Translate this page into:
Tubular variant of mammary adenomyoepithelioma: Diagnostic challenges and cytomorphological correlation in two cases
*Corresponding author
-
Received: ,
Accepted: ,
This is an open access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 License, which allows others to remix, tweak, and build upon the work non-commercially, as long as the author is credited and the new creations are licensed under the identical terms.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The authors describe the cytomorphologic features of two cases of tubular variant of adenomyoepithelioma of the breast that were first examined by fine-needle aspiration cytology (FNAC) and diagnosed as fibroadenoma. On retrospective review of the cytology, subtle features such as less cohesive epithelial clusters, intimate association of clusters of stromal cells with epithelial elements, a dominant population of plump-epithelioid naked (myoepithelial) cells, and occasional cells with intranuclear inclusions, were noted. Thus, these lesions can be diagnostically challenging and cannot be conclusively differentiated from either fibroadenoma or tubular adenoma cytologically and the pathologist may only be able to give a differential on FNAC. Recognition of the biphasic nature and the characteristic overall architecture of the tumors in combination with immunohistochemistry are essential to establish the correct diagnosis on biopsy. Although most tumors have a benign clinical course, rare instances of local recurrence, malignant transformation, and distant metastases have been reported. A complete excision with adequate margins would lower the chance of local recurrence.
Keywords
Adenomyoepithelioma
breast
fibroadenoma
fine-needle aspiration cytology
tubular adenoma
tubular variant
INTRODUCTION
Adenomyoepithelioma (AME) of the breast is a relatively rare, benign tumor characterized by a biphasic proliferation of epithelial and myoepithelial cells. Fine-needle aspiration cytology (FNAC) findings in AMEs have been described in only a few reports, possibly due to rarity of the lesion. We report two cases of breast lumps in young women who were initially diagnosed as fibroadenoma on FNAC. Incomplete excision of this lesion is associated with a greater risk of recurrence as compared to fibroadenomas.
CASE REPORT
Two women aged 28 years and 42 years presented with discrete, firm, mobile breast lumps, measuring 1.5 cm and 2.0 cm in maximum dimensions, respectively. Both were subjected to FNAC. Cytologically, both cases showed moderate cellularity with flat clusters of a biphasic cell population. Although myoepithelial cells were predominant, there were numerous benign epithelial cells too, including bare nuclei and occasional fibrous stromal elements. Both cases were diagnosed as fibroadenoma and underwent lumpectomy. The lumpectomy specimen revealed well-circumscribed, firm, globular mass with gray-white, solid appearance on cut section [Figure 1a]. On H and E sections, both cases showed an encapsulated and highly cellular lesion consisting of tubules. The tubules were lined by an attenuated lining of ductal epithelial cells with bland nuclei and small nucleoli, surrounded by a prominent layer of clear cells with low N:C ratio and hyperchromatic nuclei. No atypical mitosis or necrosis was seen [Figure 1b]. Immunohistochemistry (IHC) revealed epithelial cells positive for cytokeratin (CK) [Figure 2a] and epithelial membrane antigen (EMA) [Figure 2b], while the myoepithelial cells were clearly demonstrated by positivity with calponin [Figure 2c] and p63 [Figure 2d].
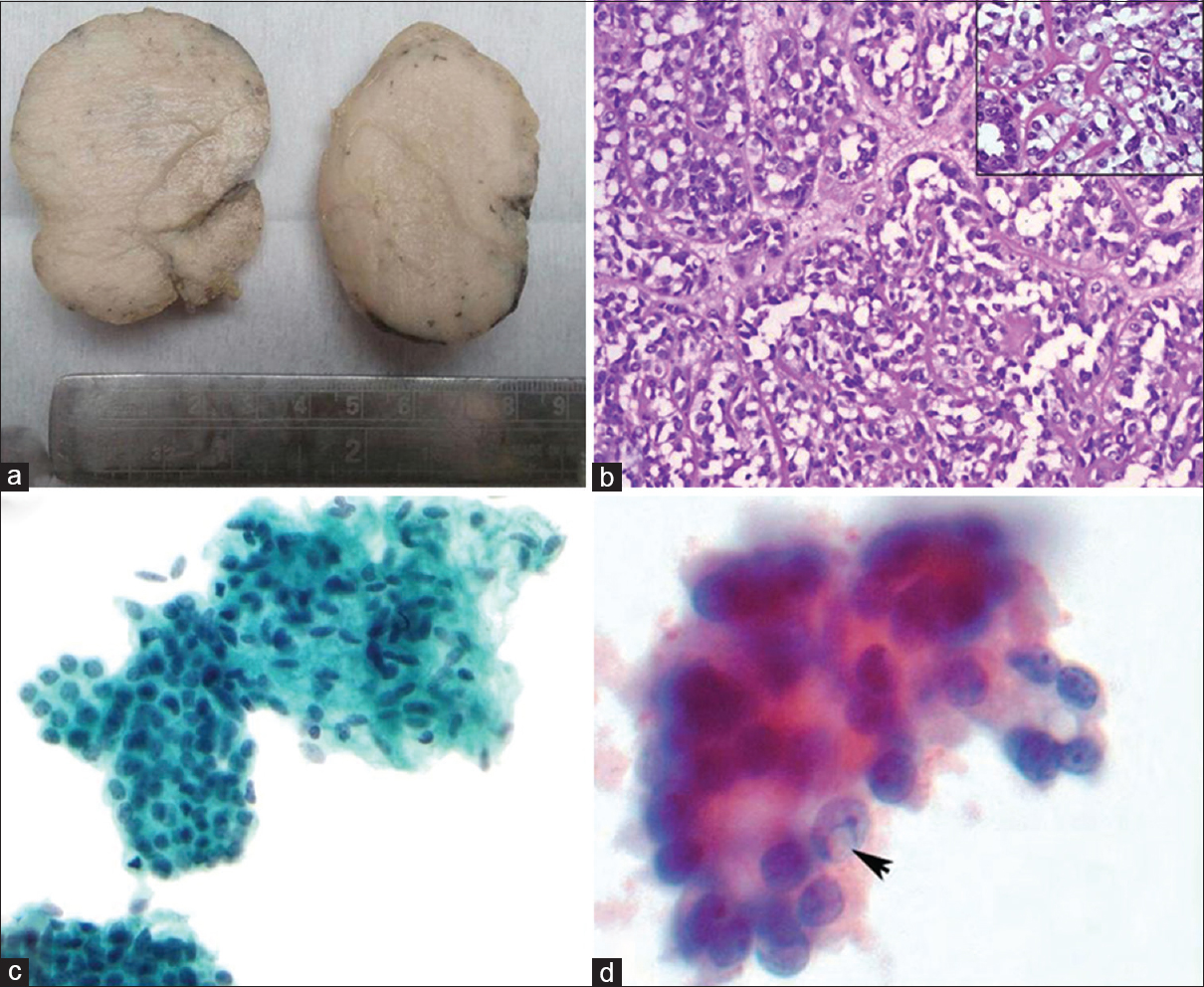
- (a) Well-circumscribed mass with gray-white homogenous appearance, (b) tubular variant of adenomyoepithelioma - dark staining cuboidal epithelial cells and outer prominent myoepithelial cells with clear cytoplasm (H and E, ×100) (inset – higher magnification shows bland nature of both epithelial and myoepithelial cells (H and E, ×200), (c) Fine-needle aspiration cytology smear with bland round epithelial cells closely admixed with abundant oval-spindled myoepithelial cells. No atypia is present (Pap stain, ×400) (d) Intranuclear inclusion (arrow) in few myoepithelial cells (Pap oil immersion)
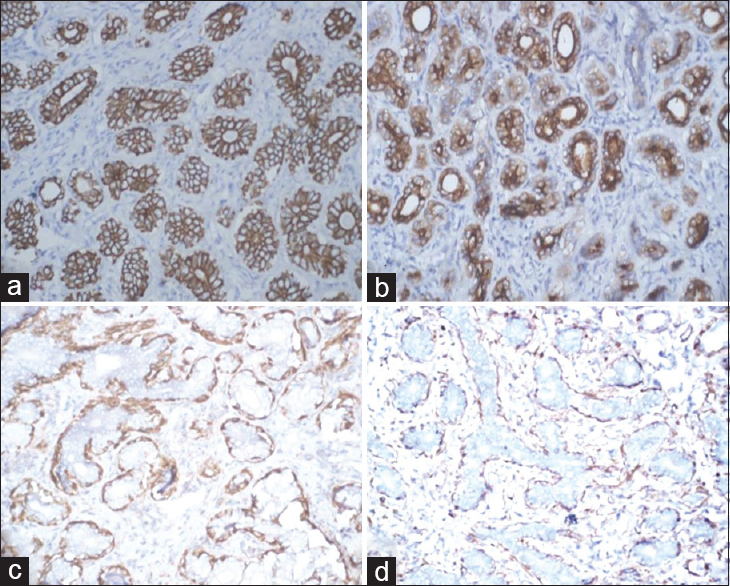
- (a) Cytokeratin positivity of ductal epithelial cells (cytokeratin, ×100), (b) epithelial membrane antigen positive epithelial cells (EMA, ×100), (c) Calponin positivity in myoepithelial cells (Calponin, ×100), (d) p63 positive staining in myoepithelial cells (p63, ×100)
On careful review of the cytology slides in both cases, it was found that the epithelial clusters were less cohesive, there was intimate association of clusters of stromal cells with the epithelial elements, sometimes encircling the latter [Figure 1c], and there was a predominant population of plump-epithelioid naked (myoepithelial) cells which showed occasional intranuclear inclusions [Figure 1d].
DISCUSSION
AME, a biphasic neoplastic proliferation of luminal and myoepithelial cells, was first described by Hamperl in 1970.[1] As Hamperl had noted, this tumor may display a heterogeneous pattern because of the variable proliferation of epithelial and myoepithelial cells. AME usually presents as a single circumscribed mammary nodule. FNAC findings in AMEs have been described in only a few reports.[234] A retrospective evaluation of cytologic findings from twelve patients with histologically proven benign AMEs at Memorial Sloan-Kettering Cancer Center showed that none of the patients had been diagnosed originally as AME and that cytologic diagnosis of this neoplasm can be very challenging.[2]
Cytologically, AMEs can be indistinguishable from fibroadenomas and tubular adenomas since all of them share common features of a bland bimodal cell population, admixed with stromal elements and with a predominance of myoepithelial cells. Some authors have studied the subtle distinguishing characters and attempted to differentiate the two cytologically.[5] However, the presence of close association of the stromal component and the epithelial component is not specific for AME, and in most instances, characteristic intranuclear inclusions may be appreciated only on careful review.[67] This was the case in our patients too.
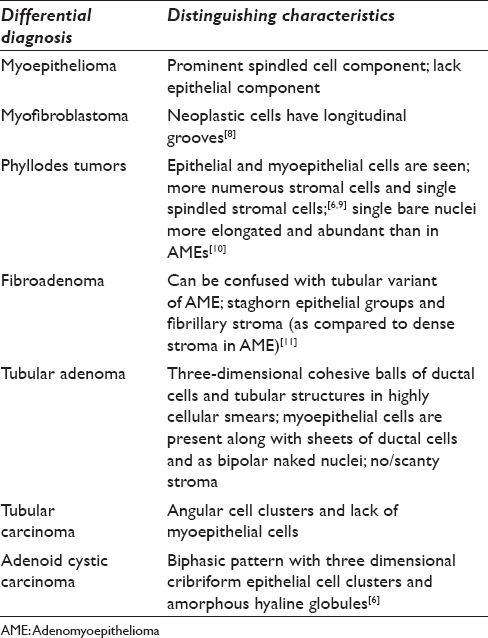
Cytology of AMEs can sometimes show atypical features in myoepithelial cells, and thus, cytopathologists must be familiar with this entity to avoid a misdiagnosis of carcinoma.[7] Benign and malignant lesions of the breast that fall into the differential diagnosis of AME and their distinguishing characteristics are summarized in Table 1.
On biopsy, AME shows a biphasic pattern of tubules lined by cuboidal or columnar-shaped epithelial cells surrounded by clear myoepithelial cells. A spectrum of histologic patterns, however, has been observed among various examples of these tumors and even in different areas of individual tumors. Tavassoli described three variants of AME: tubular, spindle cell, and lobular.[12] The tubular pattern, which is characterized by a balanced proliferation of rounded tubules as well as unusually prominent and hyperplastic myoepithelial cells, is the most common and most likely to be misinterpreted as fibroadenoma or tubular adenoma as was the case in our patients. On biopsy, accurate identification of myoepithelial cells and absence of atypical features, such as pronounced nuclear pleomorphism, mitotic activity, necrosis, invasive growth, and overgrowth of one of the two components of the lesion, is crucial to avoid misinterpretation of this lesion as tubular, myoepithelial, or micropapillary carcinoma.
The interplay between epithelial and myoepithelial cell elements is highlighted by immunohistochemical staining with antibodies specific for these two components. The epithelial component stains positive with low molecular weight CK and EMA, while the myoepithelial cell component is highlighted by smooth muscle actin, S-100 protein, calponin, p63, and smooth muscle myosin heavy chains (SMMHC).[234] Although calponin is highly sensitive for detecting myoepithelial cells, SMMHC is more specific and easier to interpret because of its low cross-reactivity with myofibroblasts.[1314] Malignant AMEs are usually characterized by cellular pleomorphism, necrosis, high mitotic activity, and invasion of the surrounding tissue.[9]
CONCLUSION
Benign AME of breast is a rare lesion that mimics fibroadenomas clinically, radiologically, and cytologically. The presence of intranuclear inclusions on cytology can be a useful identification feature but is by no means pathognomonic. Thus, cytologic diagnosis of this entity is extremely difficult and can only be suggested as a differential of fibroadenoma. Correct diagnosis is usually possible only on excisional biopsy and confirmed by demonstrating the biphasic nature of the tumor by IHC. Because of the morphologic heterogeneity of this tumor, thorough sampling of the tumor is essential to rule out atypia or infiltrative margins. This is vital as incomplete excision may lead to late recurrences. Loose et al. described a spectrum of biologic behavior in six cases of AME, two of which were malignant as they had high mitotic rates (11–14/10 high-power fields) diffusely throughout the tumors and foci of cytologically malignant cells, one of these metastasized to the lung and brain.[15]
In summary, we have tried to address the cytological features of tubular variant of AME describing two cases, both were diagnosed as fibroadenomas on prior FNA. In particular, we wish to emphasize that cytological diagnosis of this entity is extremely difficult, and that at best, the pathologist can give a differential diagnosis of this entity. Moreover, a “mistaken” diagnosis of fibroadenoma on FNAC in such cases is of no clinical significance since the two lesions have almost similar clinical behavior, other than the fact that it is prudent to have these cases on long-term follow-up after wide local excision as opposed to fibroadenomas.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors have contributed significantly and agree with the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
As this is a case report without identifiers, our institution does not require approval from the Institutional Review Board (IRB). documentation in this respect.
LIST OF ABBREVIATIONS (In alphabetic order)
AME - Adenomyoepithelioma
CK - Cytokeratin
EMA - Epithelial membrane antigen
FNAC - Fine-needle aspiration cytology
IHC - Immunohistochemistry
SMMHC - Smooth muscle myosin heavy chains
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
REFERENCES
- The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970;53:161-220.
- [Google Scholar]
- Fine-needle aspiration cytology of mammary adenomyoepithelioma: A study of 12 patients. Cancer. 2006;108:250-6.
- [Google Scholar]
- Fine-needle aspiration biopsy of benign adenomyoepithelioma of the breast: Radiologic and pathologic correlation in four cases. Diagn Cytopathol. 2007;35:690-4.
- [Google Scholar]
- Adenomyoepithelioma of the breast: A potential diagnostic pitfall on fine needle aspiration cytology. Indian J Pathol Microbiol. 2004;47:243-5.
- [Google Scholar]
- Fine needle aspiration cytology of adenomyoepitelioma of the breast: Comparison with typical fibroadenoma. Korean J Cytopathol. 1998;9:105-10.
- [Google Scholar]
- Adenomyoepithelioma of the breast. A review of three cases with reappraisal of the fine needle aspiration biopsy findings. Acta Cytol. 2002;46:317-24.
- [Google Scholar]
- Fine-needle aspiration biopsy of breast adenomyoepithelioma: A potential false positive pitfall and presence of intranuclear cytoplasmic inclusions. Diagn Cytopathol. 2012;40:1005-9.
- [Google Scholar]
- Fine needle aspiration of myofibroblastoma of the breast in a man. A report of two cases. Acta Cytol. 1992;36:194-8.
- [Google Scholar]
- Fine-needle aspiration cytology of benign and malignant adenomyoepithelioma: Report of two cases. Diagn Cytopathol. 2002;26:29-34.
- [Google Scholar]
- Adenomyoepithelioma of the breast: Report of a case initially examined by fine-needle aspiration. Diagn Cytopathol. 1993;9:547-50.
- [Google Scholar]
- Cytologic features of the tubular variant of breast adenomyoepithelioma. Acta Cytol. 2001;45:1090-2.
- [Google Scholar]
- Myoepithelial lesions of the breast. Myoepitheliosis, adenomyoepithelioma, and myoepithelial carcinoma. Am J Surg Pathol. 1991;15:554-68.
- [Google Scholar]
- Best practices in diagnostic immunohistochemistry: Myoepithelial markers in breast pathology. Arch Pathol Lab Med. 2011;135:422-9.
- [Google Scholar]
- Immunohistochemical distinction of invasive from noninvasive breast lesions: A comparative study of p63 versus calponin and smooth muscle myosin heavy chain. Am J Surg Pathol. 2003;27:82-90.
- [Google Scholar]
- Adenomyoepithelioma of the breast. A spectrum of biologic behavior. Am J Surg Pathol. 1992;16:868-76.
- [Google Scholar]








