Translate this page into:
Testicular touch preparation cytology in the evaluation of male infertility
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
Male infertility is traditionally evaluated by tissue core biopsies of the testes. Touch preparations (TP) of these biopsies have been infrequently used. The aim of this study is to report our experience with using testicular biopsy TP for the evaluation of male infertility.
Materials and Methods:
A retrospective search was performed for cases of testes biopsies with concurrent TP. These cases were evaluated for clinical information, specimen adequacy, and cytological–histological correlation.
Results:
A total of 39 cases were identified from men with a mean age of 34 years (range 23 to 50 years). TP slides were satisfactory for evaluation in 31 (89%) cases, and less than optimal in four due to low cellularity, obscuring blood or air drying artifact. Cytopathology showed concordance with the biopsy in almost all cases. In one discordant case where the biopsies showed no active spermatogenesis, a rare sperm were identified on the TP.
Conclusions:
TP of the testis is a helpful adjunct to biopsy because of its ability to clearly evaluate all stages of spermatogenesis. These data demonstrate that TP cytopathology of the testes in our experience has an excellent correlation with both normal testicular biopsies and those showing pathological spermatogenesis, and in rare cases may provide added benefit in evaluating the presence of spermatogenesis for male infertility. Albeit uncommon, cytopathologists may be required to identify and evaluate spermatogenic elements in cytology specimens being submitted from men with infertility.
Keywords
Cytology
infertility
spermatogenesis
testis
touch preparation
INTRODUCTION
Many men with male infertility may have oligozoospermia (decreased numbers of sperms in their ejaculate) or azoospermia (no sperm in their ejaculate). The etiology of azoospermia may be attributed to (a) pre-testicular causes (e.g., endocrine abnormalities causing secondary testicular failure), (b) testicular causes (e.g., primary testicular failure due to intrinsic disorders of seprmatogeneis within the testes, including maturation arrest, complete absence of germ cells or Sertoli-cell-only syndrome), or (c) post-testicular causes (e.g., ejaculatory dysfunction or ductal obstruction that prevent sperm transport).[1] To distinguish between obstructive and non-obstructive causes of azoospermia, testicular biopsy may be performed.
For patients with normal spermatogenesis, testicular sperm retrieval is relatively simple by employing surgical reconstruction of the obstruction. Therefore, proper interpretation of a diagnostic testicular biopsy is critical for preoperative counseling and subsequent patient management.[2] Surgical biopsy of the testis has generally been regarded as the ‘gold standard’ in the evaluation of male infertility.[3] For this purpose, percutaneous core needle biopsy of the testis has been associated with fewer complications and equal diagnostic value to an open biopsy.[4]
Testicular fine needle aspiration (FNA) biopsy has been advocated by several authors as an alternative or adjunct approach to surgical biopsy, to evaluate testicular morphology.[5–10] Testis FNA cytology in several studies correlated well (84 – 97% agreement) with testis biopsy histology in the evaluation of male infertility.[11–13] In some studies, FNA was even more sensitive than tissue biopsy in detecting the presence of spermatogenesis.[14] Artifacts reported by some authors in histological sections were avoided in FNA.[1516] For example, fixation of testicular tissue with buffered formalin instead of Bouin or acetic acid-zinc formalin may cause luminal sloughing of cells, thereby obscuring the cell lineages under evaluation.[2]
The cytological appearance of spermatogenic cells may show a spectrum of germ cell components, ranging from immature spermatogonia to spermatocytes and mature spermatids, typically characterized by diminution of nuclear size with maturation.[17] Secondary spermatocytes are not usually seen in cytology specimens because they have a short lifespan. Leydig cells in aspirates are also not commonly seen, except in cases with Leydig cell hyperplasia.[18] Sertoli cells tend to be bigger and are often recognized as a homogeneous cell population compared to spermatogenic cells.
Fine needle aspiration has proven to be particularly helpful in locating (mapping) and retrieving spermatozoa in men with non-obstructive azoospermia, particularly as sperm production in these men can be patchy or focal in nature.[19–21] As FNA is minimally invasive (‘testis-sparing’ technique) it is associated with fewer complications than tissue biopsies in azoospermic patients. Testicular FNA has also been used to perform cell counts and indices (e.g., sperm-Sertoli index and Johnsen's score) in patients being worked-up for infertility.[21–23] However, features like fibrosis, basement thickening, and atrophy cannot be assessed on FNA.
Compared to FNA, touch preparation (TP) of testicular biopsies has been used infrequently in the evaluation of male infertility.[2425] Kim and colleagues report that testicular TP cytology serves two main purposes: (a) It helps to differentiate maturation arrest at the late spermatid stage from normal spermatogenesis, and (b) it helps to determine if infertility is due to obstructive azoospermia.[24] At our institution, we have been evaluating testicular TP slides for several years, in addition to biopsy. The aim of this study is to report our experience with using testicular TP in the evaluation of male infertility.
MATERIALS AND METHODS
A retrospective search of our pathology computer database was conducted (from 2004 to 2011) for cases of testes biopsies where a concurrent TP was submitted to the Pathology Department. In all cases, six core biopsies were performed from the upper, middle, and lower regions of both testes with a corresponding spray fixed TP (i.e., six TP slides / case). To obtain a biopsy, an incision was made by the urologist in the direction of the scrotal ruggae. Sharp dissection was carried down to the tunica albuginea, which was incised. The testicular tissue was extruded and excised. The tissue was immediately passed across a slide in both circular and straight overlapping patterns [Figure 1]. The slide was then immediately sprayed with Cytofix™ and placed in a cardboard slide container to air dry. The slides were transported to the cytology laboratory and stained with a Papanicolaou stain. These cases were further evaluated for clinical information (patient age, indication for biopsy), specimen adequacy, and cytological–histological correlation. The captured data elements included the adequacy of the cytological specimens, presence of spermatogenesis, and any other significant histological or cytological features. Hypospermatogenesis (oligospermia) was used to define cases where all stages of spermatogenesis were present, but reduced to a varying degree. Cases were categorized as having maturation arrest, when there was a complete arrest of spermatogenesis at a particular stage. Slides from selected cases were reviewed.
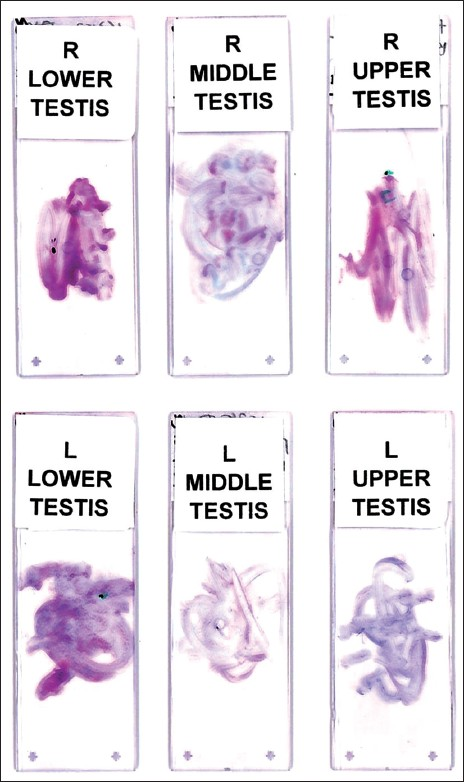
- Touch preparations. For each case, six generous touch preparations were performed corresponding to bilateral testicular biopsies (gross image, no magnification)
RESULTS
Clinical features
The study included 39 patients with a mean age of 34 years (range, 23 to 50 years). The clinical indications for testicular biopsy included, testicular failure (24 cases), azoospermia (four cases), and one case each for atrophic testes, Klinefelter syndrome, and scrotal varices. Of the 24 cases with testicular failure, 14 had an additional concern for possible carcinoma in situ.
Cytological findings
Up to 31 (89%) of the cytology specimens were adequate (satisfactory non-obscured cellularity) for evaluation. In two cases, only one of the six TPs was unsatisfactory. Four cases were felt to be less than optimal, due to low cellularity, obscuring blood or air drying artifact. The TPs contained varying testicular elements, including seminiferous tubule components [Figure 2], and interstitium (mainly Leydig cells in some cases) and supporting structures (predominantly mesothelial cells). Occasional TPs contained intact segments of seminiferous tubules. In some cases crystalloids of Charcot-Böttcher could be identified within the cytoplasm of the Sertoli cells [Figure 3]. Several cases had scattered naked nuclei from different cell types. Cytopathological evaluation showed concordance with the corresponding biopsy in all, but one case, including those specimens with normal biopsy findings, maturation arrest [Figure 4], Sertoli-cell-only syndrome [Figure 5], and one case showing Leydig cell hyperplasia [Figure 6]. The discordant case showed rare sperm on the imprint slide of one biopsy, whereas the histopathology in all tissue cores from this patient showed no active spermatogenesis [Figure 7]. The only cellular atypia identified on TPs was attributed to air drying artifact.
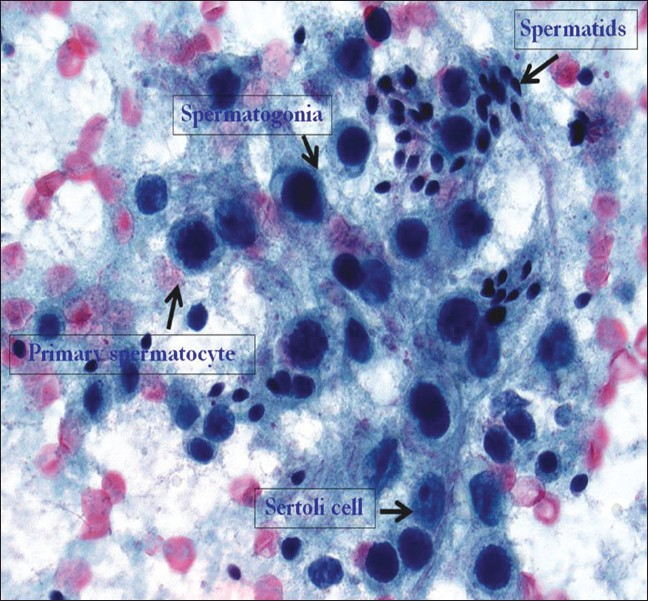
- Testicular touch preparation from a normal testis biopsy showing germ cells in varying stages of spermatogenesis. Complete maturation of germ cells is evident, beginning with spermatogonia (intermediate-sized cells with a high N : C ratio containing single, oval-to-round, darkly stained nuclei with even chromatin), followed by spermatocytes (slightly larger cells with nuclei containing more coarse or thread-like chromatin), and finally spermatids (small-sized cells with minimal cytoplasm, darkly stained small, oval nuclei) as well as spermatozoa (usually with an indistinct tail on Pap stain). Note that as the chromosomal structures become more prominent in the primary spermatocytes their nuclear outline becomes more irregular. As shown in this image, spermatids tend to be found in groups. The Sertoli cell seen in this TP has cytoplasm with poorly delineated cell borders and a vesicular, oval nucleus, with smooth contours and a distinct, large nucleolus. (×600 magnification; Pap stain)
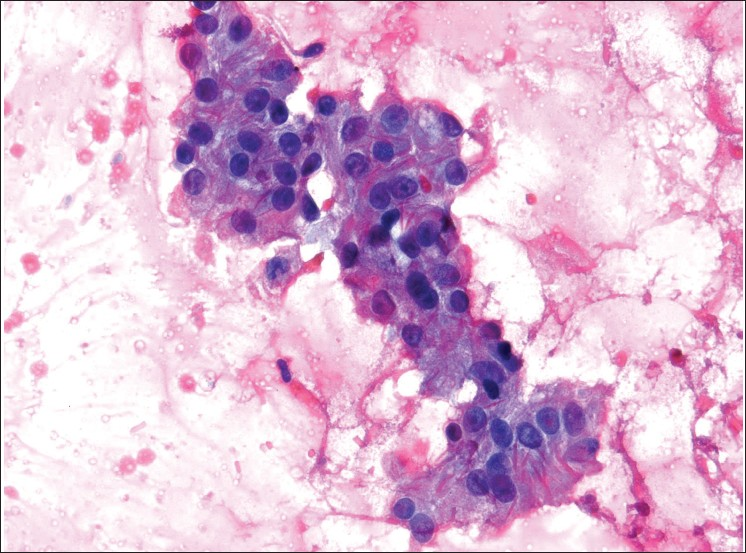
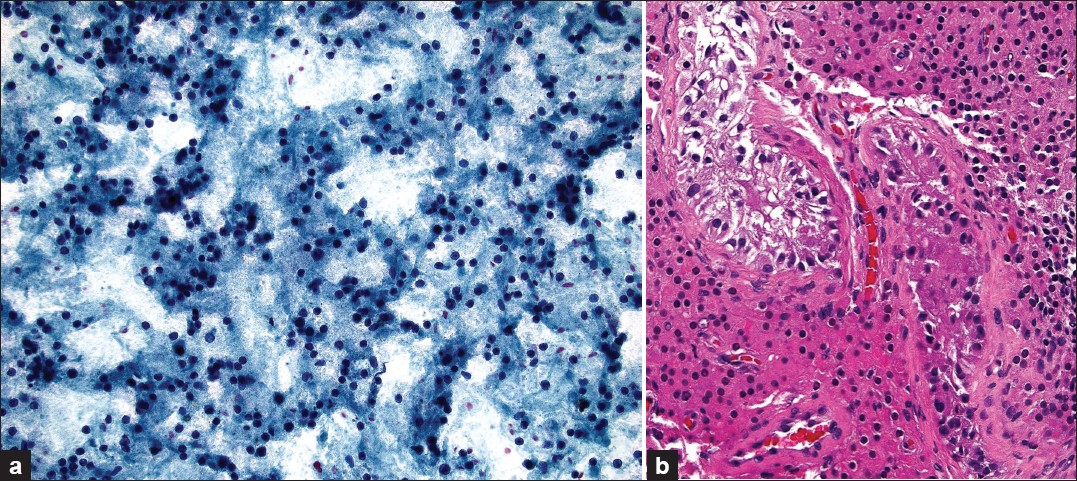
- Sertoli cells with intracytoplasmic crystalloids of Charcot-Böttcher (×400 magnification; Pap stain)
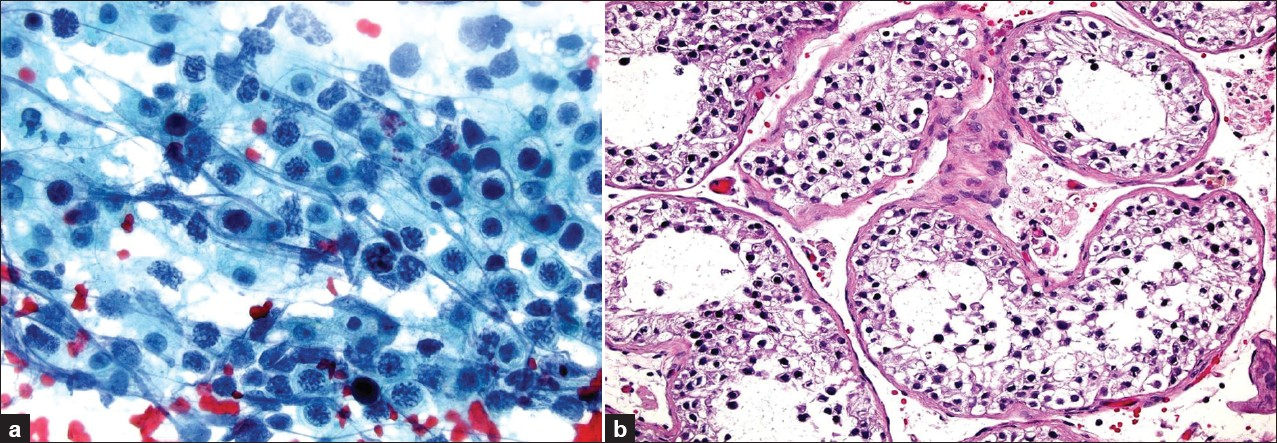
- Maturation arrest with no spermatids. Immature germ cells, including several primary spermatocytes with coarse chromatin are present. [a) touch prep, ×600 magnification, Pap stain; b) biopsy, ×200 magnification, H and E stain]
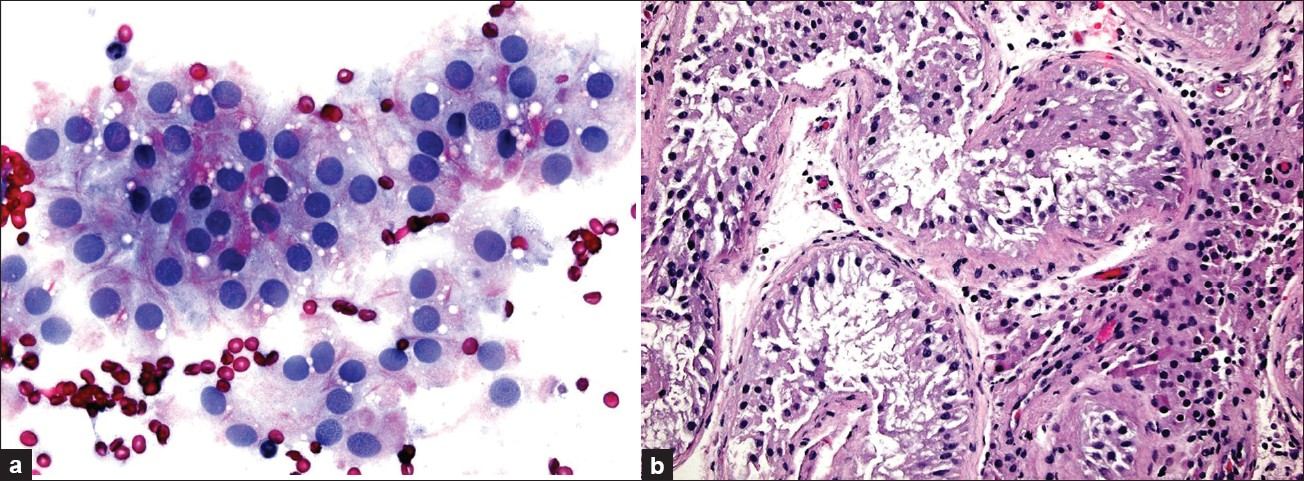
- Sertoli-cell-only syndrome (germ cell aplasia) showing the presence of only Sertoli cells. The touch preparation in this case also shows air drying artifact. [a) touch prep, ×400 magnification, Pap stain; b) biopsy, ×200 magnification, H and E stain}
- Leydig cell hyperplasia. a) In the TP most of the Leydig cells show disruption of their granular cytoplasm (×200 magnification, Pap stain). b) In the corresponding tissue core biopsy, increased numbers of Leydig cells, with abundant eosinophilic cytoplasm, can be seen packing the interstitium between tubules (×200 magnification, H and E stain)
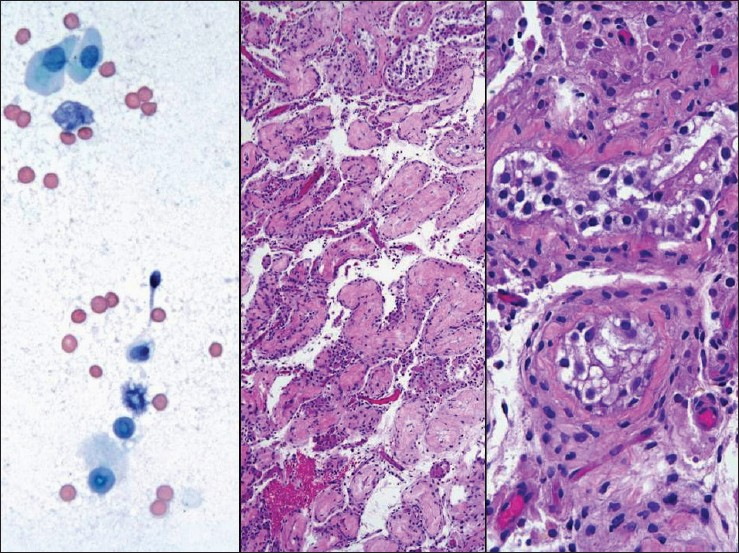
- The touch imprint specimen from this 35-year-old-man shows a rare sperm (left panel; ×600 magnification, Pap stain), whereas, the core biopsy shows sclerotic tubules (middle panel; ×200 magnification, H and E stain) with no active spermatogenesis (right panel; ×400 magnification, H and E stain)
Histopathological findings
Biopsy diagnoses were as follows: Absent spermatogenesis in 19 cases (49%), of which six (32%) were due to Sertoli-cell-only syndrome, 10 cases (29%) due to maturation arrest, hypospermatogenesis in four cases (11%), and complete spermatogenesis in six cases (15%). Although focal atypical cells were initially noted in four of the biopsies, none of these cases showed positive immunoreactivity for OCT-3/4 or C-kit to support intratubular germ cell neoplasia. No cases showed infection or malignancy.
CONCLUSION
In this study, we report our six-year experience with using touch preparations of testicular biopsies in the pathological evaluation of male infertility in 39 patients. Our data demonstrate that TP cytopathology of the testes in our experience has had excellent correlation with both normal testicular biopsies and those showing pathological spermatogenesis. TP has been particularly helpful as an adjunct to the biopsy, because of its ability to easily evaluate all stages of spermatogenesis. This corroborates the findings of other investigators, who have similarly reported that the use of testicular touch print cytology improves the diagnostic power when performed in combination with the routine histological examination of testicular tissue.[24–27]
Even though the value of adjunctive testicular TP cytology was reported by other investigators, these publications were primarily clinically oriented. Also, in these prior studies, the cytology material was stained with H and E and the authors published very few cytology images. Our study has focused mainly on the cytomorphology in a series of testicular TP cases, with histological follow-up, and has shown a similar diagnostic benefit as reported by others when performing a core biopsy with TP. In one case of our study, the TP specimen provided an added benefit of identifying a rare sperm not seen in the corresponding core biopsies, which was clinically helpful in the management of this patient with infertility.
Fine needle aspiration of the testes in azoospermia has several advantages, including the fact that this is a rapid, reliable, and minimally invasive method.[28] In some cases, FNA has proved to be more sensitive in detecting full or advanced maturation of spermatogenesis.[28] Moreover, it is believed that FNA provides more representative sampling than a tissue biopsy.[12] Hence, testicular FNA has proven to be reliable at differentiating obstructive from non-obstructive azoospermia.[9] Although a testicular tissue core biopsy is more invasive, examination of the procured tissue provides additional information on the histopathology (e.g., seminiferous tubule hyalinization due to ischemia or remote infection) and may provide adequate material for immunohistochemistry if needed (e.g., to confirm intratubular germ cell neoplasia). As alluded to before, concomitant testicular TP cytology with such core biopsies provides additional value because of its ability to differentiate between late spermatids and mature spermatozoa, as well as recognize other spermatogenic elements.[24] TP is, however, subject to air drying artifact and if performed too vigorously it is possible that sperm may be lost from the tissue specimens. Finally, computer-assisted image analysis of the air dried testicular touch imprints may be used, if available, to effectively quantify spermatogenesis.[29]
In summary, we conclude that testicular TP is a useful addition to the infertility workup in males, and recommend that these cytology specimens be prepared as a companion to testis surgical biopsy. Compared to prior publications, this article describes the testicular TP cytomorphology related to spermatogenesis. Cytologists should be familiar with testicular cytology and its interpretation, as they may be called upon to identify and evaluate spermatogenesis in cytology specimens submitted from men with infertility.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The authors declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Each author acknowledges that this final version has been read and approved. All authors qualify for authorship, as defined by ICMJE http://www.icmje.org/#author. Each author has participated sufficiently in the study and takes public responsibility for appropriate portions of the content of this article.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from our Institutional Review Board (IRB).
EDITORIAL / PEER-REVIEW STATEMENT
To ensure integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and reviewers are blinded for authors)through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2011/8/1/24/91244.
REFERENCES
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Male Reproduction and Urology. Evaluation of the azoospermic male. Fertil Steril. 2008;90(5 Suppl):S74-7.
- [Google Scholar]
- A practical approach to testicular biopsy interpretation for male infertility. Arch Pathol Lab Med. 2010;134:1197-204.
- [Google Scholar]
- Modern Cytopathology. Philadelphia: Churchill Livingstone; 2004. p. :723-4.
- Percutaneous testis biopsy: an alternative to open testicular biopsy in the evaluation of the subfertile man. J Urol. 1996;156:1647-51.
- [Google Scholar]
- Assessment of testicular cytology by fine needle aspiration as a diagnostic parameter in the evaluation of the oligospermic subject. Fertil Steril. 1992;58:1028-33.
- [Google Scholar]
- Diagnostic role of testicular fine needle aspiration biopsy in male infertility. Acta Cytol. 1997;41:1705-8.
- [Google Scholar]
- Testicular needle aspiration as an alternative to biopsy for the assessment of spermatogenesis. Hum Reprod. 1997;12:1483-7.
- [Google Scholar]
- The role of fine-needle aspiration cytology of the testis in the diagnostic evaluation of infertility. BJU Int. 1999;84:485-8.
- [Google Scholar]
- Fine needle aspiration cytology compared with open biopsy histology for the diagnosis of azoospermia. J Obstet Gynaecol. 2002;22:527-31.
- [Google Scholar]
- Testicular fine needle aspiration in evaluation of male infertility. JNMA J Nepal Med Assoc. 2009;48:78-84.
- [Google Scholar]
- Fine needle aspiration of the testis. Correlation between cytology and histology. Acta Cytol. 1999;43:991-8.
- [Google Scholar]
- Comparison of fine-needle aspiration and open biopsy of testis in sperm retrieval and histopathologic diagnosis. Andrologia. 2003;35:121-5.
- [Google Scholar]
- Testicular fine-needle aspiration in infertile men: correlation of cytologic pattern with biopsy histology. Am J Surg Pathol. 2001;25:71-9.
- [Google Scholar]
- Fine needle aspiration cytology of the testis as the first-line diagnostic modality in azoospermia: a comparative study of cytology and histology. Cytopathology. 2008;19:363-8.
- [Google Scholar]
- Fine needle aspiration of the testis and correlation with testicular open biopsy. Acta Cytol. 1993;37:67-72.
- [Google Scholar]
- The value of testicular ‘mapping’ in men with non-obstructive azoospermia. Asian J Androl. 2011;13:225-30.
- [Google Scholar]
- Heterogeneity of the testis, an important clinical fact for infertile azoospermic men undergoing fine needle aspiration of the testis. Mod Pathol. 2011;24(suppl 1):80A-1.
- [Google Scholar]
- Correlation of cell counts and indices in testicular FNAC with histology in male infertility. Acta Cytol. 1999;43:617-23.
- [Google Scholar]
- Clinical value of cell counts and indices in testicular fine needle aspiration cytology in primary infertility: diagnostic performance compared with histology. Anal Quant Cytol Histol. 2006;28:331-6.
- [Google Scholar]
- Testicular fine needle aspiration cytology in azoospermic males. Nepal Med Coll J. 2009;11:88-91.
- [Google Scholar]
- Assessment of testicular cytology by fine-needle aspiration and the imprint technique: are they reliable diagnostic modalities? Br J Urol. 1997;79:445-8.
- [Google Scholar]
- A comparative study of three techniques for the analysis of sperm recovery: touch-print cytology, wet preparation, and testicular histopathology. J Assist Reprod Genet. 2001;18:357-63.
- [Google Scholar]
- Histopathological and cytopathological correlations of percutaneous testis biopsy and open testis biopsy in infertile men. J Urol. 1995;153:1151-5.
- [Google Scholar]
- Fine needle aspiration biopsy of azoospermic testes. Could it replace histologic biopsy? Acta Cytol. 2000;44:967-75.
- [Google Scholar]
- Image analysis assessment of testicular touch preparation cytologies effectively quantifies human spermatogenesis. J Urol. 1998;160:1334-6.
- [Google Scholar]








