Translate this page into:
Survey of cytopathologists and cytotechnologists for the clinical impact of the use of atypia or follicular lesion of undetermined significance
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
The cytologic diagnosis of atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) is controversial because of variation in how it is applied in practice, as well as uncertainty about patient management. We aimed to assess the percentage of thyroid fine-needle aspiration biopsies (FNABs) with AUS/FLUS diagnoses in different North American and European practice settings (e.g. community, academic, etc.), assess whether patients were managed according to current guidelines, and determine patient outcomes.
Materials and Methods:
A detailed questionnaire survey was posted in secure websites used separately by cytopathologists and cytotechnologists. The questionnaire was posted from August 1 through December 31, 2013.
Results:
Endocrinologists and cytopathologists performed 51.7% and 37.1% of thyroid FNABs, respectively. The Bethesda reporting system for thyroid FNAB was used in 90% of practices. The rate of AUS/FLUS varied widely among institutions, with 46.1% of represented institutions reporting AUS/FLUS rates of 3–10%. The median follow-up rate of patients with an initial AUS/FLUS diagnosis was 70% (range, 10–100%). For the majority of represented institutions (86.4%), patients with initial AUS/FLUS diagnosis had follow-up with endocrinologists. Of repeat AUS/FLUS thyroid FNABs, a median of 52% was considered benign, and 18% were suspicious of or positive for malignancy (median, 10% and 7.5%, respectively).
Conclusions:
Reporting of the AUS/FLUS category varied widely among different institutions. The median follow-up rate was lower than published guidelines. The most common follow-up diagnosis was benign thyroid nodule. Improved standardization of cytologic criteria should be adopted to reduce such variation.
Keywords
Cytology
fine-needle aspiration
follicular lesion of undetermined significance
questionnaire
thyroid
INTRODUCTION
Thyroid nodules are prevalent, with palpable nodules occurring in 4–7% and nonpalpable nodules in 20–70% of the US population.[12] Thyroid fine-needle aspiration biopsy (FNAB) is a commonly used procedure worldwide for evaluating thyroid nodules. Epidemiologic studies have shown that the prevalence of palpable thyroid nodules is approximately 5% in women and 1% in men living in iodine-sufficient parts of the world.[23]
Endocrinologists, radiologists, and cytopathologists evaluate thyroid nodules using predominantly ultrasound-guided FNABs. In addition, cytopathologists evaluate these nodules microscopically using different reporting standardized terminology (e.g., nondiagnostic or unsatisfactory, benign, atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS), suspicious for neoplasm or malignancy, and malignancy).[4] The AUS/FLUS category is controversial because of variation in how this category is applied in cytopathology practice and uncertainty about how these patients should be managed.
The 2008 National Cancer Institute (NCI) state of the science conference for thyroid FNAB recommended using the FLUS category for thyroid aspirates with cytomorphologic findings that are neither convincingly benign nor sufficient for interpretation of neoplasia or malignancy.[4] According to the Bethesda System for thyroid cytopathology reporting, the rate of AUS/FLUS should be < 7% of all reported thyroid FNABs within the laboratory, but practices vary in rates of AUS/FLUS (from 1% to > 25%).[5] The recommendation for this atypical category is follow-up with ultrasound imaging and another biopsy within 6 months.[3] The AUS/FLUS category has a 5–10% estimated risk of malignancy, which is intermediate between the risk of a benign aspirate and an aspirate suspicious for neoplasm.[4]
The aim of the current study was to assess the percentage of biopsy samples being categorized as AUS/FLUS in different North American and European practice settings (e.g., community, academic), assess whether the clinicians are managing these patients according to current guidelines, and determine patient outcomes of those patients who received follow-up FNAB. We collected survey data because no data sources with this information were available from the different institutions.
MATERIALS AND METHODS
The study was approved by the institutional review board. Patient consent for this study was waived because the study did not confer any risk.
Sampling frame
We sought to survey cytopathologists, cytotechnologists, and endocrinologists who were current members of the American Society of Cytopathology (ASC) or the American Society for Cytotechnology (ASCT). Members are in North America, Europe, and other continents and practice in various settings (e.g., community hospitals, academic centers, specialized referral centers). Of the 2847 current members of ASC, approximately 1000 participate in the E-mail discussion list. Of the 560 members of the ASCT, approximately 200 participate in the E-mail discussion list.
The survey was posted in two secure web sites used separately by cytopathologists and cytotechnologists; an E-mail with information about the survey was sent to members. The survey was a 21-item questionnaire [Appendix] and was created using Survey Tracker (Training Technologies, Inc.) in collaboration with the Survey Research Center in Mayo Clinic's Department of Health Sciences Research. The survey questionnaire was pretested by 10 practicing cytopathologists (colleagues in different practice settings) through E-mail correspondence.
Sample size
We had a target of 100 respondents, with the goal of a 10% margin of error. Three E-mail reminders were sent to a different mailing list for survey participation.
Statistical methods
All analyses were descriptive. Survey data were summarized with frequencies and percentages for categorical items and with medians and ranges (or means and standard deviations) for continuous items.
RESULTS
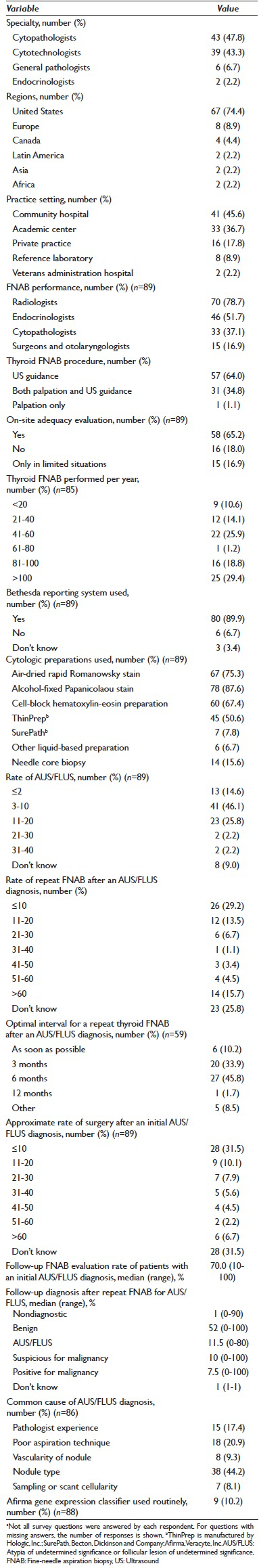
The survey questionnaire was accessible from August 1 through December 31, 2013, and promoted through the ASC and ASCT E-mail lists. The survey had 90 respondents (cytopathologists, n = 43; cytotechnologists, n = 39; general pathologists, n = 6; endocrinologists, n = 2). The majority of respondents were from community hospitals (n = 41 [45.6%]) and academic centers (n = 33 [36.7%]). Most (n = 67 [74.4%]) were from the United States, Europe (n = 8 [8.9%]), and Canada (n = 4 [4.4%]). Survey results are detailed in Table 1.
Specimen acquisition and preparation
Endocrinologists and cytopathologists performed 51.7% and 37.1% of thyroid FNABs, respectively. The majority of thyroid FNABs (64.0%) was performed using ultrasound guidance. Most institutions represented in the survey (65.2%) indicated that an on-site adequacy assessment for thyroid FNABs was performed. Thirty-four represented institutions (40%) indicated performing an average of 21–60 thyroid FNABs annually.
Specimen reporting and follow-up
Eighty represented institutions (90%) reported using the Bethesda system for thyroid cytopathology reporting. The estimated AUS/FLUS rate ranged from 3% to 10% for 41 represented institutions (46.1%). Fifty-nine represented institutions (67.0%) recommended a repeat FNAB for the AUS/FLUS category. Of represented institutions, 45.8% of those surveyed thought that the optimal interval for a repeat thyroid FNAB was 6 months, whereas 33.9% thought that 3 months were the optimal interval. In 41.6% of represented institutions, surgeons requested an intraoperative consultation after a thyroid lobectomy for a previous cytologic diagnosis of AUS/FLUS. Of represented institutions, 29.2% had a ≤10% rate of repeat FNAB after an initial cytologic diagnosis of AUS/FLUS. Of represented institutions, 31.5% had an approximate rate of surgery of ≤10% after an initial AUS/FLUS diagnosis. For the majority of represented institutions (86.4%), endocrinologists managed patients after an initial AUS/FLUS diagnosis. The median follow-up FNA evaluation rate for patients with an AUS/FLUS diagnosis was 70% among different institutions (mean [SD], 63.1% [30.6%]). The median rate of benign thyroid nodule diagnoses after an initial AUS/FLUS diagnosis was 52.0% (mean [SD], 56.2% [27.3%]) following either repeat cytologic aspiration or surgery. For 44.2% of respondents, the most common cause of an AUS/FLUS diagnosis was believed to be the type of thyroid nodule aspirated (solid vs. cystic vs. mixed). Only 10% of represented institutions routinely used the Afirma gene expression classifier (Veracyte, Inc.), a molecular test, for an AUS/FLUS diagnosis.
DISCUSSION
The reporting of AUS/FLUS was highly variable among institutions. The majority of thyroid nodule biopsies were performed by endocrinologists using ultrasound guidance. Two-thirds of represented institutions performed on-site adequacy assessment for thyroid aspirates. The Bethesda system for reporting thyroid FNABs was widely used. Most institutions performed a repeat FNAB within 3–6 months after an initial AUS/FLUS diagnosis. The median follow-up FNA evaluation rate for an initial AUS/FLUS diagnosis was 70%. The most common follow-up diagnosis following an AUS/FLUS diagnosis was benign thyroid nodule. Most respondents thought that the 2 most common causes for AUS/FLUS diagnosis were nodule type and poor aspiration technique (65.1%).
The definition of the FLUS subgroup by the Bethesda system for reporting FNAB results[4] states that it is “a heterogeneous category that includes cases in which the cytologic findings are not convincingly benign, yet the degree of cellular or architectural atypia is not sufficient for an interpretation of “follicular neoplasm” or “suspicious for malignancy.” This category was introduced as optional, and when utilized, would ideally represent <7.0% of all thyroid FNAB interpretations at an institution.[4] However, reporting rates vary widely among different laboratories, ranging from 3% to 27.2%.[67] For patients with indeterminate biopsy findings, factors such as sex, age, nodule size, and thyroid ultrasound characteristics can be considered to identify those with an elevated risk of malignancy. Indications for hemithyroidectomy in the setting of an FLUS diagnosis should include clinical and radiologic examination findings and patient preference.[8910]
The AUS/FLUS category is a diagnosis of exclusion, resulting from several factors such as borderline cellularity, preparation artifacts, or equivocal cytologic or architectural features.[6] The AUS: Malignancy ratio, which varies from 0.5% to 4.9%, has been described as a measure for assessing the AUS category use.[11] In most laboratories, the AUS: Malignancy ratio should be between 1.0 and 3.0; ratios exceeding 3.0 are most likely indicative of overcalling AUS/FLUS or undercalling the malignant category.[11]
The reported sensitivity and specificity of thyroid FNAB are 86% and 62%, respectively.[10] The diagnostic accuracy of a thyroid FNAB approaches 95%; nevertheless, thyroid FNAB can be nondiagnostic in up to 20% of cases.[1] In a questionnaire survey of endocrinologists and surgeons, respondents believed that the nodule characteristics most commonly caused a nondiagnostic FNAB (48%), followed by poor biopsy technique (29%).[1]
The percent of biopsies in the indeterminate category (including FLUS and suspicious for follicular or Hürthle cell neoplasm) ranges from 6% to 55% among different institutions, [12] with marked variation in the rate at which the indeterminate category was used among pathologists (2.5–28.6%) and institutions (3.3–14.9%).[13] In approximately 20–28% of AUS/FLUS cases, a repeat thyroid FNAB is still given an AUS/FLUS diagnosis.[7] Nodule size was found to be an independent predictor of malignancy in FLUS[8] and the “suspicious for neoplasm” category.[9] Most respondents in the current study (44.2%) believed that nodule type (cystic, solid, mixed) accounts for the majority of FLUS diagnosis in their respective institutions. The most significant cytologic features predictive of neoplasia in the FLUS category include the presence of syncytial tissue fragments, isolated microfollicles, follicles with scalloped borders, scant cytoplasm, irregular nuclear membranes, nuclear overlapping, coarse chromatin, and increased cellularity.[13]
The overall risk of malignancy in all patients with a thyroid nodule is from 5% to 7%.[14] The malignancy rate for an AUS/FLUS diagnosis varies (6–27%) among different institutions.[71314151617] The malignancy rate for the indeterminate category is higher for those with nuclear atypia (56%) than for those with architectural atypia (7.0%).[17] The reported malignancy rate of AUS/FLUS after a repeat FNAB is much greater (19–27%) than the malignancy rate of an AUS/FLUS diagnosis with the initial FNAB (2.7–12%).[18] The most common malignancy for a patient with an AUS/FLUS diagnosis is papillary thyroid carcinoma (PTC; 44%), followed by follicular variant of PTC (38%).[14] Seventy percent of malignant diagnoses in the indeterminate group with architectural atypia were follicular variants of PTC.[717] In one institution, the overall incidence of incidental PTC was 21.3%.[10]
Repeat FNAB of the AUS/FLUS category is more cost effective, with better-quality-adjusted life years than lobectomy.[18] Despite the NCI state of the science conference on thyroid FNAB, only 19–35% of patients have a repeat FNAB and 30–60% of patients go directly to surgery after an initial AUS/FLUS diagnosis.[18] However, thyroid surgery is not without inherent risks and complications, including hematoma, recurrent laryngeal nerve palsy, hypoparathyroidism, and hypothyroidism requiring life-long thyroid hormone replacement therapy.[19] The rate of hypothyroidism requiring thyroid hormone replacement therapy is 35% after hemithyroidectomy [18] and 47% after lobectomy for an indeterminate FNAB (FLUS and suspicious for follicular or Hürthle cell neoplasm).[19]
Molecular testing may be performed on AUS/FLUS lesions to further characterize the nature of the lesion. AUS/FLUS lesions with a V600E BRAF mutation likely represent classical or tall-cell variants of PTC.[20] The Afirma gene expression classifier can be used to test indeterminate thyroid nodules, including those characterized as AUS/FLUS, suspicious for follicular neoplasm, suspicious for Hürthle cell neoplasm, and suspicious for malignancy; it has high negative predictive value for discerning benign nodules.[2122] Sending indeterminate thyroid samples for molecular testing may increase the positive predictive value (BRAF testing) or negative predictive value (Afirma testing). In this study, 10% of respondents routinely sent samples for molecular testing.
The current study has several limitations. Only a select group of cytopathologists and cytotechnologists was surveyed. (Some members forwarded the survey link to endocrinologists in their respective institutions, but only two Endocrinologists responded to the survey.) Our findings have limited generalizability because of selection bias. Furthermore, despite sending 3 reminders, the overall response rate was low.
CONCLUSION
This study confirms that the reporting of the AUS/FLUS category varied widely among different institutions. Furthermore, the median follow-up rate of the AUS/FLUS category was only 70%, despite the American Thyroid Association management guidelines.[3]. The most common follow-up diagnosis after an initial AUS/FLUS diagnosis and repeat FNAB was benign thyroid nodule. Strict cytologic criteria should be adopted to minimize the variable AUS/FLUS diagnosis rates. Adherence to American Thyroid Association management recommendations and guidelines should be encouraged.
COMPETING INTEREST STATEMENT BY ALL AUTHOR
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHOR
All authors of this article declare that we qualify for authorship as defined by ICMJE. Each author has participated sufficiently in the work and take public responsibility for appropriate portions of the content of this article. AN: Design and conception of the study, data interpretation, writing of initial and final draft; JPR: Data interpretation and review of final draft; SEK: Design of study survey and review of final draft; SMJ: Statistical analysis and data interpretation and review of final draft; KAL: Statistical analysis and data interpretation and review of final draft. All authors read and approved the final manuscript. Each author acknowledges that this final version was read and approved.
ETHICS STATEMENT BY ALL AUTHOR
The study was approved by our institutional review board. Patient consent for this study was waived because the study did not confer any risk.
LIST OF ABBREVIATIONS
FNAB = Fine-Needle Aspiration Biopsy
AUS = Atypia of Undetermined Significance
FLUS = Follicular Lesion of Undetermined Significance
NCI = National Cancer Institute
ASC = American Society of Cytopathology
ASCT = American Society for Cytotechnology
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model.(authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- Management of nondiagnostic thyroid fine-needle aspiration biopsy: Survey of endocrinologists. Endocr Pract. 2004;10:317-23.
- [Google Scholar]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer Thyroid. 2009;19:1167-214.
- [Google Scholar]
- Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36:425-37.
- [Google Scholar]
- Malignancy risk for fine-needle aspiration of thyroid lesions according to the Bethesda System for Reporting Thyroid Cytopathology. Am J Clin Pathol. 2010;134:450-6.
- [Google Scholar]
- The potential for overuse of atypical thyroid diagnoses. Cancer Cytopathol. 2012;120:108-10.
- [Google Scholar]
- The atypical thyroid fine-needle aspiration: past, present, and future. Cancer Cytopathol. 2012;120:73-86.
- [Google Scholar]
- Clinical outcomes for “suspicious” category in thyroid fine-needle aspiration biopsy: Patient's sex and nodule size are possible predictors of malignancy. Arch Pathol Lab Med. 2009;133:787-90.
- [Google Scholar]
- Nodule size is an independent predictor of malignancy in mutation-negative nodules with follicular lesion of undetermined significance cytology. Surgery. 2013;154:730-6.
- [Google Scholar]
- Incidence of malignancy in thyroid nodules determined to be follicular lesions of undetermined significance on fine-needle aspiration. World J Surg. 2012;36:69-74.
- [Google Scholar]
- The atypia of undetermined significance/follicular lesion of undetermined significance: Malignant ratio: A proposed performance measure for reporting in The Bethesda System for thyroid cytopathology. Cancer Cytopathol. 2012;120:111-6.
- [Google Scholar]
- Correlation of thyroid nodule fine-needle aspiration cytology with corresponding histology at Mayo Clinic, 2001-2007: An institutional experience of 1,945 cases. Diagn Cytopathol. 2012;40(Suppl 1):E27-32.
- [Google Scholar]
- Minimizing the diagnosis of “follicular lesion of undetermined significance” and identifying predictive features for neoplasia. Diagn Cytopathol. 2011;39:737-42.
- [Google Scholar]
- The impact of atypia/follicular lesion of undetermined significance on the rate of malignancy in thyroid fine-needle aspiration: Evaluation of the Bethesda System for reporting thyroid cytopathology. Surgery. 2011;150:1234-41.
- [Google Scholar]
- Yield of repeat fine-needle aspiration biopsy and rate of malignancy in patients with atypia or follicular lesion of undetermined significance: The impact of the Bethesda System for reporting thyroid cytopathology. Surgery. 2012;152:1037-44.
- [Google Scholar]
- Fine-needle aspiration of follicular patterned lesions of the thyroid: Diagnosis, management, and follow-up according to National Cancer Institute (NCI) recommendations. Diagn Cytopathol. 2010;38:731-9.
- [Google Scholar]
- Thyroid follicular lesion of undetermined significance: Evaluation of the risk of malignancy using the two-tier sub-classification. Diagn Cytopathol. 2012;40:410-5.
- [Google Scholar]
- Cost-effectiveness analysis of repeat fine-needle aspiration for thyroid biopsies read as atypia of undetermined significance. Surgery. 2012;152:423-30.
- [Google Scholar]
- Thyroid lobectomy for indeterminate FNA: Not without consequences. J Surg Res. 2013;184:189-92.
- [Google Scholar]
- BRAF mutation detection in indeterminate thyroid cytology specimens: Underlying cytologic, molecular, and pathologic characteristics of papillary thyroid carcinoma. Cancer Cytopathol. 2013;121:197-205.
- [Google Scholar]
- Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705-15.
- [Google Scholar]
- afirma® gene expression classifier for preoperative identification of benign thyroid nodules with indeterminate fine needle aspiration cytopathology. PLoS Curr. 2013;5
- [Google Scholar]
APPENDIX (21-ITEM QUESTIONARE)
Please respond by circling one answer per question.
What is your specialty?
Endocrinologist Cytopathologist Cytotechnologist Other__________ In what region of the United States do you practice?
Northeast Southeast East Coast Midwest Southwest West Coast Other__________ In what setting are you currently practicing? Mark all or select one as needed
Academic center Community hospital Private practice Reference laboratory Other__________ Who performs the thyroid fine-needle aspiration (FNA) in your practice? Mark all that apply:
Cytopathologists Endocrinologists Radiologists Other__________ What is the most common method used for performing a thyroid FNA procedure at your institution and/or practice?
Ultrasound guidance Palpation Both On-site adequacy evaluation is a process where a cytopathologist and/or cytotechnologist and/or trainee will immediately evaluate the specimen for adequacy as soon as the thyroid specimen is obtained. Does your practice or institution have an on-site adequacy evaluation available during the performance of thyroid FNA procedure?
Yes No Only in limited situations Not sure Please check all types of cytologic preparations that are used to evaluate thyroid FNAs in your practice and/or institution.
Air-dried (eg, Rapid Romanowsky [Diff-Quik]) Alcohol-fixed (Papanicolaou) Cell-block ThinPrep SurePath Other liquid-based preparation Needle core biopsy I don’t know What is the most common type of cytologic preparation that is used routinely (for the majority of FNAs) in your practice (please check one answer only):
Air-dried (eg, Rapid Romanowsky [Diff-Quik]) Alcohol-fixed (Papanicolaou) Cell-block ThinPrep SurePath Other liquid-based preparation Needle core biopsy I don’t know How many thyroid FNAs do you personally or as a practice evaluate and/or interpret annually?
≤20 21-40 41-60 61-80 81-100 >100 Does your institution use the Bethesda reporting system for thyroid FNA?
Yes No → What other system do you use?_________ I don’t know According to published guidelines, the rate of atypia or follicular lesion of undetermined significance (AUS/FLUS) category should be less than 7% of all reported thyroid FNAs within the laboratory. What is the estimated rate of AUS/FLUS at your practice and/or institution?
≤2% 3%-10% 11%-20% >20% I don’t know Do you think a repeated thyroid FNA biopsy is helpful for a FLUS diagnosis?
Yes__________ No__________ (If you answered “No,” go to question 14) If a repeat FNA biopsy is performed after an AUS/FLUS diagnosis, what is the optimal interval?
As soon as possible 3 months 6 months 12 months 1-3 years Other__________ When is surgery appropriate follow-up for an AUS/FLUS diagnosis?
Almost never Only if there is high clinical suspicion for malignancy Depends on patient preference Almost always Other__________ Do surgeons at your institution use frozen-section interpretation during thyroid surgery? That is, do they first perform a thyroid lobectomy for frozen section and decide whether to proceed with total thyroidectomy for nodules with indeterminate FNA?
Yes No I don’t know According to published guidelines, individuals with a diagnosis of AUS/FLUS should be followed-up within 6.12 months. At your institution or practice, what is the approximate rate for repeat FNA biopsy if the initial diagnosis was FLUS?
≤10% 11%-20% 21%-30% 31%-40% 41%-50% 51%-60% > 60% I don’t know At your institution, what is the approximate rate of surgery after an initial AUS/FLUS diagnosis?
≤10% 11%-20% 21%-30% 31%-40% 41%-50% 51%-60% > 60% I don’t know Do your endocrinologists follow-up these patients?
Yes__________ No__________ (If you answered “No,” go to question 20) If yes, what is the follow-up rate on the AUS/FLUS subset of patients? Indicate approximate percentage rate. What is the follow-up diagnosis on patients with initial AUS/FLUS who underwent repeat aspiration or surgery? For each of the following diagnostic categories, write the percentage rate of follow-up diagnoses on patients with initial AUS/FLUS who underwent repeat aspiration or surgery. If none, write “0%.”
Nondiagnostic Benign FLUS Suspicious for malignancy Malignancy In your opinion, what is the most common cause of an AUS/FLUS diagnosis? Mark only one choice.
Pathologist's experience Poor technique Vascularity of nodule Nodule type (excluding simple cysts) Anxious patient Other________














