Translate this page into:
Spectrum of clinicohematological profile and its correlation with average parasite density in visceral leishmaniasis
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Leishmaniasis is the prevalent in tropical and subtropical regions of the world. Demonstration of Leishman-Donovan (LD) bodies in the bone marrow aspirates (BMA) is vital to diagnosis of visceral leishmaniasis (VL). In the present study, we studied the clinicohematological parameters encountered in VL and correlated them with parasite load on BMA.
Methods:
Retrospective analysis over 3 years was done; clinical details, biochemical profile, complete hemogram with peripheral smear findings, and BMA smears were reviewed and average parasite density (APD) calculated in each case. Multivariate analysis and tests of significance were applied.
Results:
The study included 28 patients. Splenomegaly showed a positive trend with APD. rK39 antigen detection test was 100% positive in select cases. A strong negative correlation was observed between albumin to globulin ratio and grade of APD. BMA revealed hemophagocytosis (HPS) in 78.57% cases and it had a significant strong correlation with APD (P = 0.014). A significant correlation was also observed between APD and bone marrow plasma cell percentage (P = 0.01). LD bodies were noted in unusual locations such as within myelocytes (14.2%), plasma cells (7.1%), and megakaryocytes (10.7%).
Conclusion:
HPS and bone marrow plasmacytosis were two statistically significant findings, which showed positive correlation with parasite load. The presence of these two findings should prompt hematopathologists for more focused search of hemoparasites in BMA to arrive at a definitive diagnosis. This will avoid unnecessary workups and improve the prognosis. To the best of our knowledge, a statistical correlation between APD and clinicohematological parameters has never been previously studied.
Keywords
Average parasite density
bone marrow aspirate
hemophagocytosis
Leishman-Donovan body
visceral leishmaniasis
INTRODUCTION
Visceral leishmaniasis (VL) (also called as Kala-azar) is a fatal zoonotic systemic disease caused by the protozoan complex Leishmania donovani and transmitted by the bite of Phleobotomine sand fly. The disease is endemic in over 98 countries worldwide and prevalent in areas of the tropics, subtropics, and Southern Europe. More than 90% of new cases are reported from six countries: India, Bangladesh, Brazil, Ethiopia, South Sudan, and Sudan.[1] Of these countries, India accounts for the maximum burden with approximately 146,700–282,800 new cases being reported each year.[2] Clinical presentation of VL lacks specificity. Confirmatory tests are therefore, needed for the diagnosis and management of this disease. Demonstration of amastigote forms of the parasite and their load assessment by calculating average parasite density (APD) through bone marrow aspiration remains a vital investigation in disease diagnosis. In the present study, we analyzed the spectrum of clinicohematological presentations of VL and correlated these findings with APD.
MATERIALS AND METHODS
In the present retrospective study, data of consecutive bone marrow samples diagnosed as Leishmaniasis over a period of 3 years (August 2013–July 2016) was retrieved and reviewed. Sample size was calculated taking into account minimum 80% power and 5% significance level (significant at 95% confidence level). Assuming that, at 95% confidence level, 0.80 probability of success, and a margin of error (confidence interval) of ± 15%. The minimum sample calculated was 27.
Clinical presentation, biochemical profile with complete hemogram, and peripheral smear findings of cases retrieved were analyzed. A total of 28 case files including 16 adults and 12 pediatric cases were included in the study. Bone marrow aspiration bone marrow aspirates (BMA) smear slides were assessed for APD by counting Leishman-Donovan (LD) bodies. Smears were graded on × 400 as (0):0 LD bodies/1000 fields, (1+): 1–10 ld bodies/1000 fields, (2+): 1–10 ld bodies/100 fields, (3+): 1–10 LD bodies/10 fields, (4+): 1–10 LD bodies/field, (5+): 10–100 ld bodies/field, and (6+): >100 ld bodies/field [Figure 1a and b]. Hemophagocytosis (HPS) was graded as- (0): Absent; (1+) (Mild): <2 histiocytes with HPS/Slide; (2+) (Moderate): 2–5 histiocytes with HPS/Slide; (3+) (Severe): >5 histiocytes with HPS/Slide.[34]

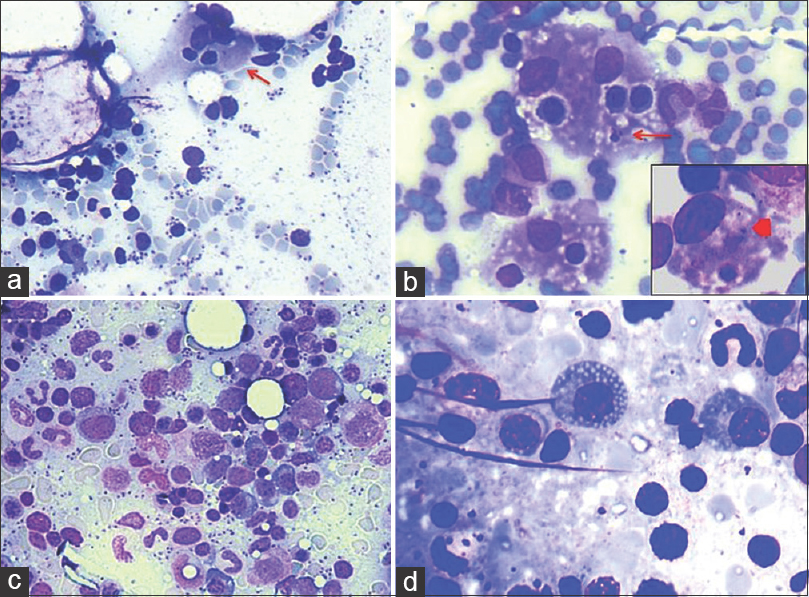
- (a) Low average parasite density. Occasional Leishman-Donovan body (arrow) (Giemsa; ×400). (b) High parasite density (Giemsa; ×200). (c) Leishman-Donovan body aggregate within and adjacent to histiocyte (Giemsa; ×1000). (d) Single scattered extracellular Leishman-Donovan bodies. Free cytoplasmic body loaded with Leishman-Donovan bodies (Giemsa; ×1000)
Statistical analysis using descriptive statistics was done. A multivariate analysis was performed and Pearson's correlation coefficient calculated for different variables such as hepatosplenomegaly, A: G ratio, peripheral blood, and bone marrow findings including HPS and APD. Chi-square test was applied to test the significance of correlation between two variables. Statistical testing was performed at 0.05 level of significance.
Ethical statement
This study was conducted with approval and as per the directives of Institutional Review Board. The corresponding author takes responsibility to maintain relevant documentation in this respect.
RESULTS
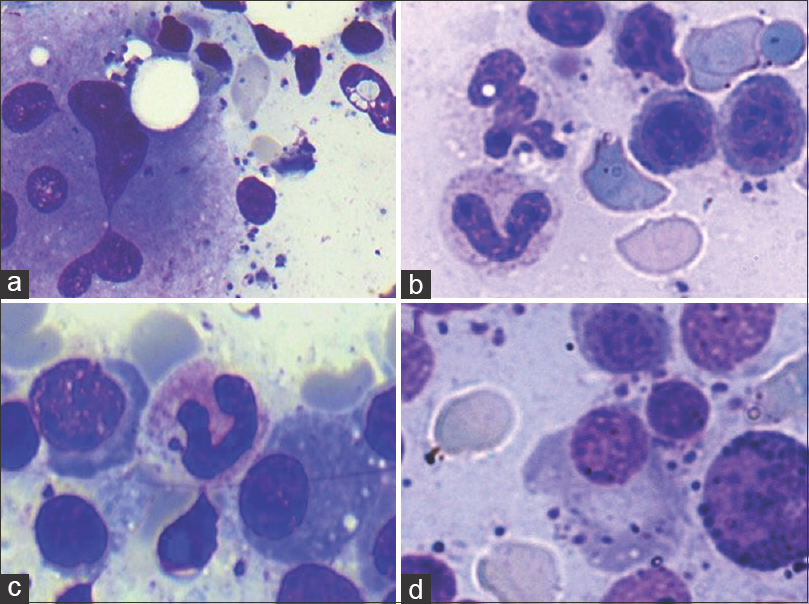
The study included 28 patients of which, 16 were adults and 12 were children. The patient characteristics are enlisted in Table 1. Mean overall age of presentation was 24.2 years. Continuous low-grade fever (89.2% cases) was the most common symptom followed by abdominal pain (17.8% cases), loss of appetite (16.4%), weakness and easy fatigability (16%). Splenomegaly, graded as per Hacketts’ classification[5] showed a positive trend with APD; however, this correlation was not statistically significant (P = 0.533). Mild hepatomegaly was noted in 82% cases. Mean albumin to globulin (A: G) ratio was 0.8 and a strong negative correlation was observed between A: G ratio and grade of APD. A descriptive analysis of hemogram findings has been enlisted in Table 2. Pancytopenia was observed in 39.3% of the patients. 96.4% of our patients were anemic and 75% had leukopenia with decreased absolute lymphocyte count (82.1%), absolute neutrophil count (71.4%), absolute monocyte count (89.3%) and absolute eosinophil count (60.7%). No significant correlation of APD was observed with total leukocyte count (P = 0.2), absolute neutrophil count (P = 0.778), absolute lymphocyte count (P = 0.312), absolute monocyte count (P = 0.276), and absolute eosinophil count (P = 0.351). Peripheral blood picture varied from normocytic normochromic to dimorphic and microcytic hypochromic morphology. Table 3 enumerates bone marrow findings in the slides reviewed. BMA were cellular in 18 (64.2%) cases, hypocellular in seven (25%) cases, and hemodilute in three (10.7%) cases. Erythroid hyperplasia was seen in 10 (35.7%) of the cases with. APD was graded as 1+ in 14 (14%) cases, 2+ in six (21.4%) cases, 3+ in three (10.7%) cases, 4+ in three (10.7%) cases, and 5+ in two (7.14%) cases. HPS was noted in 22 (78.6%) cases along with a significant strong correlation between APD and HPS (P = 0.014) [Figure 2a and b]. All of our cases revealed intracellular LD bodies. However, 22 (78.6%) cases also displayed presence of extracellular forms [Figure 1c and d]. In addition, LD bodies were also noted within unusual locations such as myelocytes four (14.2%) cases, plasma cells two (7.1%) cases, and megakaryocytes three (10.1%) cases [Figure 3]. Mean bone marrow plasma cell percentage was found to be 5.36% with a significant correlation between APD and bone marrow plasma cell percentage (P = 0.01) [Figure 2c and d]. Dysplastic features involving erythroid (nuclear budding, nuclear bridging, and atypical mitosis), myeloid (hypogranulosis, pseudo Pelger-Huet anomaly), and megakaryocytic (hypolobation, separation of nuclear lobes) lineages were noted in the BMA. Dyserythropoiesis, dysgranulopoiesis, and dysmegakaryopoiesis were seen in 18 (64.3%), 4 (14.3%), and 15 (53.6%) of the cases, respectively [Figure 4].



- (a) Hemophagocytosis (arrow) in a background of Leishman-Donovan bodies (Giemsa; ×200). (b) Hemophagocytosis along with Leishman-Donovan body within histiocytes (arrow); Inset-hemophagocytosis (arrow head) (Giemsa; ×400). (c) Increased marrow plasma cell percentage (Giemsa; ×200). (d) Mott cell
- Uncommon findings (Giemsa; ×400). Intracytoplasmic Leishman-Donovan bodies noted within (a) megakaryocyte (b) neutrophil (c) band form (d) plasma cell
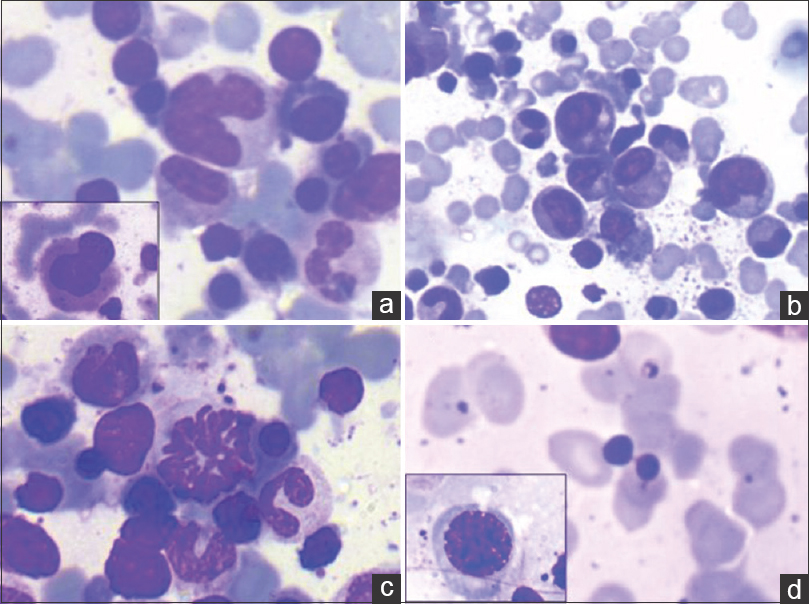
- Dyshemopoietic changes (Giemsa; ×400). (a) Dysplastic megakaryocytes. Hypolobated form (inset). (b) Dysgranulopoiesis. Hypogranular myeloid cells (inset). (c) Dyserythropoiesis (mitotic form). (d) Dyseythropoiesis (nuclear bridging) and megaloblastic forms (inset)
DISCUSSION
VL is the most severe form of leishmaniasis and if left untreated, is usually fatal.[6] It was first described by Dr. Wiliam Boog Leishman and Dr. Charles Donovan, separately, in the year 1903.[7] Leishmaniasis is characterized by prolonged fever, splenomegaly, hepatomegaly, substantial weight loss, progressive anemia, pancytopenia, and hypergammaglobulinemia. It is often complicated by secondary infections. It may also mimic a variety of hematological disorders such as other causes of pancytopenia, myelofibrosis, myelodysplasia, and HPS.[8] The diagnosis of VL is further complicated by certain clinical features which are shared in common with other febrile illnesses such as malaria, typhoid, and tuberculosis. Furthermore, the clinical course can be unpredictable in immunocompromised patients and may be be masked by other associated opportunistic infections. Sequestration of the parasites in the spleen, bone marrow, or lymph nodes further makes the diagnosis difficult.[9]
In the present study, persistent low-grade fever (range: 5 days–12 months; mean 3.24 months) was the most common presenting complaint, seen in all cases, as was also observed by Chakrabarti et al.[10] in their study. Dhingra et al.[8] and Chufal et al.[11] also concurred similar findings, however, the latter did not specify duration of fever. Development of fever in leishmaniasis is possibly due to activation of innate and adaptive cellular immunity of the host. Primary cells to be affected are the macrophages. Promastigotes from female sand fly proboscis enter bloodstream and attach to the reticuloendothelial system and mount a Th1 response leading to subsequent IL-2, IL-3, and interferon-gamma production by CD4+ T cells along with several proinflammatory cytokines such as IL12 and tumor necrosis factor-alpha (TNF-α).[12] This leads to reactive proliferation of cells of the reticuloendothelial system causing splenomegaly and sequestration of red blood cells (RBCs) in spleen. In the present study, splenomegaly was observed in 94.6% of patients along with an increasing trend of correlation between spleen size and APD; however, this correlation was not statistically significant (P = 0.12). Schaefer et al.[13] investigated 2,941 patients in an endemic zone of Leishmaniasis and found significant correlation between marked splenomegaly (Hackett Grade 3 or more) and previous or current Leishmania infection. Deranged liver profile along with jaundice, ascites, altered albumin to globulin ratio, and coagulation abnormalities may occur in late stages of VL. Liver dysfunction may be caused directly by protozoa itself or indirectly related to immune response against parasites.[14] el Hag et al.[15] documented kupffer cell colonization by parasites, ballooning degeneration of hepatocytes, pericellular fibrosis, fibrosis of the terminal hepatic venules, periportal, and lobular mononuclear inflammatory infiltrate as liver changes in cases of VL. In the present study, mean A: G ratio was 0.8 due to raised globulins and a strong negative correlation was observed with increasing parasite density (P = 0.737). In the present study, rK39 antigen dipstick test, an immunochromatographic assay was performed on 19 patients and showed 100% positivity. This finding was well in consonance with results mentioned in literature.[1617] Eight (28.5%) of our patients also presented with peripheral lymphadenopathy, however, LD bodies were demonstrated in the lymph node aspirate of only one patient. This case was quite interesting, as the patient had enlarged conglomerate of mesenteric lymph nodes along with persistent low-grade fever raising a strong clinical suspicion of tuberculosis. Fine-needle aspiration of peripheral lymph nodes also showed epithelioid cell granulomas in addition to LD bodies. This patient was immunocompromised and HIV positive. Diagnosis of Leismaniasis by LD body demonstration in lymph node aspirate is extremely unusual. A high APD on BMA was noted in this patient. VL is now included as an AIDS-defining condition in virtually all VL-treatment guidelines.[18]
In the present study, mean Hb levels were found to be 7.3 gm%; it was comparatively lower in pediatric population (mean Hb 7.05 gm%) when compared to adults (mean Hb 8.03 gm%). This result is similar to average hemoglobin levels of 8.3 gm% and 7.8 gm% reported in two large series of VL patients. [1920] However, Marwaha et al.[21] in their study observed an overall lower Hb values with mean of 6.4% in pediatric population and mean of 7.3% in adults. Woodruff et al.[22] demonstrated decreased RBC life span in patients of Leishmaniasis and possible role of autoimmune mechanisms in kala-azar associated anemia. They observed that patients with active VL had raised immune-agglutinin titers causing agglutination of erythrocytes. In addition, other causes of anemia include sequestration and destruction of RBCs RBC in enlarged spleen, nutritional deficiency, alterations in RBC membrane permeability, and replacement of bone marrow elements by reticuloendothelial hyperplasia.[1723] Pancytopenia was noted in 11 (39%) of our patients as opposed to the finding of Chufal et al.[11] and Kumar et al.[24] who had 100% patients presenting with pancytopenia in their study. Cartwright et al.[19] have discussed that as the duration of the leishmaniasis increases, spleen tends to become larger and the anemia, leukopenia, and thrombocytopenia become progressively more severe. Leukopenia generally appears first, followed by anemia, and finally thrombocytopenia. Occasional report has also mentioned thrombocytosis in VL patients.[25] Sula and Tekin suggested that this increase in bone marrow megakaryocytes and relative thrombocytosis in VL patients may be a result of ongoing inflammation.[26] Normocytic normochromic anemia has been documented to be the most common type of anemia in this study (12 cases; 42.9%), as has also been documented in other studies in literature.[1419] Microcytic hypochromic peripheral blood picture was seen in six (21.4%) cases and dimorphic picture was seen in 10 (35.7%) cases (8 cases - macrocytic normochromic to normocytic normochromic and two cases - microcytic hypochromic to normocytic normochromic).
Demonstration of parasites on BMA smears is an established reliable method for VL diagnosis; however, at the disease onset, parasite load is low, and can often be overlooked. In the present study, cellular bone marrow was noted in 64.3% patients along with presence of erythroid hyperplasia in majority of the patients (70%). Erythropoiesis was normoblastic in 71.4% cases and megaloblastic change was seen in 28.5% cases. Features of dyserythropoiesis were seen in 18 (64.3%) cases. Wickramasinghe et al.[27] indicated dyserythropoiesis and ineffective erythropoiesis have a role in the pathogenesis of the anemia of at least some cases of kala-azar while Kafetzis and Maltezou[28] observed that marrow-related changes in VL are mostly related with prominent dyserythropoiesis and may even simulate congenital dyserythropoietic anemia in pediatric population. In a recent study, Temiz et al.[29] also observed dyserythropoietic changes in BMA smears in approximately 50% of the cases. In addition to dyserythropoiesis, dysgranulopoeisis was observed in 4 out of 28 (14.3%) cases and and dysmegakaryopoiesis was observed in 15 out of 28 (53.4%) cases. It has been postulated that elevated levels of TNF-α may cause trilineage myelodysplasia in the patients with VL.[30] Moreover, dyshemopoietic changes in the present study may have also been contributed by underlying nutritional anemia. HPS was the most prominent finding in our study, observed in 75% cases. A statistically significant association was observed between APD and frequency of HPS; this association has not been studied in the past to the best of our knowledge. Bhatia et al.[3] also noted significant HPS, however, the strength of association has not been tested. Although VL-related hemophagocytic lymphohistiocytosis (HLH) has been reported, it is an uncommon entity.[31] In the present retrospective study, S. ferritin and triglyceride levels were not available in a significant number of cases, so a confirmation of HLH could not be made. However, in view of presence of frequent hemophgaocytosis, fever, and splenomegaly in majority of the cases, further studies are required to identify the syndrome of VL associated HLH in an endemic country like India. Similar to the study by Daneshbod et al.,[32] the present study showed presence of intracellular and extracellular LD bodies in various aggregates - flower such as - ball-like, platelet-like clusters and within free cytoplasmic bodies; however, no statistical significant association of these arrangements with either APD or HPS was observed. In addition to the presence of LD bodies within histiocytes, they were also noted within megakaryocytes, myelocytes, and plasma cells. It corroborated well with findings of Bhatia et al.[3] Bone marrow lymphocytes were not a prominent finding in the present study and a mean lymphocyte percentage of 2.4% was noted. Although no significant statistical correlation was noted between bone marrow lymphocyte count and APD, a negative trend of bone marrow lymphocyte count with APD was noted. These findings compared favorably with those of Shahriar et al.[33] who also observed hypolymphopoiesis as one of the cytological clues for diagnosis of VL in BMA. Bone marrow plasmacytosis showed a statistical significant correlation with APD (P = 0.01) in this study along with presence of several reactive forms including flame cells and mott cells. Increased bone marrow plasmacytosis has been observed in literature;[710] however, to the best of our knowledge, no data showing its association with APD has been published so far.
CONCLUSION
The present study is a comprehensive review of clinicohematological findings in VL. A correlation of various clinicohematological parameters with APD has revealed significant statistical correlation of HPS (P = 0.014) and plasmacytosis (P = 0.01) with APD in the bone marrow. These significant observations highlight the significance of more focused search of hemoparasites in BMA to arrive at a definitive diagnosis. This will avoid unnecessary workups and improve the patient outcome. Although no statistical significant correlation with reference to A: G ratio, total leukocyte count, differential leukocyte count, and LD body aggregates was noted in the present study, larger studies are required for further validation of these findings. To the best of our literature search, no similar study showing statistical correlation between APD and different clinicohematological parameters has been published so far.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors declare that there are no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
VK: Conceptualized the study, was involved in editing and proof reading the manuscript; PA: Conceptualized, collected data, analyzed, wrote and proof read the manuscript; SM: Was involved in data collection and proof reading; ASN: was involved in data collection and proof reading; AT: was involved in data collection
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval and as per the directives of Institutional Review Board. The corresponding author takes responsibility to maintain relevant documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
APD - Average parasite density
HPS - Hemophagocytosis
LD - Leishman Donovan
VL - Visceral Leishmaniasis.
EDITORIAL/PEER REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double-blind model (authors are blinded for reviewers and vice versa) through automatic online system.
ACKNOWLEDGEMENT
The authors are grateful to the extremely dedicated physicians and pediatricians at our hospital for providing bone marrow examination material and discussing patient details.
REFERENCES
- Bi-regional meeting on leishmaniasis to strengthen cross border collaboration for the control of leishmaniasis in central Asian countries (WHO regional office for Europe) and middle east countries (WHO regional office for the eastern mediterranean), WHO and turkmenistan (Avaza, Turkmenbashi, Turkmenistan, 18-20 November 2014) Med Parazitol (Mosk). 2015;3:62-3.
- [Google Scholar]
- Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671.
- [Google Scholar]
- A case series highlighting the relative frequencies of the common, uncommon and atypical/unusual hematological findings on bone marrow examination in cases of visceral leishmaniasis. Mediterr J Hematol Infect Dis. 2011;3:e2011035.
- [Google Scholar]
- Infection-associated haemophagocytosis: The tropical spectrum. Clin Lab Haematol. 2005;27:312-5.
- [Google Scholar]
- Splenomegaly. Chamberlaine's Symptoms and Signs in Clinical Medicine. In: Ogilvie C, Evans C, eds. ELBS (11th ed). Oxford: Brucks, Butterworth; 1987. p. :490.
- [Google Scholar]
- Clinical and hematological manifestations of visceral leishmaniasis in Yemeni children. Turk J Haematol. 2009;26:25-8.
- [Google Scholar]
- The Social History of Health and Medicine in Colonial India. (1st ed). London: Routledge; 2009. p. :99.
- [Google Scholar]
- Morphological findings in bone marrow biopsy and aspirate smears of visceral Kala Azar: A review. Indian J Pathol Microbiol. 2010;53:96-100.
- [Google Scholar]
- Laboratory Diagnosis of Visceral Leishmaniasis. Clin Diagn Lab Immunol. 2002;9(5):951-8.
- [Google Scholar]
- Clinico-hematological profile of visceral leishmaniasis among immunocompetent patients. Southeast Asian J Trop Med Public Health. 2013;44:143-9.
- [Google Scholar]
- Role of haematological changes in predicting occurrence of leishmaniasis- A study in Kumaon region of Uttarakhand. J Clin Diagn Res. 2016;10:EC39-43.
- [Google Scholar]
- Splenomegaly in Baringo district, Kenya, an area endemic for visceral leishmaniasis and malaria. Trop Geogr Med. 1995;47:111-4.
- [Google Scholar]
- Kala-azar presenting as isolated cervical lymphadenopathy in an HIV- infected child. S Afr J Child Health. 2012;6:88-9.
- [Google Scholar]
- Liver morphology and function in visceral leishmaniasis (Kala-Azar) J Clin Pathol. 1994;47:547-51.
- [Google Scholar]
- Evaluation of two rK39 dipstick tests, direct agglutination test, and indirect fluorescent antibody test for diagnosis of visceral leishmaniasis in a new epidemic site in highland Ethiopia. Am J Trop Med Hyg. 2011;84:102-6.
- [Google Scholar]
- Hematologic changes in visceral leishmaniasis/Kala Azar. Indian J Hematol Blood Transfus. 2010;26:78-82.
- [Google Scholar]
- Visceral leishmaniasis as an AIDS defining condition: Towards consistency across WHO guidelines. PLoS Negl Trop Dis. 2014;8:e2916.
- [Google Scholar]
- Haematological investigations in Kala-Azar patients in Bihar. Indian J Med Res. 1979;70:571-82.
- [Google Scholar]
- Clinico-hematological characteristics in patients with Kala Azar. A study from North-West India. Trop Geogr Med. 1991;43:357-62.
- [Google Scholar]
- Studies of the anemia of Kala-Azar in 68 childhood cases. Specific antiparasitic chemotherapy is the most effective treatment. Clin Pediatr (Phila). 1977;16:733-6.
- [Google Scholar]
- Kala-azar – A case series from non endemic area, Uttarakhand. J Commun Dis. 2012;44:145-9.
- [Google Scholar]
- Visceral leishmaniasis in Southwestern Iran: A Retrospective clinico-hematological analysis of 380 consecutive hospitalized cases (1999-2014) PLoS One. 2016;11:e0150406.
- [Google Scholar]
- Use of hematological parameters in evaluation of treatment efficacy in cutaneous leishmaniasis. J Microbiol Infect Dis. 2015;5:167-72.
- [Google Scholar]
- Ultrastructure of bone marrow in patients with visceral leishmaniasis. J Clin Pathol. 1987;40:267-75.
- [Google Scholar]
- An association of leishmaniasis and dyserythropoiesis in children. Indian J Hematol Blood Transfus. 2014;30:19-21.
- [Google Scholar]
- Visceral leishmaniasis associated hemophagocytic lymphohistiocytosis – Case report and systematic review. J Infect. 2008;56:381-8.
- [Google Scholar]
- Cytological clues of bone marrow findings in Kala-Azar. Diagn Cytopathol. 1999;20:208-11.
- [Google Scholar]








