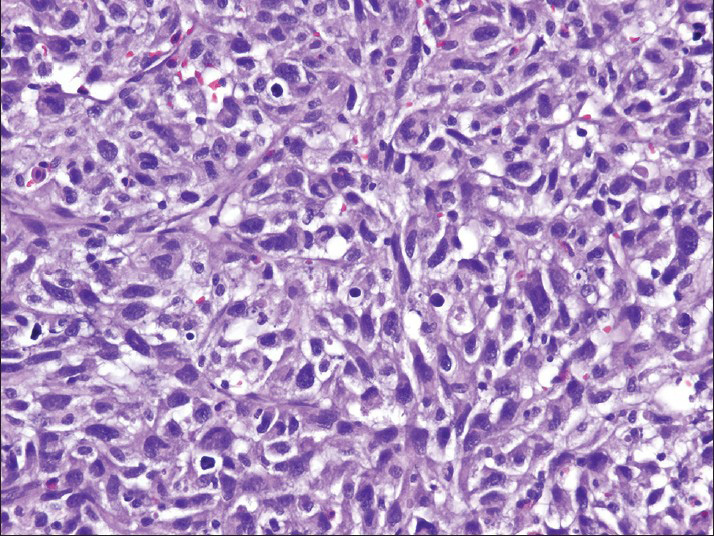
- The cell block preparation contains pleomorphic cells with spindled and epithelioid features (H and E)
Immunohistochemical studies performed on the cell block showed that the tumor cells were positive for vimentin, focally weak for CD31 and ERG, and nonreactive for pan-keratin, HMB-45, pan melanoma cocktail, smooth muscle actin (SMA), leukocyte common antigen, S-100 protein, and CD34. The tumor was diagnosed as a pleomorphic spindle and epithelioid cell neoplasm, consistent with a sarcoma of the pulmonary artery.
The pulmonary artery resection showed a 5.9 cm × 5.7 cm × 4.1 cm mass involving the right main pulmonary artery and the right upper lobe of the lung [Figure 5]. Histologically, some areas showed atypical spindled cells arranged in a storiform pattern [Figure 6] while other areas contained both pleomorphic spindled and epithelioid cells. Immunohistochemical results revealed that the tumor cells were negative for SMA, desmin, keratin and melanoma cocktail, SOX10, BRAFV600E, CD31, and CD34.
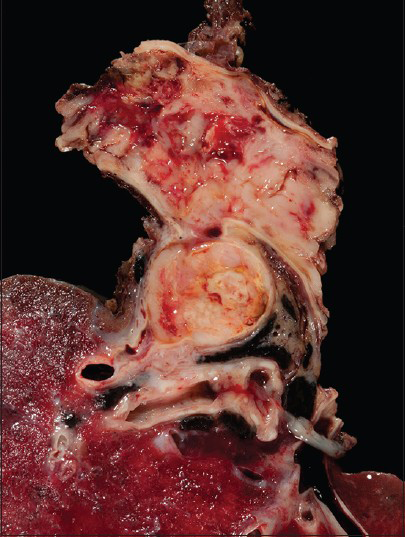
- The gross resection shows the lumen of the pulmonary artery completely occulted by tumor
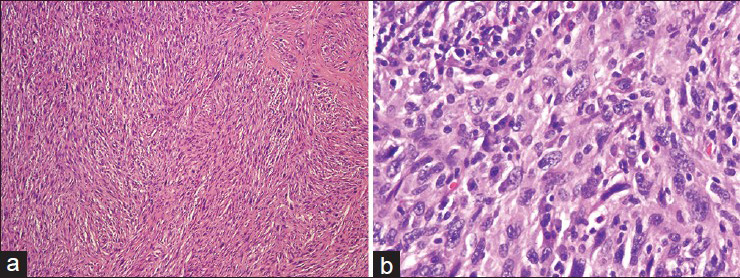
- (a) The resected tumor demonstrates spindled cells with a storiform pattern (H and E). (b). Higher magnification shows pleomorphic cells with convoluted to spindled nuclei, often containing nucleoli (H and E)
DISCUSSION
We present an unusual case of PAS that was discovered during restaging of a patient after treatment for multifocal large cell lymphoma. The PET-CT scan showed a hypermetabolic mass in the main pulmonary artery and the segmental division of the right anterior upper lobe pulmonary artery, and these features raised the possibility of a PAS. However, a primary lung carcinoma invading the pulmonary artery could not be entirely excluded. Therefore, an EBUS-TBNA was performed to evaluate this mass further. Immediate assessment of the aspirate smears showed pleomorphic malignant spindled and epithelioid cells, and additional material was obtained for immunohistochemical studies. The differential diagnosis included sarcomatoid carcinoma, melanoma, and sarcoma. Although there was immunostaining for vimentin, there was no evidence of epithelial, melanocytic, smooth muscle, or vascular differentiation. The diagnosis of sarcoma was based on the cytomorphologic features and immunoprofile. The tumor's pulmonary artery origin was supported radiographically by the presence of extensive intraluminal involvement, thus excluding a mediastinal sarcoma with arterial invasion that often encases and compresses the vessel. In addition, there was no mediastinal mass.
Imaging studies are valuable in detecting and diagnosing PAS. On CT scans, the tumors are hyperdense, and there is vascular distention when the tumor fills the lumen. Angiographic findings include a lobular or polypoid mass that moves with the heart rhythm. Since these tumors are metabolically active, they show more FDG positivity on PET-CT scans than do thrombi,[812] as imaging studies of our patient demonstrated. Similarly, gadolinium-enhanced magnetic resonance imaging is helpful in identifying pulmonary artery intimal sarcomas because these tumors enhance with gadolinium compared with thrombi. Furthermore, the degree of enhancement may correlate with tumor differentiation and the myxoid matrix.[13]
Endobronchial ultrasound-guided transbronchial needle aspiration allows visualization of the lesion, evaluation of vascular flow via Doppler ultrasonography and sampling of the lesion for diagnosis. Park et al.[10] reported two cases in which EBUS-TBNA was performed in an attempt to obtain diagnostic material. Cytological analysis revealed a spindled cell malignancy in one case but showed only fibrous exudate in the other case. Medalie et al.[9] described the cytomorphologic features in the case of metastatic PAS to the lung diagnosed by FNA. The aspirate showed single and loosely cohesive clusters of pleomorphic and spindled malignant cells that were embedded in an extracellular metachromatic matrix. Although our case showed tumor cells with similar cytomorphologic features, an extracellular matrix was not identified.
On microscopic examination, the majority of intimal sarcomas arising in the pulmonary circulation system are composed of poorly differentiated malignant cells with fibroblastic or myofibroblastic differentiation. The spindled tumor cells can show varying degrees of nuclear pleomorphism, mitotic activity, and necrosis.[1] Often there is tumor heterogeneity,[3] and there may be large myxoid areas.[1] Occurring less frequently are tumors with leiomyosarcomatous, rhabdomyosarcomatous, or angiosarcomatous differentiation as supported by immunophenotyping.[35] In contrast, the poorly differentiated tumors are typically positive for vimentin, variably positive for SMA, and negative for vascular markers. Frequent findings in pulmonary artery intimal sarcomas are overexpression of MDM2 and gains and amplification of the 12q13-14 region.[14]
Surgical resection remains the optimal treatment for patients with intimal sarcoma involving the pulmonary artery. However, treatment may involve one or several interventions including chemotherapy, radiation therapy, and/or surgery. The rarity of the tumor and the varied treatment modalities used make it difficult to analyze therapeutic outcomes. Nevertheless, even with multimodality treatment including surgery, the prognosis for patients with pulmonary artery intimal sarcoma is dismal.[8]
In summary, we describe an unusual case of PAS that was diagnosed on cytological material obtained by EBUS-TBNA with ROSE. The cytologic diagnosis helped expedite treatment of this rare and aggressive tumor.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
None.
AUTHORSHIP STATEMENT BY ALL AUTHORS
NC drafted the manuscript. DS participated in the design and contributed cytology figures. MD performed on the histologic assessment and discussion. RM performed the EBUS procedure and contributed the radiographic figures and legends. GL participated in the design and coordination. All authors read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
All authors have read and agreed to its content. IRB approval was waived for this case report.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Intimal sarcoma. In: Fletcher CD, Bridge JA, Hogendoorn P, Mertens F, eds. World Health Organization Classification of Tumours of Soft Tissue and Bone (4th ed). Lyon, France: IARC Press; 2002. p. :223-4.
- [Google Scholar]
- Pulmonary artery sarcomas. A review and report of a case. Arch Pathol Lab Med. 1985;109:35-9.
- [Google Scholar]
- Pulmonary artery sarcoma: A histologic and follow-up study with emphasis on a subset of low-grade myofibroblastic sarcomas with a good long-term follow-up. In: Am J Surg Pathol. Vol 32. 2008. p. :1751-61.
- [Google Scholar]
- Primary pulmonary artery sarcoma. Report of two autopsy cases studied by immunohistochemistry and electron microscopy, and review of 110 cases reported in the literature. Acta Pathol Jpn. 1988;38:883-96.
- [Google Scholar]
- Pulmonary artery sarcoma: A clinicopathologic and immunohistochemical study of 12 cases. Am J Clin Pathol. 2006;125:419-24.
- [Google Scholar]
- Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg. 2013;43:787-93.
- [Google Scholar]
- Metastatic pulmonary artery sarcoma. Report of a case with diagnosis by fine needle aspiration. Acta Cytol. 1998;42:968-72.
- [Google Scholar]
- EBUS-TBNA in the differential diagnosis of pulmonary artery sarcoma and thromboembolism. Eur Respir J. 2011;38:1480-2.
- [Google Scholar]
- Diagnostic usefulness of 18F-FDG PET/CT in the differentiation of pulmonary artery sarcoma and pulmonary embolism. Ann Nucl Med. 2009;23:671-6.
- [Google Scholar]
- Primary angiosarcoma of the pulmonary arteries: Dynamic contrast-enhanced MRI. J Comput Assist Tomogr. 1998;22:687-91.
- [Google Scholar]
- Gains of 12q13-14 and overexpression of mdm2 are frequent findings in intimal sarcomas of the pulmonary artery. Virchows Arch. 2001;438:57-65.
- [Google Scholar]








