Translate this page into:
Two smalls in one: Coincident small cell carcinoma and small lymphocytic lymphoma in a lymph node diagnosed by fine-needle aspiration biopsy
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background:
B-cell chronic lymphocytic leukemia / small lymphocytic lymphoma (CLL / SLL) is one of the most common lymphoproliferative disorders in western countries. Patients with SLL / CLL are at increased risk of site-specific secondary cancers. We present a unique case of a 71-year-old male, with a history of SLL / CLL, who presented with pulmonary symptoms and a mediastinal mass. Fine needle aspiration (FNA) of the mediastinal lymph node revealed synchronous SLL / CLL and small cell carcinoma (SCC).
Materials and Methods:
The patient underwent a computed tomography (CT) scan of the chest and endobronchial ultrasound-guided transbronchial fine needle aspiration of the mediastinal lymph node (4R). The sample was submitted for cytopathology, immunohistochemical stains, and flow cytometry evaluation.
Results:
Fine needle aspiration of the mediastinal lymph node revealed neoplastic cells, in clusters and singly, with cytological features suggestive of small cell carcinoma. The immunohistochemistry results confirmed this diagnosis. Small-to-medium, mature-appearing lymphocytes were also present in the background. Flow cytometry analysis revealed that these lymphocytes possessed an immunophenotype consistent with CLL / SLL.
Conclusions:
This case illustrates the importance of a pathologist's awareness of the possibility of concurrent lymphoma and metastatic carcinoma in a lymph node. When evaluating lymph nodes, pathologists must strive to identify both foreign cells and subtle lymphoid changes. As demonstrated by our case, ancillary techniques (such as immunohistochemistry and flow cytometry) can be critical to making a complete and accurate diagnosis. The diagnosis of small cell carcinoma in the enlarged lymph node, primarily harboring CLL / SLL, is of critical importance for decision-making and treatment purposes, in addition to having a significant adverse impact on the overall survival.
Keywords
Small cell carcinoma
lymphoma
FNA
INTRODUCTION
It is well-established that certain individuals are at increased risk for multiple primary malignant tumors (MPMT).[1] Hematopoietic neoplasms have been known to be frequent components of MPMT.[2] Low-grade lymphoproliferative disorders such as chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and lymphoma arising from mucosa associated lymphoid tissue (MALT) occur more often than high-grade lymphomas in MPMT.[34] Among the non-hematopoietic component of MPMT melanoma, squamous cell carcinoma and adenocarcinoma have been found to be the most frequent.[56] Small cell carcinoma of the lung, metastatic to a lymph node that harbors CLL/SLL, is very rarely documented in medical literature. We report a case of CLL/SLL and metastatic small cell carcinoma of the lung, occurring synchronously in the same mediastinal lymph node.
Clinical Summary
The patient was a 71-year-old Caucasian man, with a three-year-history of CLL/SLL. He presented with a four-month history of progressive dyspnea, exertional chest pain, decreased appetite, and a 15-pound weight loss. A CT scan showed a large 8 cm mass in the right hilum [Figure 1]. The mass was encroaching on the right upper lobe bronchus as well as on the middle and lower bronchi. Massively enlarged lymph nodes were noted in the subcarinal and paratracheal locations, left hilum, and left supraclavicular area. These findings suggested a differential diagnosis of transformation of his CLL/SLL to large cell lymphoma versus primary bronchogenic carcinoma, with lymph node metastasis. The patient was referred to the Pulmonary Clinic for bronchoscopy.
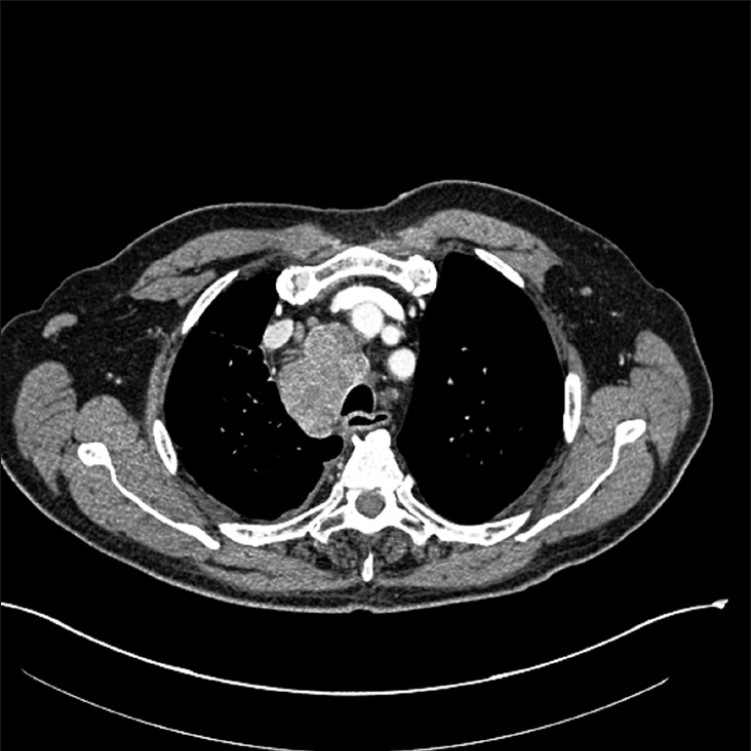
- CT image of chest showing right hilar mass and enlarged mediastinal lymph nodes
MATERIALS AND METHODS
Sample procurement and processing
Endobronchial ultrasound-guided transbronchial fine needle aspiration was performed on a mediastinal lymph node (4R). The sample was immediately smeared by a cytotechnologist and the needle was rinsed into an alcohol-based fixative solution. The smears were evaluated on-site by the cytotechnologist, to determine sample adequacy. After the sample was deemed adequate, three additional passes were obtained, placed into the RPMI medium, and submitted for flow cytometry evaluation. Alcohol-fixed cytology slides were stained with the Papanicolaou stain, and the air-dried slides were stained with the Diff Quik stain. The cell block was prepared from the needle rinse and stained with hematoxylin and eosion (H and E).
Immunohistochemistry
The cell block tissue sections were deparaffinized in xylene, rehydrated in descending grades of ethanol (100 – 70%) and subjected to heat-induced epitope retrieval using a microwave oven (GE Profile Sensor), for 15 minutes, at 100°C, in 10 mM citrate buffer (pH 6). Endogenous peroxidase activity was blocked and sections were stained with a labeled streptavidin-biotin peroxidase detection system, using a Dako Automated Immunostainer (Dako Corporation, Carpinteria, CA, USA). Sections were immunostained with cytokeratins (AE1/AE3 and CAM 5.2), synaptophysin, chromogranin, TTF-1, CD45, CD3, CD20, and CD79a. All antibodies used for immunohistochemical staining were obtained from Dako (Carpinteria, CA, USA).
Flow cytometry
A portion of the fresh FNA from the lymph node was submitted to routine flow cytometric immunophenotyping. Briefly, the lymph node was processed and a single cell suspension was incubated with a panel of antibodies specific for CD5, CD10, CD19, CD20, CD23, CD38, CD45, FMC7, and kappa and lambda light chains, and labeled with different flurochromes. The samples were then analyzed using a conventional five-color FACSCalibur flow cytometer with CellQuest software (Becton Dickinson, San Jose, CA).
RESULTS
Hematological Results
A complete blood count (CBC) performed at the time of biopsy revealed a white blood cell count of 13.2 × 0.109, hemoglobin value of 11.6 g/dl, and platelet count of 186 × 106/L. The white blood cell differential showed 22% neutrophils, 73% lymphocytes, 3% monocytes, and 2% eosinophils. In addition, the peripheral blood smear showed a mild lymphocytosis, with occasional smudge cells. The above-mentioned features are consistent with chronic lymphocytic leukemia (CLL).
Cytology Results
The smears were cellular and showed neoplastic cells in clusters and singly. These cells were two to four times the size of the lymphocytes with a high nuclear-to-cytoplasmic ratio and scant cytoplasm. The nuclei had fine granular chromatin (salt and pepper), without nucleoli. Marked nuclear fragility of the malignant cells was manifested by a nuclear ‘molding’ and ‘smearing’ artifact. The background of the lymphocytes and the presence of carbon-laden macrophages confirmed that the specimen was indeed obtained from a lymph node. The lymphocytes were small-to-medium in size and had slightly irregular nuclear contours, moderately dispersed chromatin, and inconspicuous nucleoli [Figure 2].
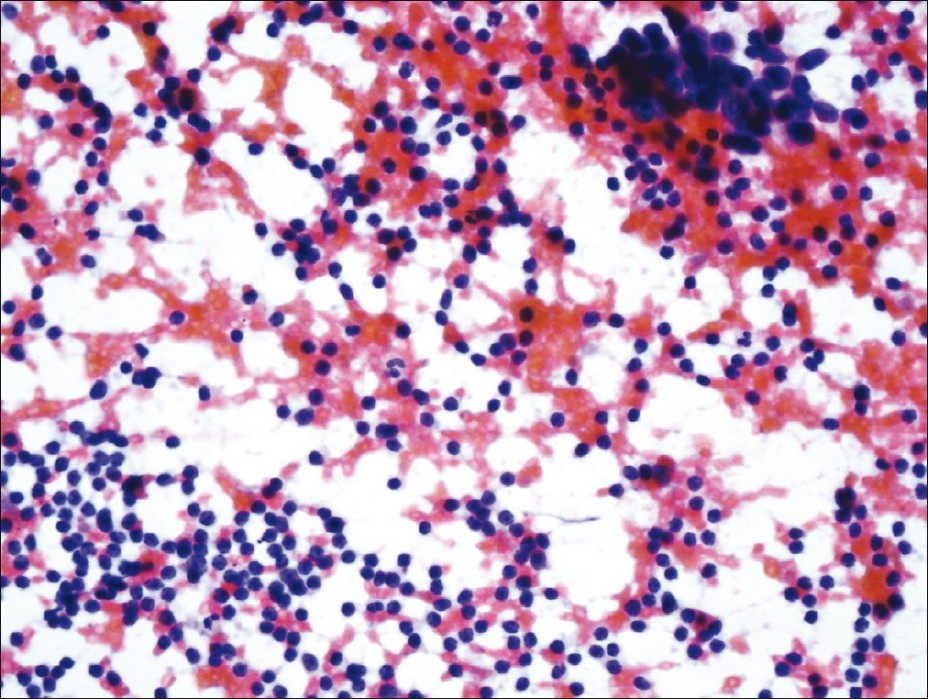
- FNA smear (PAP stain) showing malignant cells in clusters and numerous lymphocytes in the background
Cell block tissue sections showed clusters of malignant cells and lymphocytes [Figure 3]. The morphology of the malignant cells suggested a differential diagnosis of small cell carcinoma versus large cell lymphoma. The tumor cells showed positivity for cytokeratins (AE1/AE3 and CAM 5.2), synaptophysin, chromogranin, and TTF-1. They were negative for CD45, CD3, CD20, and CD79a, [Figures 4a and 4b]. The few background lymphocytes stained positive with CD45. Based on the cytomorphology and immunohistochemistry results, the diagnosis of small cell carcinoma was rendered. Although the background lymphocytes showed features suspicious for CLL/SLL, a definitive diagnosis could not be made without further immunophenotyping.
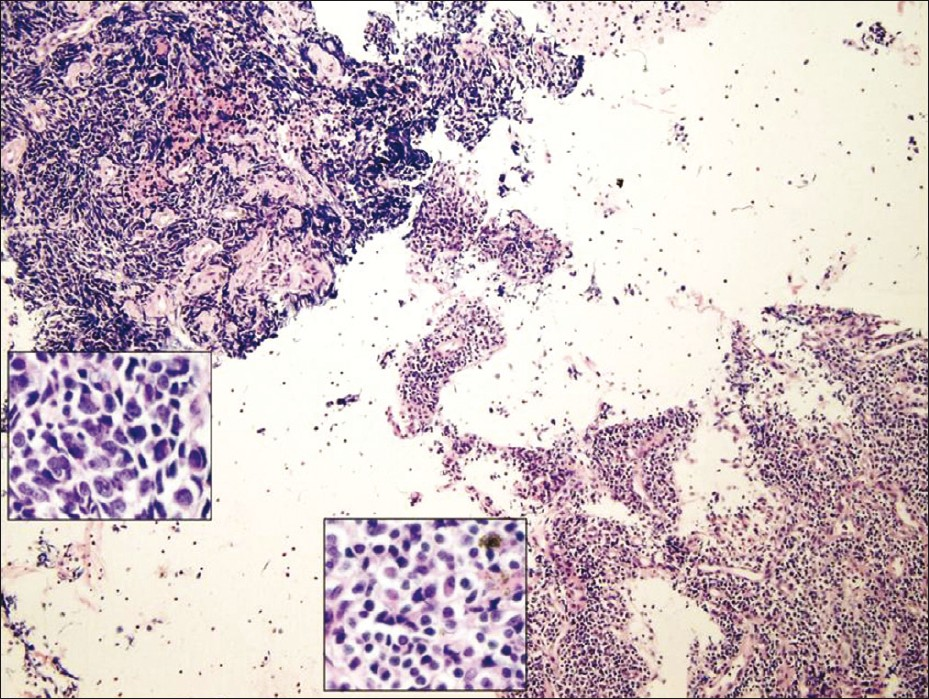
- Cell block tissue section showing small cell carcinoma (upper left corner) and lymphocytes (lower right corner)
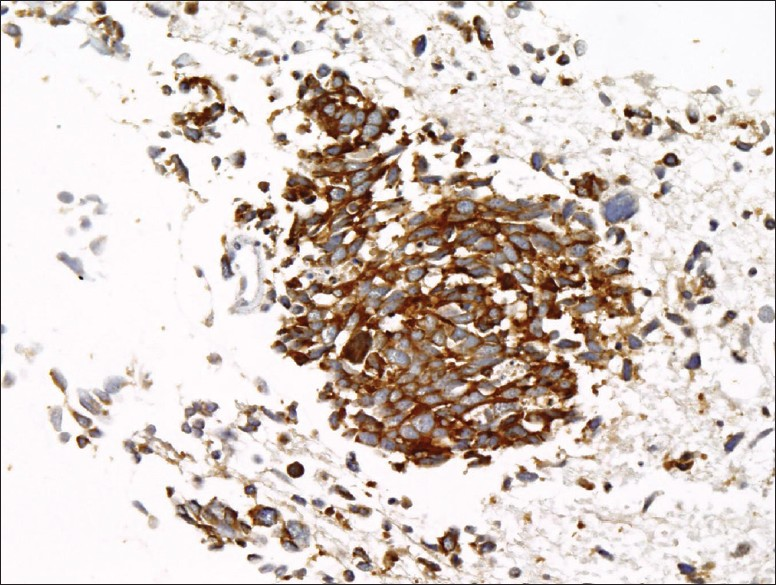
- Cell block tissue section showing small cell carcinoma positive for synaptophysin
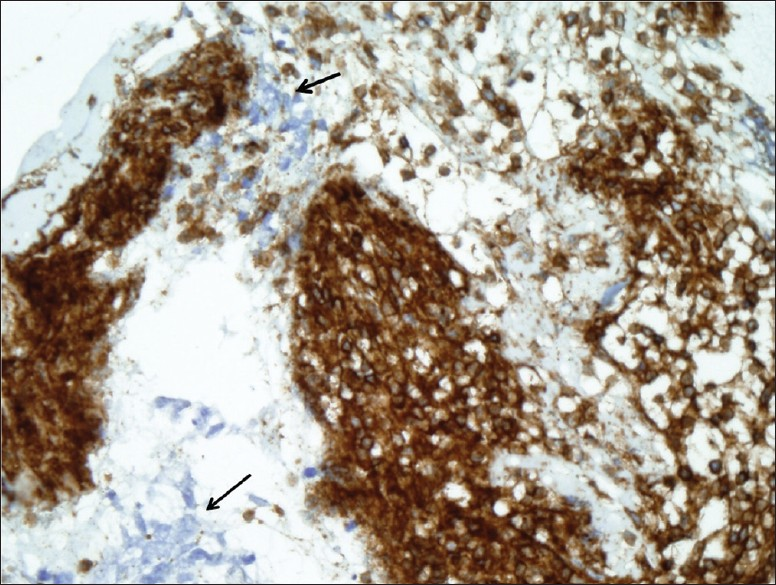
- Cell block tissue section showing small cell carcinoma negative for CD45 (arrows) and numerous lymphocytes positive for CD45
Flow cytometry results
Cytospin preparation showed numerous mature appearing lymphocytes with clumped chromatin and some nuclear membrane irregularities. A second larger population of cells, morphologically consistent with small cell carcinoma, was also present. The flow cytometry studies revealed a distinctly abnormal B-cell population (78% of the analyzed cells) that co-expressed CD19 and CD5. CD20 (dim) and CD23 cells were also expressed. There was no significant expression of CD10, FMC-7 or CD138 antigens. No definite immunoglobulin light chain restriction was detected. These flow cytometry findings were consistent with CLL/SLL.
The possibility that the abnormal lymphocytes reflected peripheral blood contamination should be considered. However, a significant amount of lymphocytes (some noted in aggregates), virtual absence of neutrophils, and the presence of carbon-laden macrophages and metastatic SCC, strongly favored that the abnormal lymphocytes were truly from the lymph node and were not on account of peripheral blood contamination. Based on the cytomorphological, immunohistochemical, and flow cytometry results, the final diagnosis rendered in this case was pulmonary small cell carcinoma (SCC) metastatic to a lymph node, with underlying CLL/SLL.
DISCUSSION
In this report, we describe a unique case of synchronous SLL/CLL and metastatic pulmonary SCC, diagnosed by fine-needle aspiration. Although rare, carcinomas metastatic to the lymphomatous lymph nodes have been reported.[7] In most cases, hematopoietic malignancy has been a low-grade lymphoma, such as SLL/CLL, and the carcinoma has been squamous cell carcinoma.[89] Adenocarcinoma, melanoma, merkel cell carcinoma, and glioblastoma have also been reported.[10] According to the National Cancer Institute, there is an overall increase in the incidence of second cancers in SLL/CLL patients of about 8.7%.[11]
Royle et al., reported that in Australia the risk of any second coincidental cancer in patients with CLL/SLL was more than double that of the general population.[3] A large study by Parekh et al., showed that approximately 2% of the patients with CLL/SLL developed lung carcinomas.[12] Another retrospective study by Schollkopf et al, in Denmark, involved 12,373 patients with CLL/SLL. It showed a statistically significant increase in the relative risk for lung cancer, particularly adenocarcinoma and squamous cell carcinoma, in these patients.[13] Given the prolonged survival of patients with CLL, it was prudent for physicians to be alert to the possibility of second cancers, particularly when new symptoms or physical findings arose, as the diagnosis of a secondary malignancy could change the patient's prognosis, require alteration of clinical management or affect patient eligibility for entry into clinical trials.
It is also possible that an initial diagnosis of CLL/SLL may be made when evaluating lymph nodes for metastatic carcinoma. Such a diagnosis presents several challenges. The presence of metastatic carcinoma may distort the lymph node architecture or replace most of the node. Numerous reactive germinal centers in the lymph nodes, induced by metastatic carcinoma, could easily lead to the mistaken conclusion that the changes in the lymph nodes are reactive. Additionally, certain types of lymphoma, such as CLL/SLL, are very difficult to diagnose based solely on the cytomorphological features. The diagnosis of SLL/CLL often relies on finding a small lymphoid population with the characteristic immunophenotype (co-expression of B-cell markers CD19, CD 79a with CD5, and CD23; negative for CD10 and FMC-7). Ancillary testing, particularly in flow cytometry analysis, is instrumental.
The etiology of multiple synchronous malignancies is not clear. Environmental factors, treatments (e.g., alkylating agent chemotherapy), immunological alterations, genetic susceptibilities, and other risk factors (e.g., viral infections, tobacco use) are likely to play varying roles in this phenomenon. Interestingly, certain organs, such as the gastrointestinal tract, skin, liver, and lungs, seem to have an increased predilection for synchronous epithelial and hematopoietic neoplasms. This predilection may reflect site-specific exposure to certain environmental factors that predispose to both cancer and lymphoma, such as, Helicobactor pylori infection, the Epstein–Barr virus (EBV), and the hepatitis C virus.[14–16]
In conclusion, we describe an unusual case of small cell carcinoma and CLL/SLL diagnosed on an FNA specimen. When evaluating lymph nodes for metastatic carcinoma, it is important to identify both foreign cells and subtle lymphoid changes. Ancillary studies, such as, immunohistochemistry, flow cytometry, and molecular analyses, are often essential for an accurate and complete diagnosis. In this case, the discovery of small cell carcinoma in the setting of concomitant CLL/SLL was critical for rational medical decision-making and implementation of appropriate treatment, in addition to having a significant adverse impact on the overall survival.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The author(s) declare that they have no competing interests.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from Institutional Review Board (IRB) of all the institutions associated with this study. All authors take responsibility to maintain relevant documentation in this respect.
AUTHORS’ CONTRIBUTIONS
AA: Has conceived the study, reviewed the cytology and immunohistochemistry stains, and has written the manuscript and given the final approval of the version to be published.
SD: Has evaluated the cytological sample and immunohistochemistry, reviewed the manuscript and literature, and drafted the manuscript.
CM: Has reviewed the flow cytometry, and reviewed and revised the manuscript.
EDITORIAL / PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through automatic online system.
Available FREE in open access from: http://www.cytojournal.com/text.asp?2012/9/1/5/93280
REFERENCES
- Occurrence of second primary malignancies in man-a second look. Cancer Treat Rev. 1985;12:77-94.
- [Google Scholar]
- Risk of subsequent solid tumors after non-Hodgkin's lymphoma: Effect of diagnostic age and time since diagnosis. J Clin Oncol. 2008;26:1850-7.
- [Google Scholar]
- Second cancer incidence and cancer mortality among chronic lymphocytic leukaemia patients: a population-based study. Br J Cancer. 2011;105:1076-81.
- [Google Scholar]
- Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol. 2010;28:4935-44.
- [Google Scholar]
- Synchronously diagnosed lymph nodal collision tumor of malignant melanoma and chonic lymphocytic leukemia/small lymphocytic lymphoma: case report. Diagn Pathol. 2007;2:34.
- [Google Scholar]
- Fine-needle aspiration diagnosis of squamous cell carcinoma in a lymph node involved with small lymphocytic lymphoma: case report and review of the literature. Diagn Cytopathol. 2009;37:48-50.
- [Google Scholar]
- Concomitant lymphoma and metastatic carcinoma in a lymph node: diagnosis by fine-needle aspiration biopsy in two cases. Diagn Cytopathol. 1996;17:287-91.
- [Google Scholar]
- Coexistence of non-Hodgkin's lymphoma and non-small cell lung carcinoma: diagnosis and treatment. Thorac Cardiovasc Surg. 2002;50:59-61.
- [Google Scholar]
- Merkel cell carcinoma and chronic lymphocytic leukemia (collision tumor) of the arm: A diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol. 2007;35:293-5.
- [Google Scholar]
- Second cancers in patients with chronic lymphocytic leukemia. J Natl Cancer Inst. 1992;84:1422-7.
- [Google Scholar]
- The clinical course of lung carcinoma in patients with chronic lymphocytic leukemia. Cancer. 1999;86:1720-3.
- [Google Scholar]
- Risk of second cancer after chronic lymphocytic leukemia. Int J Cancer. 2007;1:151-6.
- [Google Scholar]
- Synchronous gastric adenocarcinoma and MALT lymphoma in a patient with H.pylori infection. Could the two neoplasms share a common pathogenesis. Hepatogastroenterology. 2001;48:104-6.
- [Google Scholar]
- Hepatitis C infection and risk of malignant lymphoma. Int J Cancer. 2008;122:1885-90.
- [Google Scholar]
- Intrinsic and treatment-related immune alterations in chronic lymphocytic leukaemia and their impact for clinical practice. Expert Opin Pharmacother. 2008;9:1481-94.
- [Google Scholar]








