Translate this page into:
Upper tract urinary cytology to detect upper tract urothelial carcinoma: Using the Johns Hopkins Hospital template and evaluation of its feasibility
*Corresponding author
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Introduction:
Primary upper urinary tract (UT) urothelial carcinoma (UC) is rare. UT washing cytology is often used during UT surveillance. The Johns Hopkins Hospital template (JHHT) is primarily designed to use on lower tract urine cytology and the data on applying JHHT on UT cytology is limited. We herein study the value of UT cytology in detecting UTUC using JHHT in a cohort.
Materials and Methods:
One hundred UT cytologic specimens were retrieved from our database during a 10-year period (2001–2011). For each patient, the cytology specimen with the highest degree of abnormality was selected. Histologic sections of these cases were also studied.
Results:
Seventy-six cases of UT cytology had histologic follow-up by either serial (>2) endoscopic biopsies or nephroureterectomy or ureterectomy. Among them, the cytologic diagnosis of positive or suspicious for high-grade UC (HGUC) was made in 15 cases; suspicious for low-grade UC (LGUC) in 3 cases; atypical urothelial cells (AUCs) of undetermined significance in 19 cases; and negative in 39 cases. Of the 15 cases with diagnosis of positive for HGUC or AUC-HGUC (AUC-H), 10 had histologically confirmed HGUC, 1 had LGUC, and 4 had benign histology. All 3 cases of cytologically suspicious for LGUC had LGUC on concomitant histology. Among the 19 washings with AUCs with unknown significance, 7 were LGUC, 1 was HGUC, and 11 were benign on histology. Six of 39 cases with negative cytology had UC (3 low-grade and 3 high-grade) on histology. Combining positive and AUC-H for UC diagnoses, sensitivity, and specificity for detecting HGUC were 71.4% and 91.9%, while for LGUC were 21.4% and 100%, respectively.
Conclusions:
UT washing cytology has high specificity for detecting UC, especially HGUC. Using JHHT on UT washing cytology is feasible, but the category of LGUC may need modification.
Keywords
Johns Hopkins Hospital template
upper urinary tract
urine cytology
urothelial carcinoma
INTRODUCTION
Urothelial carcinomas (UCs) of the upper tract (UT) have an epidemiology similar to those of the bladder. However, UTUC only accounts for 5–10% of the UC in the urinary tract.[1] The standard management of high-grade UTUC is nephroureterectomy, either open or laparoscopic, with excision of a cuff of the bladder mucosa.[2] Selected patients with small low-grade UTUC may be treated with endoscopic management using laser resection. The basic requirement for such a conservative approach is the confirmation of a low-grade UTUC on ureteropyeloscopy with endoscopic biopsy of the UT.[3456] However, recent studies have shown that the accuracy of endoscopic biopsy for UT tumors is not optimal.[78] UT urine cytology has been widely used and plays an essential role to detect UTUC.[9101112] There are wide ranges of the sensitivity of UT urine cytology on detecting UTUC in the literature.[131415] Several studies have shown that high-grade UTUCs are detected quite reliably by UT cytology.[1416] On the other hand, the sensitivity of UT cytology for low-grade lesions is poor.[1617] A combined endoscopic biopsy and urine cytology approach has been shown to improve the sensitivity of detecting UTUC.[1819]
The Johns Hopkins Hospital template (JHHT) of reporting urinary cytopathology was proposed recently, and it is a seven-tiered diagnostic system.[2021] This template is intended to improve the predictability of indeterminate urine cytology diagnoses. It acknowledges that the primary goal of urinary cytology is to diagnose high-grade UC (HGUC) rather than low-grade UC (LGUC).[20] This template is primary designed to use on lower tract urine cytology and the literature of using this template on UT cytology is limited.
Our institution has been using an almost identical template as the JHHT with slight modification using “Suspicious for LGUC” rather than “LGUC.” The main objective of the present study is to investigate the diagnostic value of UT cytology. In addition, we applied the JHHT to this cohort of UT urine cytology to evaluate the feasibility of adopting the JHHT for UT cytology.
MATERIALS AND METHODS
This retrospective, cytohistologic correlation study was approved by the Cleveland Clinic's Institutional Review Board. From January 1, 2001 to June 30, 2011, we retrieved 100 UT cytology specimens (67 ureter washings and 33 renal pelvic washings), 76 of which had histologic follow-up either by serial endoscopic biopsies (>2) or nephroureterectomy or ureterectomy from our pathology database. These 76 patients formed our study cohort. Patients with previous or concomitant diagnosis of bladder UC were excluded from the study.
The UT urine cytology cases were reviewed and the most severe cytologic diagnosis was recorded. The JHHT of reporting urinary cytopathology was applied on all the UT cytology cases. In our institution, we routinely used a similar diagnostic terminology with the slight modification of replacing “LGUC” with “suspicious for LGUC.” Thus, it was relatively easy to apply the JHHT to our study.
The diagnostic categories were: (1) Positive for HGUC [Figure 1]; (2) atypical urothelial cells, cannot exclude HGUC (AUC-H) [Figure 2]; (3) AUCs of unknown significance (AUC-US); (4) LGUC [Figure 3]; and (5) negative for HGUC [Figure 4]. The diagnosis of AUC-H was rendered when there were rare (<5 cells) with cytologic features of HGUC. The diagnosis of LGUC was made when a concomitant biopsy showed LGUC or a true fibrovascular core was observed and there was mild cytologic atypia.
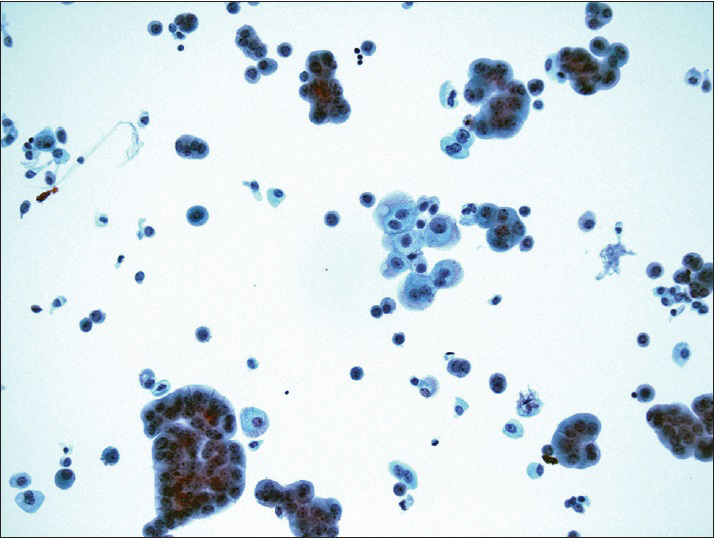
- Positive for high-grade urothelial carcinoma. Papillary clusters of malignant urothelial cells show nuclear hyperchromasia, nuclear enlargement, and irregular nuclear membrane (ThinPrep®, Pap stain ×40)
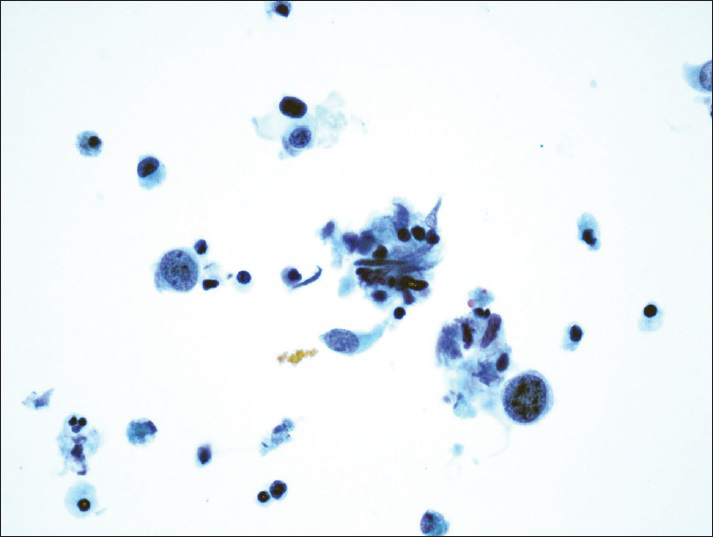
- Atypical urothelial cells, cannot exclude high-grade urothelial carcinoma. Rare atypical urothelial cells with cytomorphologic features suggestive of high-grade urothelial carcinoma (ThinPrep®, Pap stain ×40)
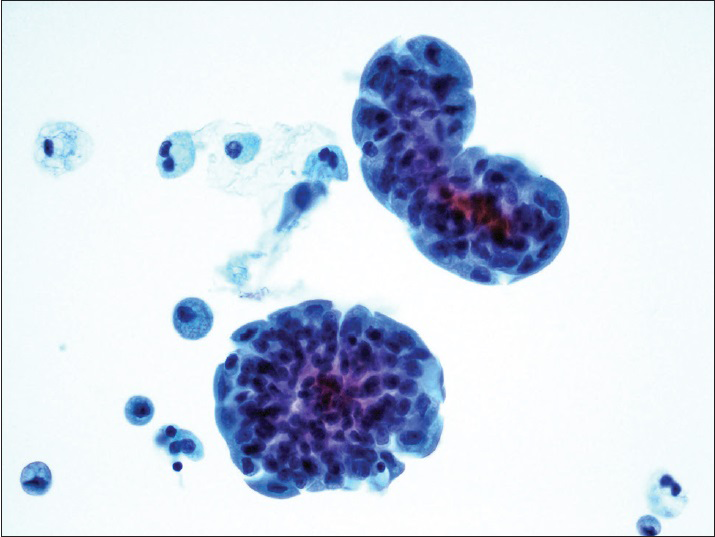
- Low-grade papillary urothelial carcinoma. Tight clusters of hyperchromatic urothelial cells show nuclear membrane irregularity. The cells lack nuclear pleomorphism. The concurrent histology revealed low-grade papillary urothelial carcinoma (ThinPrep®, Pap stain ×40)
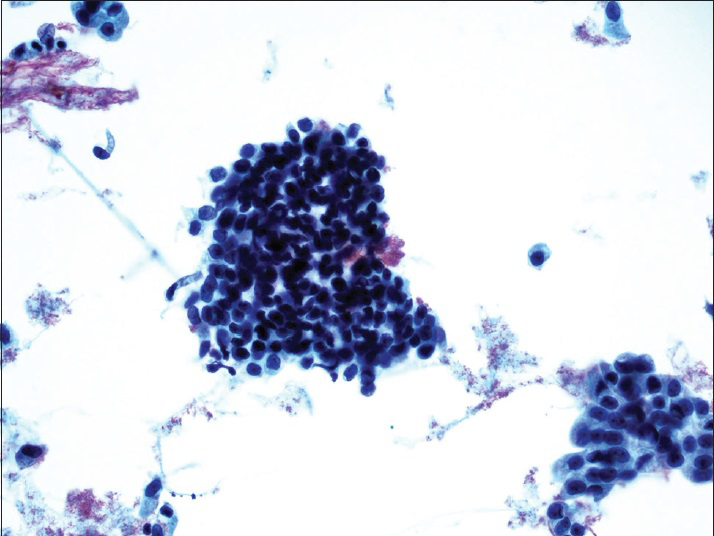
- Benign urothelial cells from the upper tract show tight pseudopapillary clusters, typically due to instrumentation. The urothelial cells have relatively low N/C ratio, no nuclear membrane irregularity, and no nuclear hyperchromasia (ThinPrep®, Pap stain ×20)
All available histologic follow-up diagnoses from serial endoscopic UT biopsies, nephroureterectomies, or ureterectomies were also reviewed. The most severe histologic diagnosis was recorded when multiple UT endoscopic biopsies were performed. UC was graded according to the 2004 world health organization/1998 International Society of Urologic Pathology consensus classification.[22]
The sensitivity and specificity of UT urine cytology detecting UTUC were calculated. For practical purpose, the groups of HGUC and AUC-H were combined when calculating the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of UT urine cytology for HGUC, since the clinical management for both groups was the same at our institution. Statistical comparisons were performed using the Fisher exact test, based on 2 × 2 contingency tables. Calculations were conducted with the SAS software system (SAS Inc., Cary, NC, USA).
RESULTS
Seventy-six patients (100 UT cytology specimens) with a mean age of 53 years (29–87) were identified who met the inclusion criteria. There were 32 women and 44 men. Twenty-eight patients underwent radical nephroureterectomy or ureterectomy and 48 patients had more than 2 endoscopic UT biopsies. The UT cytology results showed that 8 cases were reported as positive for HGUC. Seven cases were reported as AUC-H, 3 were reported as LGUC, 19 were AUC-US, and 39 were negative for HGUC. The histologic follow-up for both the HGUC and AUC-H groups showed 10 patients with HGUC, 1 patient with LGUC, and 4 patients without UC (2 with reactive atypia and 2 with dysplasia). For the 3 patients with the UT cytology diagnosis of “LGUC,” the follow-up nephroureterectomy or ureterectomy specimens demonstrated LGUC on all 3 cases. These 3 cases also had concomitant endoscopic biopsies showing LGUC. For the 19 patients with UT cytology diagnosis of AUC-US, the histologic follow-up was 1 with HGUC (by resections), 7 with LGUC (by resections, either radical or endoscopic), and 11 with nonneoplastic lesions (by serial biopsies). The histologic follow-up for the 39 patients with negative for HGUC UT cytology revealed: Three patients with HGUC on radical nephroureterectomy or ureterectomy, 3 with LGUC on resections, and the remaining 33 patients with negative histology on serial biopsies. The results of the histologic follow-up of the 76 patients with UT urine cytology are summarized in Table 1.
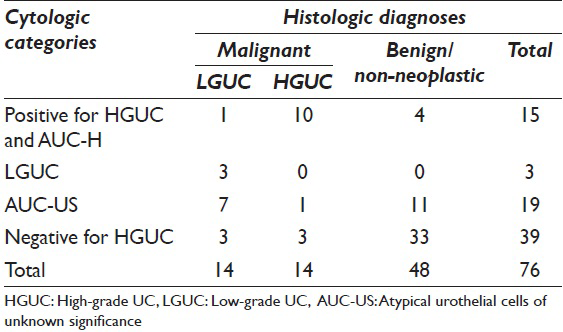
Sensitivity, specificity, PPV, and NPV of UT urine cytology for HGUC by combining the HGUC and AUC-H groups were 71.4%, 91.9%, 66.7%, and 93.4%, respectively. Since the cytology diagnosis of LGUC was rarely used (3 out of 76) and all 3 cases had concomitant biopsy histology showing LGUC, the sensitivity, specificity, PPV, and NPV of UT urine cytology was not calculated.
DISCUSSIONS
The JHHT for reporting urinary cytology (JHHT) has been proposed recently to be used in lower tract urine cytology. JHHT acknowledges that the strength of urinary cytology is the accurate and reliable detection of HGUC. Urinary cytology demonstrates limited accuracy for low-grade urothelial neoplasms. Thus indeterminate diagnoses are unavoidable.[2021] The main goal of JHHT is to improve the predictability of indeterminate urine cytology diagnoses. The main objective of this study is to evaluate the feasibility of applying JHHT on UT urine cytology, since it is primarily designed for bladder urine cytology.
UTUC is relatively uncommon, but is associated with significant morbidity and mortality. Current management of UTUC depends largely on patient's risk stratification.[16] UT cytology and endoscopic biopsy play a pivotal role in UTUC treatment paradigms. A recent study by Smith et al.[8] from our institution shows that a single endoscopic biopsy is not adequate for diagnosis of UTUC. In that study, more than one-third of patients with UTUC have a change of grade and/or stage in subsequent biopsies. Due to the fact that a single endoscopic biopsy is not the “gold standard” for cytology-histology correlation, we designed our study accordingly by including only those patients with more than 2 endoscopic biopsies to correlate with their UT urine cytology.
Our study demonstrates that a high percentage (66.7%) of HGUC and AUC-H cytologic specimens reveal HGUC on surgical resections. The specificity and NPV of UT urine cytology for HGUC are 91.9% and 93.4%. These results are in keeping with recent studies of UT urine cytology.[111416] The sensitivity of UT cytology for detecting high-grade UTUC ranges from 69% to 89% in previous studies.[111423] Our study reveals a sensitivity of 71.4%, which falls into the above range as well. We calculated the UT cytology sensitivity by combining positive and AUC-H cases without including AUC-US cases. By itself, AUC-US has a very low sensitivity (25%) for detecting high-grade UTUC in our study. This result is in contrast with a previous study by Muus Ubago et al.,[24] which showed that AUC on UT cytology has higher predictive value for developing UTUC. On the other hand, 4 of 7 cases (57%) with AUC-H diagnosis showed high-grade UTUC in our study, justifying the similar clinical management to the positive for UC patients. The AUCs in the “AUC-H” category are similar to those described in the JHHT,[20] defined as rare (<5) abnormal cells with larger than normal and usually pleomorphic nuclei with prominent nucleoli. Nuclear contours are irregular and the nucleus is usually hyperchromatic with clumped, irregular chromatin. The cytoplasm ranges from scant to abundant, with occasional cytoplasmic vacuoles. However, the cellularity and cytomorphologic features of AUC-H cases fall short of the threshold of calling “positive for HGUC.” Our result supports the JHHT proposal to separate AUC-H from AUC-US.
Our results on low-grade UTUC are misleading, but interesting. First, we rarely use the diagnostic category of “LGUC” (4%) on UT urine cytology specimens; second, all 3 of these cases with cytologic diagnosis of “LGUC” have concurrent endoscopic biopsies, which show LGUC; finally, 7 of the 19 AUC UT cytology cases show LGUC on histology. It has been well-documented in the literature that UT urine cytology has much lower sensitivity and specificity for detecting UT LGUC as compared to HGUC.[111716] This is mainly due to overlapping cytomorphologic features between LGUC cells and benign/reactive urothelial cells. As expected, in our study, “LGUC” is only used when there is concomitant histology showing LGUC, while 7 of 19 AUC-US cytology cases turn out to be LGUC on histology. This is in agreement with a recent study on bladder urine cytology by Morency and Antic,[25] which showed that 48 of the 144 AUC-US cases have LGUC on histology. Upon further review of these seven cases, we found that the AUCs showing a slight variation in nuclear size, mild increase of nuclear to cytoplasmic ratio, and irregular nuclear membrane with homogenous cytoplasm. Interestingly, the cellularity of these cases is not significantly higher than those negative cases as UT cytology cases are usually quite cellular. These data suggest that since there are no reliable morphologic features to distinct LGUC from reactive/instrumentation effect, cytopathologists tend to include these subtle changes into the AUC-US category, rather than use the “LGUC” terminology. The main purpose of including the AUC-US in the JHHT is to separate the rather large and heterogenous atypical urine cytology cases into different categories with risk of malignancy and limit the over-use of atypical urine cytology. Only rare AUC-US cases (1 of 19) in our study demonstrate HGUC on histology, on the other hand, a relative high percentage (36.8%) of AUC-US cases show LGUC. Modification of the “LGUC” terminology to “AUCs, suspicious or cannot exclude LGUC” may be indicated. Witte et al., have proposed a similar terminology for UT urine cytology on detecting LGUC in their study.[11]
UroVysion/fluorescent in-situ hybridization (FISH) is the only molecular-based Food and Drug Administration (FDA) approved a test for surveillance of UC in voided urine.[26] Several studies have shown that FISH in conjunction with UT urine cytology can improve the sensitivity of detecting UTUC, especially LGUC.[272829] However, since FISH has not been approved by FDA at this moment to use on UT urine cytology specimens, we do not routinely do FISH in our institution on UT urine cytology specimens.
CONCLUSION
Our study demonstrates that using the JHHT, UT urine cytology has a relatively high sensitivity in detecting high-grade UTUC. In addition, the “positive for HGUC” and “AUC-H” categories have a high specificity for HGUC. On the other hand, the diagnostic category of “LGUC” is rarely used and the AUC-US category tends to have a high sensitivity in detecting low-grade UTUC. A modification of the terminology of “LGUC” is suggested.
COMPETING INTERESTS STATEMENT BY ALL AUTHORS
All authors declare no competing interests.
AUTHORSHIP STATEMENT BY ALL AUTHORS
All authors of this article declare that they qualify for authorship as defined by the ICMJE. All authors are responsible for the conception of this study, have participated in its design and coordination, and helped to draft the manuscript. All authors have read and approved the final manuscript.
ETHICS STATEMENT BY ALL AUTHORS
This study was conducted with approval from the Institutional Review Board (IRB) of the institution. Authors take responsibility to maintain relevant documentation in this report.
LIST OF ABBREVIATIONS
UT - Urinary Tract
UC - Urothelial Carcinoma
JHHT - Johns Hopkins Hospital template
HGUC - High-grade UC
LGUC - Low-grade UC
AUCs - Atypical Urothelial Cells
PPV - Positive Predictive Value
NPV - Negative Predictive Value
FDA - Food and Drug Administration
IRB - Institutional Review Board.
EDITORIAL/PEER-REVIEW STATEMENT
To ensure the integrity and highest quality of CytoJournal publications, the review process of this manuscript was conducted under a double blind model (authors are blinded for reviewers and vice versa) through the automatic online system.
REFERENCES
- Prognostic factors, recurrence, and survival in transitional cell carcinoma of the upper urinary tract: A 30-year experience in 252 patients. Urology. 1998;52:594-601.
- [Google Scholar]
- Urothelial carcinoma of the upper urinary tract: Surgical approach and prognostic factors. Eur Urol. 2008;53:720-31.
- [Google Scholar]
- Ureteroscopic treatment and surveillance of upper urinary tract transitional cell carcinoma. J Urol. 1997;157:1560-5.
- [Google Scholar]
- Is nephroureterectomy necessary in all cases of upper tract transitional cell carcinoma? Long-term results of conservative endourologic management of upper tract transitional cell carcinoma in individuals with a normal contralateral kidney. Urology. 2001;58:174-8.
- [Google Scholar]
- Endoscopic management of upper urinary tract transitional cell carcinoma: Long-term experience. Cancer. 2003;98:55-60.
- [Google Scholar]
- Ureteroscopic management of upper-tract urothelial cancer: An exciting nephron-sparing option or an unacceptable risk? J Endourol. 2008;22:1237-9.
- [Google Scholar]
- Small endoscopic biopsies of the ureter and renal pelvis: Pathologic pitfalls. Am J Surg Pathol. 2009;33:1540-6.
- [Google Scholar]
- Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: Implications for conservative management. Urology. 2011;78:82-6.
- [Google Scholar]
- Papillary transitional-cell carcinoma of the upper urinary tract: A cytological review. Diagn Cytopathol. 1990;6:204-9.
- [Google Scholar]
- Cytologic diagnosis of upper urinary tract neoplasms by ureteroscopic sampling. Acta Cytol. 1995;39:733-40.
- [Google Scholar]
- Transitional cell carcinoma of the renal pelvis the diagnostic role of pelvic washings. Am J Clin Pathol. 2002;117:444-50.
- [Google Scholar]
- Urine cytology in the evaluation of upper tract urothelial lesions. J Urol. 2004;172:2406.
- [Google Scholar]
- Surveillance of upper urinary tract transitional cell carcinoma: The role of ureteroscopy, retrograde pyelography, cytology and urinalysis. J Urol. 2000;164:1901-4.
- [Google Scholar]
- Comparison of ureteral washing and biopsy specimens in the community setting. Cancer. 2006;108:45-8.
- [Google Scholar]
- The predictive value of positive urine cytology for outcomes following radical nephroureterectomy in patients with primary upper tract urothelial carcinoma: A multi-institutional study. Urol Oncol. 2014;32:48.e19-26.
- [Google Scholar]
- Diagnosis of upper tract urothelial carcinoma – A comparative study of urine cytology and surgical biopsy. J Am Soc Cytopathol. 2015;4:3-9.
- [Google Scholar]
- The cytologic diagnosis of low-grade transitional cell carcinoma. Am J Clin Pathol. 2000;114(Suppl):S59-67.
- [Google Scholar]
- Cytologic analysis of ureteral washings is informative in patients with grade 2 upper tract TCC considering endoscopic treatment. Urology. 2003;61:1146-50.
- [Google Scholar]
- Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol. 2008;22:71-6.
- [Google Scholar]
- The Johns Hopkins Hospital template for urologic cytology samples: Part I-creating the template. Cancer Cytopathol. 2013;121:15-20.
- [Google Scholar]
- The Johns Hopkins Hospital template for urologic cytology samples: Parts II and III: Improving the predictability of indeterminate results in urinary cytologic samples: An outcomes and cytomorphologic study. Cancer Cytopathol. 2013;121:21-8.
- [Google Scholar]
- The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435-48.
- [Google Scholar]
- Urinary cytology has a poor performance for predicting invasive or high-grade upper-tract urothelial carcinoma. BJU Int. 2011;108:701-5.
- [Google Scholar]
- Evaluation of atypical urine cytology progression to malignancy. Cancer Cytopathol. 2013;121:387-91.
- [Google Scholar]
- Atypical urine cytology and the Johns Hopkins Hospital template: The University of Chicago experience. J Am Soc Cytopathol. 2014;3:295-302.
- [Google Scholar]
- A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol. 2000;164:1768-75.
- [Google Scholar]
- Fluorescence in situ hybridization for detecting upper urinary tract tumors – A preliminary report. Urology. 2007;70:753-7.
- [Google Scholar]
- Utility of fluorescence in situ hybridization in the diagnosis of upper urinary tract urothelial carcinoma. Cancer Genet Cytogenet. 2009;189:93-7.
- [Google Scholar]
- Comparison of urine cytology and fluorescence in situ hybridization in upper urothelial tract samples. Cancer Cytopathol. 2014;122:459-67.
- [Google Scholar]








