Translate this page into:
Updates in processing of anterior fat pad aspirate for amyloid (with video and sketches)

-
Received: ,
Accepted: ,
How to cite this article: Shidham VB. Updates in processing of anterior fat pad aspirate for amyloid (with video and sketches). CytoJournal 2020;17:15.
Abstract
Fat pad aspiration is a commonly used method for detecting amyloid in tissue. Amyloid is detected in the small blood vessels of the aspirated adipose tissue. Optimum evaluation of amyloid with electron microscopy requires at least 15 blood vessels in the fat pad aspirate. The presence of a significant proportion of adipocytes in the aspirate dilutes the fibrovascular portion. This may compromise the evaluation for amyloid with electron microscopy and in FFPE with proteomic studies by mass spectroscopy for confirmation of the amyloid subtype. This video article describes the updated protocol for processing the anterior fat pad aspirate. It demonstrates how to remove the interference of blood and fatty component in the fat pad aspirate performed by the previously reported procedure.
Keywords
Amyloid
FNA
Cell-block
Cell Blockistry
Congo Red stain
Electron microscopy
Mass spectroscopy
Laser microdissection
Polarizing microscopy
Anterior fat pad aspirate
INTRODUCTION
Fat pad aspiration has been a simple and minimally invasive method for the evaluation of systemic amyloidosis at a relatively lower cost with minimal morbidity.[1-3] The procedure for performing anterior fat pad aspiration was reported previously in detail as a video article.[1] In that article, the protocol suggested for processing of the aspirate for formalin-fixed paraffin- embedded FFPE and electron microscopy has shown some limitations. This article is to communicate the updates in processing of the fat pad aspirates so that the problems noted are resolved. This is critical for the best outcome with electron microscopic evaluation and preparation of FFPE for Congo red staining with polarizing microscopy with laser microdissection for mass spectrophotometric testing as indicated.[1-3]
A commonly used method for the detection of amyloid in tissue sections is Congo red stain with the application of polarizing microscopy to detect apple green birefringence in the walls of small blood vessels in the fibrovascular tissue fragments in fat pad aspirates. Similarly, for electron microscopy, the requirement of at least 15 blood vessels may be compromised by the predominance of fat in the glutaraldehyde fixed aspirate. The abundance of the fatty component diluting the fibrovascular portion may lead to frequent suboptimal outcomes with inadequate sample quantity for electron microscopy and the FFPE material required for proteomic analysis with mass spectroscopy to confirm the amyloid subtype.[3,4] This demands a proper approach to remove most of the adipocyte component and get more fibrovascular tissue with relative predominance of blood vessels.
DISCUSSION
The diagnosis of amyloidosis is based on tissue evaluation. It was dependent mostly on relatively invasive tissue biopsies such as from the kidney, liver, and/or heart with the potential for complications including hemorrhage.[2] Some relatively less invasive approaches such as rectal, gingival, and bone marrow biopsies have been practiced as well.[5-7] However, the introduction of abdominal fat pad aspiration was reported for the tissue diagnosis of systemic amyloidosis as a simple, minimally invasive, safe, and less expensive procedure.[8] This procedure was refined further as a video article with the step- by-step procedure along with the suggested protocol of how to process the fat pad aspirate.[1]
However, the fatty component in variable proportion may dilute and interfere with the evaluation of amyloid in the diagnostic fibrovascular component. This is especially problematic for the evaluation of microfragments with electron microscopy due to the need to get more than 15 blood vessels as recommended to be evaluated by electron microscopy.[2] If the fatty component is not removed, the number of small vessels required for the evaluation of amyloid in their walls may not be sufficient in tiny electron microscopy sections of fibroadipose tissue fragments. Congo red staining for the evaluation of anterior fat pad aspirates for amyloid by light microscopy and polarizing microscopy is relatively less sensitive, especially when a representative number of blood vessels is scant in proportion to the fatty component.[9] Similarly, FFPE sections required for laser microdissection in positive cases for mass spectrophotometry to subtype the amyloid for proper clinical management also need a greater number of vessels in fibrovascular tissue than the fatty component.[3]
This technical update reports the modifications in processing of anterior fat pad aspirate by removing most of the adipocyte component for the evaluation of amyloidosis as reported previously.[1] This is especially important in cases with early disease with scant amyloid. Based on our ongoing experience with the procedure,[1] a standardized protocol for processing FNA of anterior fat pad for amyloid as demonstrated in the video 1 (please visit the article in HTML to watch the video at https://dx.doi.org/10.25259/Cytojournal_31_2020) with the commentary in reference to the video counter [Table 1] is presented in this methodology article.
| Counter | Commentary with subtitle |
|---|---|
| 00.00–00.06 | e title slide |
| 00.07–00.14 | Arrange the cart surface for processing the aspirate by spreading waterproof sheet |
| 00.15–00.25 | Remove the needle and dispense a small drop of the aspirate on the glass slide for preparing direct smears |
| 00.25–00.35 | Disperse the aspirate in two nylon mesh tissue bags. One with smaller quantity for electron microscopy |
| 00.36–00.43 | e other bag for more remaining quantity for formalin-fixed paraffin-embedded block |
| 00.44–00.52 | Lay the nylon mesh bag with larger quantity of fat pad aspirate flat on the firm surface with absorbent pad |
| 00.53–01.09 | Squeeze out most of the blood and fatty component in the aspirate to get predominantly fibrovascular content in the bag |
| 01.10–01.19 | Transfer this bag after folding properly to the labeled container with 10% formalin |
| 01.20–01.32 | Similarly, squeeze out the blood and fatty component from the other bag with lesser quantity of aspirate for electron microscopy |
| 01.33–01.35 | Rip open the nylon mesh bag |
| 01.36–01.38 | Lay flat on the firm surface |
| 01.39–02.08 | With the help of the needle of the removed needle, swipe the fibrovascular fragments from the fat aspirate to collect and transfer to the labeled glutaraldehyde tube for electron microscopy |
| 02.09–02.19 | Shake the tube after capping to disperse the microfragments of fibrovascular tissue component for proper fixation in glutaraldehyde for electron microscopy |
| 02.20–02.28 | Discard any sharp consumables such as needles to the sharp container |
| 02.29–02.37 | Discard all the contaminated consumables into the biohazard bag for proper disposal |
| 02.38–02.48 | Spread the initial drop of the aspirate on the glass slide to make direct smear for onsite adequacy evaluation |
| 02.49–02.58 | Let the direct smears dry. Stain one of the smears with DiffQuik for rapid onsite adequacy evaluation |
| 02.58–02.59 | End ©vshidham |
Perform FNA of anterior fat pad with 18G needle as a mini- liposuction with aspiration of as many microfragments of fibroadipose tissue as possible with accumulation of at least 1.5 mL aspirate in the syringe as reported previously.[1] The updated procedure explains how to remove the interference due to blood and fatty component with the help of a nylon mesh tissue bag [Figure 1]. This improved protocol to process the anterior fat pad aspirates for electron microscopy and paraffin embedding is demonstrated in the video with Figures 2-4.
- Nylon mesh tissue bag.
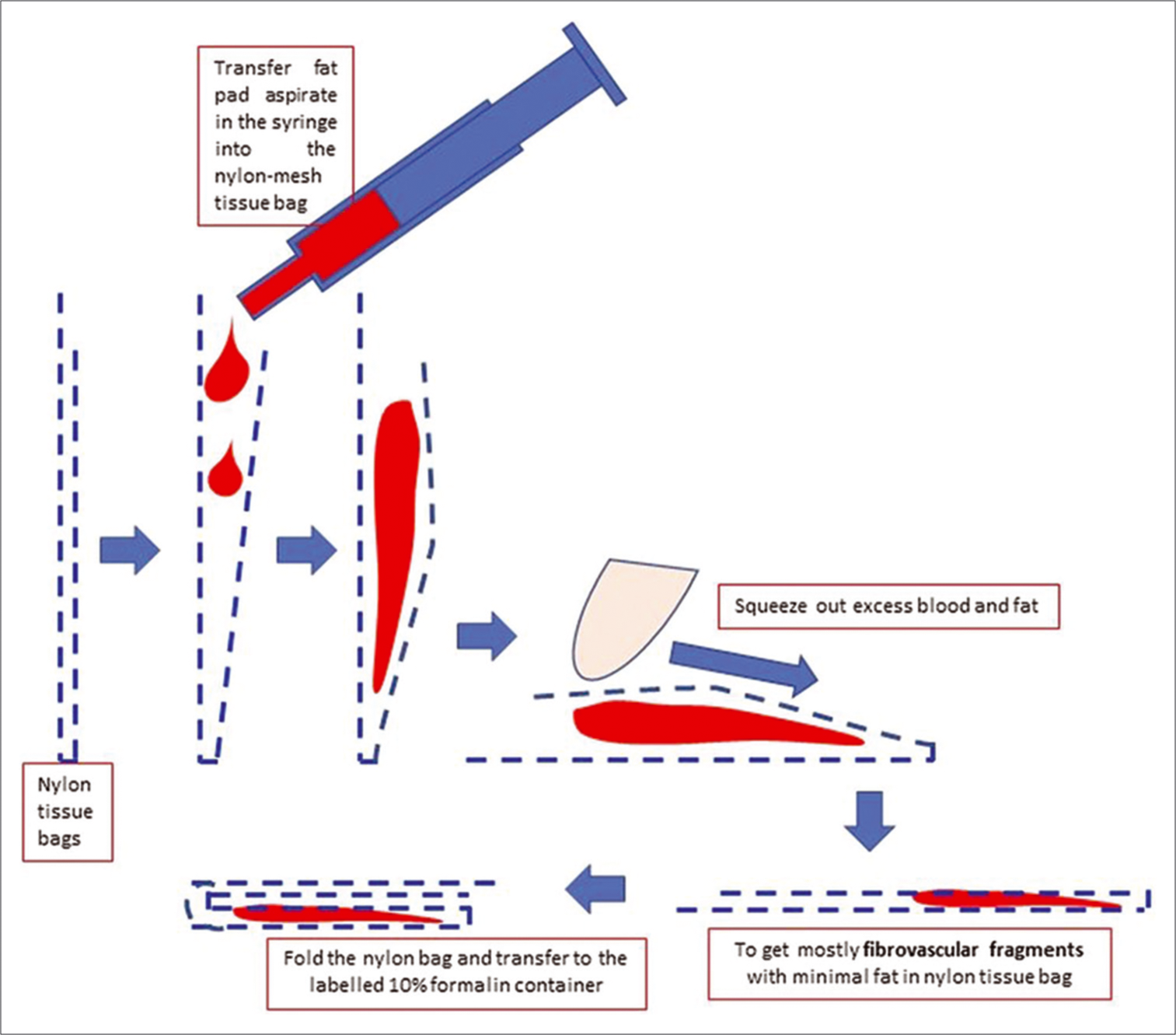
- Processing of anterior fat pad aspirate using nylon tissue bag to submit it for paraffin embedding after tissue processing.
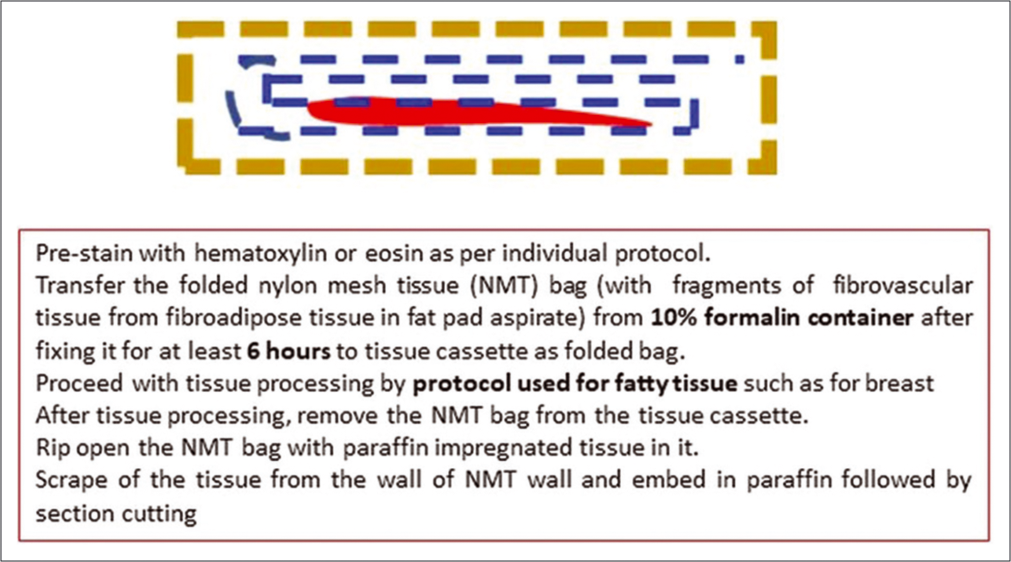
- Processing of anterior fat pad aspirate in nylon tissue bag for paraffin embedding after tissue processing.
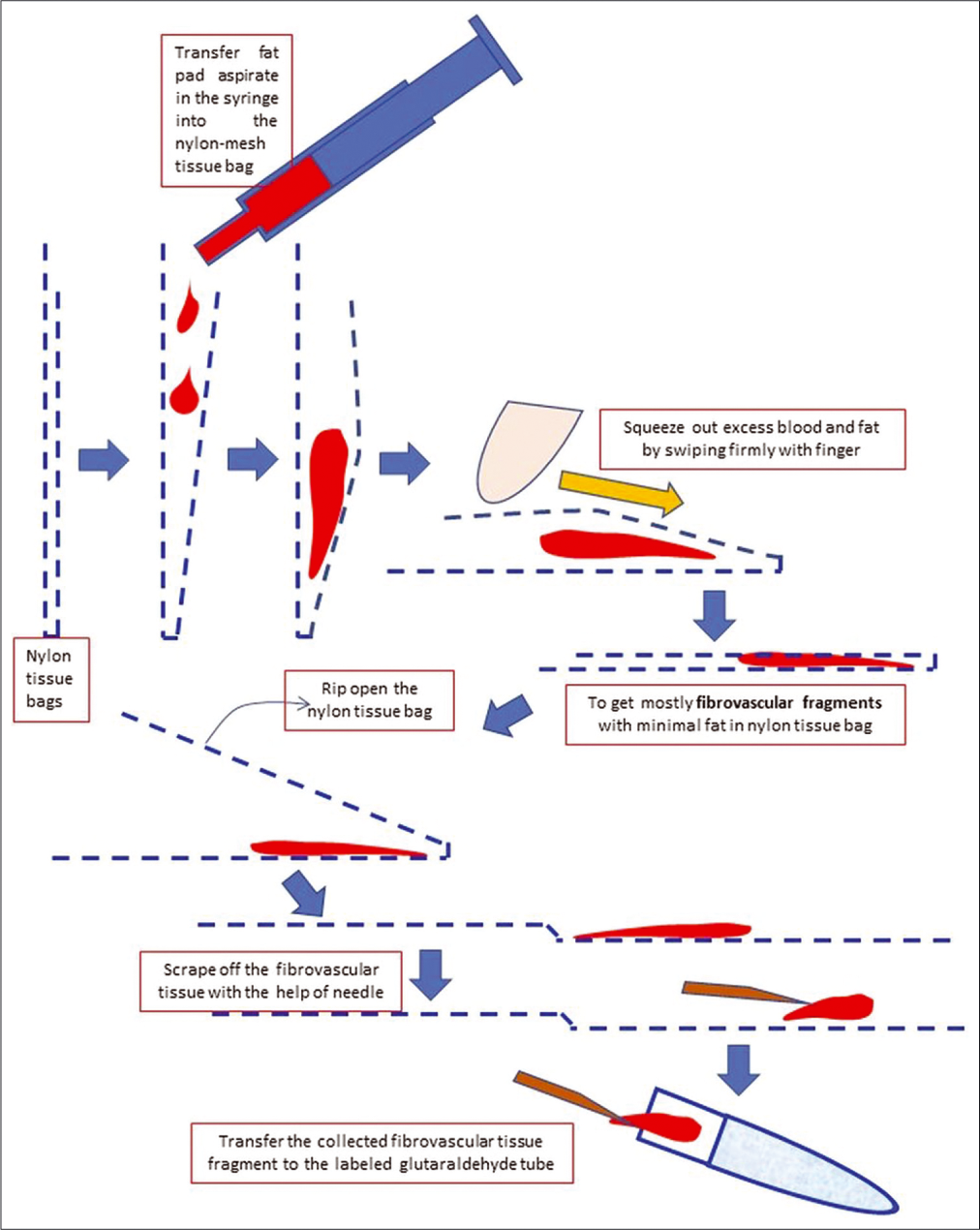
- Processing of anterior fat pad aspirate using nylon tissue bag to submit it for electron microscopy.
First, remove the needle from the syringe nozzle. Transfer a tiny portion of the aspirate in the syringe on to the glass slide. Detach the fibroadipose tissue fragment from the nozzle of the syringe with the help of the tip of the used needle if required. Next, transfer about one-fourth of the fibroadipose tissue fragments in the aspirate to one nylon mesh tissue bag and remaining aspirate to the other nylon mesh tissue bag. Most of the contaminant fresh blood and fatty portion in the aspirate are squeezed out from both the nylon mesh bags by swiping with the index finger on an absorbent pad supported by a flat firm surface.
For the portion to be submitted for the preparation of FFPE, the nylon mesh bag with a larger amount of aspirate with squeezed out blood and fat is folded and transferred to the labeled container with 10% formalin fixative [Figures 2 and 3, Video 1 (please visit the article in HTML to watch the video at https://dx.doi.org/10.25259/Cytojournal_31_2020) at counter: From 0.44 to 1.19]. Achieving maximum proportion of fibrovascular tissue in the final FFPE of the cell block is important for a successful outcome with further evaluation for subtyping of amyloid in positive cases with mass spectrophotometry after laser capture microdissection[3] and other evolving technologies.
To process for electron microscopy, the nylon mesh bag with smaller amount of aspirate is ripped open to expose the remaining fibrovascular tissue after squeezing out the fat and blood component. The fibrovascular tissue is swiped out with the needle used for the FNA procedure from the wall of the nylon mesh bag and transferred to the labeled container with glutaraldehyde fixative for electron microscopy. Close the cap and shake the tube to disperse the fibrovascular tissue fragments for optimum fixation in glutaraldehyde [Figure 4, Video 1 (please visit the article in HTML to watch the video at https://dx.doi.org/10.25259/Cytojournal_31_2020) at counter: From 1.20 to 2.19].
After this, the drop of the fibroadipose tissue on the glass slide is spread between the two glass slides to get direct smears. As the aspirate is rich in fat, the material does not dry in the time required for completing the processing to submit in 10% formalin for FFPE and in glutaraldehyde for electron microscopy. However, depending on the situation, the drop on the glass slide may be spread before starting the process for submitting in 10% formalin and in glutaraldehyde.
Both direct smears are air-dried. One of the smears is stained with DiffQuik for immediate onsite adequacy before the termination of the anterior fat pad aspiration procedure.
The other air-dried smears are rehydrated and post-fixed for Pap staining later in the Cytoprep Lab.[10] Depending on the institutional choices and biases, the second direct smear may be wet fixed for Pap staining. However, the wet direct smears with fatty material may float and a predominant portion of the material on the glass slide may be lost with suboptimal smears made with scant tissue in addition to the usual problem of interference due blood contamination in wet fixed smears.[10] The direct smears may also be used for the evaluation of amyloid in the smear with special stains, autofluorescence method, etc., as reported by some studies.[11]
Acknowledgments
The author thanks Anjani Shidham, BS and Anushree Shidham, BS, for their critical input in scientific draft editing and participation in improving this article is greatly appreciated.
COMPETING INTEREST STATEMENT BY ALL AUTHORS
The author (VS) does not have any competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
Author has reviewed this article.
ETHICS STATEMENT BY ALL AUTHORS
As this is a methodology article without identifiers, our institution does not require approval from Institutional Review Board.
LIST OF ABBREVIATION (In alphabetic order)
FFPE: Formalin-fixed paraffin-embedded.
EDITORIAL/PEER-REVIEW STATEMENT
CytoJournal editorial team thanks the academic editor: Tamara Giorgadze, MD, PhD, Professor of Pathology, Director of Cytopathology and Cytopathology Fellowship Program, Department of Pathology, Medical College of Wisconsin, Milwaukee, WI, USA for organizing and completing the peer-review process in timely manner for this manuscript as per CytoJournal’s peer-review policy.
Video available on:
References
- Performing and processing FNA of anterior fat pad for amyloid. J Vis Exp. 2010;44:1747.
- [CrossRef] [PubMed] [Google Scholar]
- Detection of amyloid in abdominal fat pad aspirates in early amyloidosis: Role of electron microscopy and Congo red stained cell block sections. Cytojournal. 2011;8:11.
- [CrossRef] [PubMed] [Google Scholar]
- Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957-9.
- [CrossRef] [PubMed] [Google Scholar]
- Proteomics and mass spectrometry in the diagnosis of renal amyloidosis. Clin Kidney J. 2015;8:665-72.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnostic accuracy of subcutaneous abdominal fat tissue aspiration for detecting systemic amyloidosis and its utility in clinical practice. Arthritis Rheum. 2006;54:2015-21.
- [CrossRef] [PubMed] [Google Scholar]
- Abdominal fat pad aspiration biopsy for tissue confirmation of systemic amyloidosis: Specificity, positive predictive value, and diagnostic pitfalls. Diagn Cytopathol. 2001;24:181-5.
- [CrossRef] [Google Scholar]
- Diagnosing amyloidosis. Scand J Rheumatol Suppl. 1995;24:327-9.
- [CrossRef] [PubMed] [Google Scholar]
- A new method for the diagnosis of systemic amyloidosis. Arch Intern Med. 1973;132:522-3.
- [CrossRef] [PubMed] [Google Scholar]
- Diagnosis and typing of systemic amyloidosis: The role of abdominal fat pad fine needle aspiration. Cytojournal. 2010;6:24.
- [CrossRef] [PubMed] [Google Scholar]
- Routine air drying of all the smears prepared during fine needle aspiration and intraoperative cytology studies: An opportunity to practice a unified protocol, offering the flexibility of choosing variety of staining methods. Acta Cytol. 2001;45:60-8.
- [CrossRef] [PubMed] [Google Scholar]
- Improved detection of amyloid in fat pad aspiration: An evaluation of Congo red stain by fluorescent microscopy. Diagn Cytopathol. 2004;31:300-6.
- [CrossRef] [PubMed] [Google Scholar]








